Published online May 26, 2020. doi: 10.4330/wjc.v12.i5.161
Peer-review started: February 29, 2020
First decision: April 7, 2020
Revised: April 29, 2020
Accepted: May 5, 2020
Article in press: May 5, 2020
Published online: May 26, 2020
Processing time: 86 Days and 16.8 Hours
Pulmonary embolism (PE) is an important public health problem. In August 2019, the European Society of Cardiology in collaboration with the European Respiratory Society released new guidelines for the diagnosis and management of PE. We discuss the basic changes between these recent guidelines and the previous guidelines that were published in 2014. Regarding diagnosis, the new guidelines propose the use of an age-adjusted cut-off level of D-dimers instead of a fixed cut-off value. A D-dimer test adapted to clinical possibility should also be considered instead of fixed cut-off level of D-dimer. Detailed recommendations for the diagnosis of PE during pregnancy are also provided. Regarding risk stratification, assessment of PE-related early mortality risk is recommended. Moreover, the importance of right ventricular dysfunction is emphasized in low-risk patients. For further risk stratification of the severity of PE in patients without hemodynamic instability, use of validated scores that combine clinical, imaging and laboratory PE-related prognostic factors might also be considered. Regarding treatment, the possibility of early discharge is mentioned in patients without severe comorbidities, who are not of high risk for sudden death and in whom proper medical management at home and proper medical follow up can be ensured. The new guidelines also suggest that pro-brain natriuretic peptide levels, right ventricular function and the presence of thrombus in the right heart could be useful for guiding the decision of early discharge. Overall, these new guidelines introduce several key changes and knowledge and adherence to them will improve the outcome of patients with PE.
Core tip: We discuss the basic changes between the recent guidelines published in August 2019 by the European Society of Cardiology in collaboration with the European Respiratory Society regarding the diagnosis and management of pulmonary embolism and the previous guidelines that were published in 2014. The use of age-specific cut-off levels of D-dimers, detailed recommendations for risk stratification and the possibility of outpatient management are some of the key changes. Knowledge and adherence to these new guidelines will improve the outcome of patients with pulmonary embolism.
- Citation: Erythropoulou-Kaltsidou A, Alkagiet S, Tziomalos K. New guidelines for the diagnosis and management of pulmonary embolism: Key changes. World J Cardiol 2020; 12(5): 161-166
- URL: https://www.wjgnet.com/1949-8462/full/v12/i5/161.htm
- DOI: https://dx.doi.org/10.4330/wjc.v12.i5.161
Venous thromboembolism (VTE), which includes both deep vein thrombosis (DVT) and pulmonary embolism (PE), is an important public health problem. The annual incidence of first-time VTE in the United States is 71-117 cases per 100000[1]. Moreover, the 28-d case fatality rate after a first episode of VTE is approximately 11%[2]. However, PE-related mortality rates have declined recently[3,4]. This decrease could be due to improvements in the diagnosis and managements of PE[4]. However, the decrease in PE-mortality might also be related to the overdiagnosis of PE, due to introduction and overuse of computed tomographic pulmonary angiography, in which non-clinically important PE is diagnosed and treated[5].
In August 2019, the European Society of Cardiology (ESC) in collaboration with the European Respiratory Society released the new guidelines for the diagnosis and management of PE[6]. This editorial will focus on the basic changes between the recent guidelines of the ESC for the diagnosis and management of PE and the previous guidelines that were published in 2014.
