Published online Apr 26, 2020. doi: 10.4330/wjc.v12.i4.110
Peer-review started: December 8, 2019
First decision: January 7, 2020
Revised: March 3, 2020
Accepted: March 12, 2020
Article in press: March 12, 2020
Published online: April 26, 2020
Processing time: 135 Days and 16.2 Hours
The human gut is colonized by a community of microbiota, primarily bacteria, that exist in a symbiotic relationship with the host. Intestinal microbiota-host interactions play a critical role in the regulation of human physiology. Deleterious changes to the composition of gut microbiota, referred to as gut dysbiosis, has been linked to the development and progression of numerous diseases, including cardiovascular disease (CVD). Imbalances in host-microbial interaction impair homeostatic mechanisms that regulate health and can activate multiple pathways leading to CVD risk factor progression. Most CVD risk factors, including aging, obesity, dietary patterns, and a sedentary lifestyle, have been shown to induce gut dysbiosis. Dysbiosis is associated with intestinal inflammation and reduced integrity of the gut barrier, which in turn increases circulating levels of bacterial structural components and microbial metabolites, including trimethylamine-N-oxide and short-chain fatty acids, that may facilitate the development of CVD. This article reviews the normal function and composition of the gut microbiome, mechanisms leading to the leaky gut syndrome, its mechanistic link to CVD and potential novel therapeutic approaches aimed towards restoring gut microbiome and CVD prevention. As CVD is the leading cause of deaths globally, investigating the gut microbiota as a locus of intervention presents a novel and clinically relevant avenue for future research.
Core tip: As cardiovascular diseases (CVD) remain the leading cause of mortality, this article reviews the current literature dysbiosis and its role in CVD progression to present a novel therapeutic avenue. In this paper, we provide a comprehensive review on the composition and development of gut microbiota, its changes (dysbiosis) due to endogenous and exogenous factors and the mechanistic association of dysbiosis with development of CVD. Additionally, we explore the potential therapeutic approaches focused at restoring gut microbiota and their impact on CVD.
- Citation: Novakovic M, Rout A, Kingsley T, Kirchoff R, Singh A, Verma V, Kant R, Chaudhary R. Role of gut microbiota in cardiovascular diseases. World J Cardiol 2020; 12(4): 110-122
- URL: https://www.wjgnet.com/1949-8462/full/v12/i4/110.htm
- DOI: https://dx.doi.org/10.4330/wjc.v12.i4.110
The human body hosts trillions of microorganisms, and together they form an interactive ecosystem within and without outside world. The changes and interactions within this ecosystem affect the human body in health and diseases. The entourage of the associated microflora in the host is referred to as the microbiome. Majority of the microflora colonizing human body are found in the gastrointestinal tract, especially in the colon. The gut microbiota plays a major role in maintaining nutrition and immune system, which, in turn, affects the host's susceptibility and response to pathologic conditions. Imbalance in the intestinal microbiome, also known as gut dysbiosis, is associated with several conditions including gastrointestinal disorders, asthma, allergies, central nervous system disorders, metabolic syndrome, cancers and cardiovascular disease (CVD)[1,2].
CVD, a leading cause of death worldwide, stems from risk factors like smoking, lipid metabolism, diabetes and unregulated blood pressure. Atherosclerosis, the key pathophysiologic mechanism underlying the development of CVD, involves a complex interaction of vasculature, immune system and lipid metabolism. The gut microbiome affects all the component risk factors of atherosclerosis - both directly and indirectly, thus playing an important, albeit poorly understood role, in CVD[2]. In this review, we outline the role of gut microbiota in CVD and areas of future research and potential interventions.
It is estimated that the human gut is home to approximately 1000 to 1150 microbial species[3]. The microbial gene pool has been shown to exceed the size of the human genome and is termed as metagenome[4]. The international Metagenomics of the Human Intestinal Tract Project identified the gene database of the human gut microbiome, from stool samples of 124 individuals who were healthy, overweight and obese and patients with inflammatory bowel disease. This study found 3.3 million non-redundant microbial genes, derived from 576.7 gigabases of sequence, which is approximately 150 times larger than the human genome size[3]. The two major phyla, Bacteroidetes and Firmicutes accounted for 90% of microbial species inhabiting human gut, with the rest comprised of Actinobacteria, Cyanobacteria, Fusobacteria, Proteobacteria and Verrucomicrobia[3,5,6].