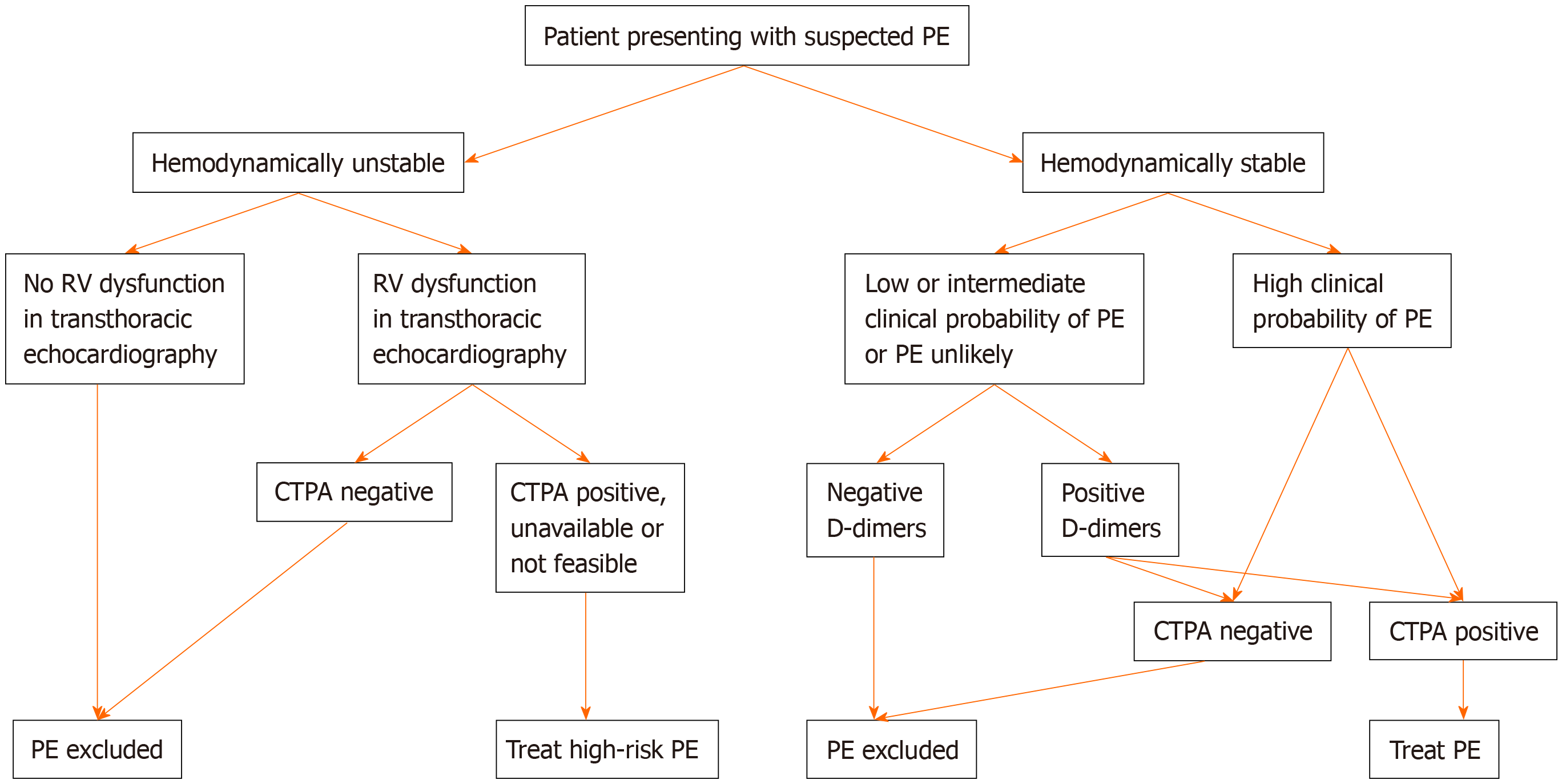
Flow chart for the diagnosis of pulmonary embolism was shown in Figure 1. Based on the new ESC guidelines, instead of a fixed-cut off level of D-dimers (500 ng/mL), an age-adjusted cut-off level of D-dimers should be considered to exclude PE in patients with low or intermediate clinical possibility for PE and in those where PE is unlikely[7,8]. The age-adjusted cut-off level of D-dimers is calculated by multiplying the age of the patient by 10 (for patients older than 50 years). Thus, in a 60-year-old patient who has a low or intermediate clinical possibility for PE or who is unlikely to have PE, D-dimers levels < 600 ng/mL (i.e., age × 10) instead of D-dimers levels < 500 ng/mL (i.e., the fixed-cut off level) excludes PE. On the other hand, in a 40-year-old patient who has a low or intermediate clinical possibility for PE or who is unlikely to have PE, the fixed-cut off D-dimers level of < 500 ng/mL should be used, since the patient is younger than 50 years.
A D-dimer test adapted to clinical possibility should also be considered instead of fixed cut-off level of D-dimer[6]. Based on the YEARS study, if D-dimer levels are < 1000 ng/mL and none of the 3 clinical items of Wells score (signs of DVT, hemoptysis or PE being the most likely diagnosis) are present or if D-dimer levels are < 500 ng/mL and one or more clinical items of Wells score are present, then a diagnosis of PE should be excluded[9].
There is also a change in the class of recommendation for the use of D-dimer levels during pregnancy and the post-partum period. According to the new guidelines, D-dimer measurement and clinical prediction rules should be considered to exclude PE during pregnancy and post-partum period[6]. Moreover, in case of suspected PE during pregnancy or the first 6 weeks post-partum, a specific diagnostic workup is provided to rule out or confirm the diagnosis of PE[6]. This updated diagnostic algorithm is based on recently published multicenter trials[10,11].
Furthermore, the 2019 guidelines summarize not only the advantages and disadvantages of the various diagnostic imaging tests but also describe and compare the exposure to radiation with the different tests.
Another change refers to the use of lower limb compression ultrasonography (CUS). The previous guidelines mentioned that, if CUS reveals proximal DVT in a patient and there is clinical suspicion of PE, a diagnosis of PE is established. However, the new recommendation in the 2019 guidelines, is that, if a positive CUS is used for the confirmation of PE, then risk assessment for PE severity and early mortality should be consider to guide further management[6].
In the 2019 guidelines, the role of ventilation/perfusion SPECT in the diagnosis of PE is emphasized more compared with the 2014 guidelines. In the new guidelines, it is mentioned that ventilation/perfusion SPECT may be considered for the diagnosis of PE[6]. However, more studies are needed to define the best SPECT technique.
In the 2019 guidelines, there is a definition of haemodynamic instability, which indicates acute high-risk PE. Three clinical manifestations of haemodynamic instability are mentioned (cardiac arrest, obstructive shock and persistent hypotension) and for each one, a clear definition is given, so that clinicians can decide if the patient is hemodynamically unstable or not. More specifically, haemodynamic instability is defined as: (1) Cardiac arrest i.e., need for cardiopulmonary resuscitation; (2) Obstructive shock i.e., systolic blood pressure (SBP) < 90 mmHg (or need for vasopressors to achieve SBP ≥ 90 mmHg) despite adequate filling status and end-organ hypoperfusion (altered mental status, cold/clammy skin, oliguria/anuria or increased serum lactate); or (3) Persistent hypotension i.e. SBP < 90 mmHg or SBP drop ≥ 40 mmHg, lasting > 15 min and not caused by new-onset arrhythmia, hypovolemia or sepsis.
Although the first risk stratification is based on the clinical manifestations of haemodynamic instability, assessment of PE severity and PE-related, early mortality risk is also recommended for patients with PE but without symptoms and signs of haemodynamic instability[6]. The prognostic criteria, on which the further risk stratification is based, are separated into 2 categories: (1) Clinical, imaging and laboratory parameters, the most important of which is right ventricular dysfunction; and (2) Comorbidities and other conditions that have an adverse effect on early prognosis.
In the 2019 guidelines, emphasis is given to right ventricular dysfunction, which is associated with increased risk for short-term mortality in hemodynamically stable patients with PE. Right ventricular dysfunction should be evaluated either with ultrasound or with laboratory prognostic biomarkers [cardiac troponins, brain natriuretic peptide (BNP) or proBNP], even if the Pulmonary Embolism Severity Index (PESI) is low or the simplified PESI (sPESI) is zero[6,12,13].