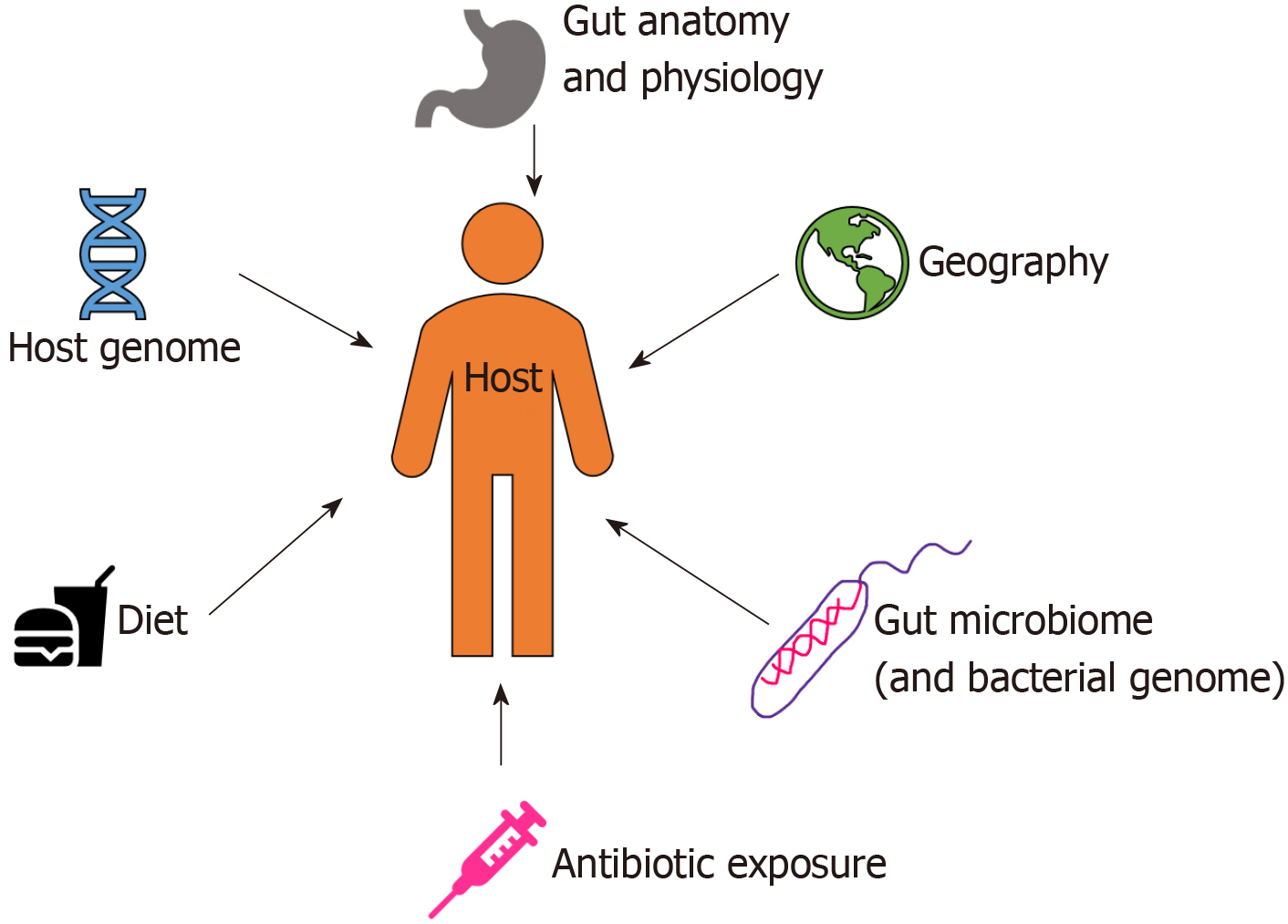