For further risk stratification of the severity of PE in patients without hemodynamic instability, use of validated scores (the Bova and the H-FABP scores) that combine clinical, imaging and laboratory PE-related prognostic factors might also be considered[6,14,15].
Patients with PE are treated according to their hemodynamic status and their risk profile. More specifically, thrombolysis is recommended in patients with PE who are hemodynamically unstable and at high risk. If thrombolysis is contraindicated or unsuccessful, surgical pulmonary embolectomy or percutaneous catheter-directed therapy might be considered[6,16,17]. Even though reperfusion therapy might be lifesaving, it is not indicated in all patients with PE because of the increased bleeding risk[6,16,18].
The new guidelines also mention the possibility of early discharge (i.e., at 24 h) in patients without severe comorbidities, who are not of high risk for sudden death and in whom proper medical management at home and proper medical follow up can be ensured[6]. This recommendation is based on the results of the multi-center HESTIA trial, which evaluated the out-of-hospital treatment in patients with low-risk PE and showed that they could safely be treated at home. In fact, only 2% of these patients experienced recurrent VTE and none of these episodes occurred during the first 7 d of treatment[19]. In another study, patients with PE and a low PESI score were treated at home and had a very low PE-related and all-cause mortality[20]. The new guidelines also suggest that proBNP levels, right ventricular function and the presence of thrombus in the right heart could be useful for guiding the decision of early discharge[6,21].
Long-term treatment of patients with PE includes anticoagulant therapy for at least 3-6 mo[6]. Whether the treatment should be extended beyond this period depends on the risk of recurrence[6]. In patients with PE due to a treatable or transient risk factor, discontinuation of anticoagulation at 3 mo is recommended[6].
Direct-acting oral anticoagulants are the treatment of choice in patients with PE, except during pregnancy and in patients with severe renal impairment or the antiphospholipid syndrome[6]. In patients with antiphospholipid syndrome, vitamin K antagonists indefinitely are the treatment of choice[6]. In pregnant women and in patients with severe renal impairment, low-molecular weight heparin is the recommended treatment[6]. Patients with cancer should also be treated with low-molecular weight heparin even though Direct-acting oral anticoagulants can also be considered based on the results of recent trials[22,23].
The use of vena cava filters is suggested only in patients with absolute contraindications to anticoagulant treatment[6]. However, they do not appear to reduce the risk of PE recurrence or PE-related mortality[24,25].
Finally, all patients with PE should be followed-up regularly because of the increased incidence of cancer (which might not be detectable at the time of PE), the risk of bleeding complications and the risk for development of chronic thromboembolic pulmonary hypertension[6].
The recent guidelines for the diagnosis and treatment of PE include several key changes which facilitate the management of this common and potentially life-threatening medical emergency (Table 1). Knowledge and adherence to these guidelines will improve the outcome of these patients.
| Diagnosis | An age-adjusted cut-off level of D-dimers can be used instead of a fixed cut-off value |
| Risk assessment | Assessment of PE-related early mortality risk is recommended |
| The importance of right ventricular dysfunction is emphasized in low-risk patients | |
| Treatment | The possibility of early discharge is mentioned in patients without severe comorbidities, who are not of high risk for sudden death and in whom proper medical management at home and proper medical follow up can be ensured |
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fabbian F, Fiore M, Yu XJ S-Editor: Dou Y L-Editor: A E-Editor: Qi LL
| 1. | White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107:I4-I8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 950] [Cited by in RCA: 1112] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 2. | Cushman M, Tsai AW, White RH, Heckbert SR, Rosamond WD, Enright P, Folsom AR. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 588] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 3. | Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979-1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163:1711-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 443] [Article Influence: 20.1] [Reference Citation Analysis (1)] |
| 4. | Jiménez D, de Miguel-Díez J, Guijarro R, Trujillo-Santos J, Otero R, Barba R, Muriel A, Meyer G, Yusen RD, Monreal M; RIETE Investigators. Trends in the Management and Outcomes of Acute Pulmonary Embolism: Analysis From the RIETE Registry. J Am Coll Cardiol. 2016;67:162-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 256] [Article Influence: 28.4] [Reference Citation Analysis (1)] |
| 5. | Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med. 2011;171:831-837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 322] [Cited by in RCA: 336] [Article Influence: 24.0] [Reference Citation Analysis (2)] |
| 6. | Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, Huisman MV, Humbert M, Jennings CS, Jiménez D, Kucher N, Lang IM, Lankeit M, Lorusso R, Mazzolai L, Meneveau N, Ní Áinle F, Prandoni P, Pruszczyk P, Righini M, Torbicki A, Van Belle E, Zamorano JL; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 2610] [Article Influence: 652.5] [Reference Citation Analysis (1)] |
| 7. | Schouten HJ, Geersing GJ, Koek HL, Zuithoff NP, Janssen KJ, Douma RA, van Delden JJ, Moons KG, Reitsma JB. Diagnostic accuracy of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013;346:f2492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 209] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 8. | Righini M, Van Es J, Den Exter PL, Roy PM, Verschuren F, Ghuysen A, Rutschmann OT, Sanchez O, Jaffrelot M, Trinh-Duc A, Le Gall C, Moustafa F, Principe A, Van Houten AA, Ten Wolde M, Douma RA, Hazelaar G, Erkens PM, Van Kralingen KW, Grootenboers MJ, Durian MF, Cheung YW, Meyer G, Bounameaux H, Huisman MV, Kamphuisen PW, Le Gal G. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA. 2014;311:1117-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 590] [Article Influence: 53.6] [Reference Citation Analysis (1)] |
| 9. | van der Hulle T, Cheung WY, Kooij S, Beenen LFM, van Bemmel T, van Es J, Faber LM, Hazelaar GM, Heringhaus C, Hofstee H, Hovens MMC, Kaasjager KAH, van Klink RCJ, Kruip MJHA, Loeffen RF, Mairuhu ATA, Middeldorp S, Nijkeuter M, van der Pol LM, Schol-Gelok S, Ten Wolde M, Klok FA, Huisman MV; YEARS study group. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 338] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 10. | Righini M, Robert-Ebadi H, Elias A, Sanchez O, Le Moigne E, Schmidt J, Le Gall C, Cornuz J, Aujesky D, Roy PM, Chauleur C, Rutschmann OT, Poletti PA, Le Gal G; CT-PE-Pregnancy Group. Diagnosis of Pulmonary Embolism During Pregnancy: A Multicenter Prospective Management Outcome Study. Ann Intern Med. 2018;169:766-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 11. | van der Pol LM, Tromeur C, Bistervels IM, Ni Ainle F, van Bemmel T, Bertoletti L, Couturaud F, van Dooren YPA, Elias A, Faber LM, Hofstee HMA, van der Hulle T, Kruip MJHA, Maignan M, Mairuhu ATA, Middeldorp S, Nijkeuter M, Roy PM, Sanchez O, Schmidt J, Ten Wolde M, Klok FA, Huisman MV; Artemis Study Investigators. Pregnancy-Adapted YEARS Algorithm for Diagnosis of Suspected Pulmonary Embolism. N Engl J Med. 2019;380:1139-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 198] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 12. | Barco S, Mahmoudpour SH, Planquette B, Sanchez O, Konstantinides SV, Meyer G. Prognostic value of right ventricular dysfunction or elevated cardiac biomarkers in patients with low-risk pulmonary embolism: a systematic review and meta-analysis. Eur Heart J. 2019;40:902-910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 174] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 13. | Coutance G, Cauderlier E, Ehtisham J, Hamon M, Hamon M. The prognostic value of markers of right ventricular dysfunction in pulmonary embolism: a meta-analysis. Crit Care. 2011;15:R103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 188] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 14. | Hobohm L, Hellenkamp K, Hasenfuß G, Münzel T, Konstantinides S, Lankeit M. Comparison of risk assessment strategies for not-high-risk pulmonary embolism. Eur Respir J. 2016;47:1170-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Dellas C, Tschepe M, Seeber V, Zwiener I, Kuhnert K, Schäfer K, Hasenfuß G, Konstantinides S, Lankeit M. A novel H-FABP assay and a fast prognostic score for risk assessment of normotensive pulmonary embolism. Thromb Haemost. 2014;111:996-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, Jenkins JS, Kline JA, Michaels AD, Thistlethwaite P, Vedantham S, White RJ, Zierler BK; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123:1788-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1460] [Cited by in RCA: 1566] [Article Influence: 111.9] [Reference Citation Analysis (0)] |
| 17. | Beckerman Z, Bolotin G. Surgical Treatment of Acute Massive Pulmonary Embolism. In: Islam M. Thrombosis and Embolism: from Research to Clinical Practice. Adv Exp Med Biol. 2016;906. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Meyer G, Vicaut E, Danays T, Agnelli G, Becattini C, Beyer-Westendorf J, Bluhmki E, Bouvaist H, Brenner B, Couturaud F, Dellas C, Empen K, Franca A, Galiè N, Geibel A, Goldhaber SZ, Jimenez D, Kozak M, Kupatt C, Kucher N, Lang IM, Lankeit M, Meneveau N, Pacouret G, Palazzini M, Petris A, Pruszczyk P, Rugolotto M, Salvi A, Schellong S, Sebbane M, Sobkowicz B, Stefanovic BS, Thiele H, Torbicki A, Verschuren F, Konstantinides SV; PEITHO Investigators. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014;370:1402-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 921] [Cited by in RCA: 1062] [Article Influence: 96.5] [Reference Citation Analysis (0)] |
| 19. | Zondag W, Mos IC, Creemers-Schild D, Hoogerbrugge AD, Dekkers OM, Dolsma J, Eijsvogel M, Faber LM, Hofstee HM, Hovens MM, Jonkers GJ, van Kralingen KW, Kruip MJ, Vlasveld T, de Vreede MJ, Huisman MV; Hestia Study Investigators. Outpatient treatment in patients with acute pulmonary embolism: the Hestia Study. J Thromb Haemost. 2011;9:1500-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 259] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 20. | Aujesky D, Obrosky DS, Stone RA, Auble TE, Perrier A, Cornuz J, Roy PM, Fine MJ. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172:1041-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Klok FA, Mos IC, Huisman MV. Brain-type natriuretic peptide levels in the prediction of adverse outcome in patients with pulmonary embolism: a systematic review and meta-analysis. Am J Respir Crit Care Med. 2008;178:425-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 253] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 22. | van Es N, Coppens M, Schulman S, Middeldorp S, Büller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124:1968-1975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 594] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 23. | van der Hulle T, Kooiman J, den Exter PL, Dekkers OM, Klok FA, Huisman MV. Effectiveness and safety of novel oral anticoagulants as compared with vitamin K antagonists in the treatment of acute symptomatic venous thromboembolism: a systematic review and meta-analysis. J Thromb Haemost. 2014;12:320-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 373] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 24. | Bikdeli B, Wang Y, Minges KE, Desai NR, Kim N, Desai MM, Spertus JA, Masoudi FA, Nallamothu BK, Goldhaber SZ, Krumholz HM. Vena Caval Filter Utilization and Outcomes in Pulmonary Embolism: Medicare Hospitalizations From 1999 to 2010. J Am Coll Cardiol. 2016;67:1027-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 25. | White RH, Brunson A, Romano PS, Li Z, Wun T. Outcomes After Vena Cava Filter Use in Noncancer Patients With Acute Venous Thromboembolism: A Population-Based Study. Circulation. 2016;133:2018-2029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |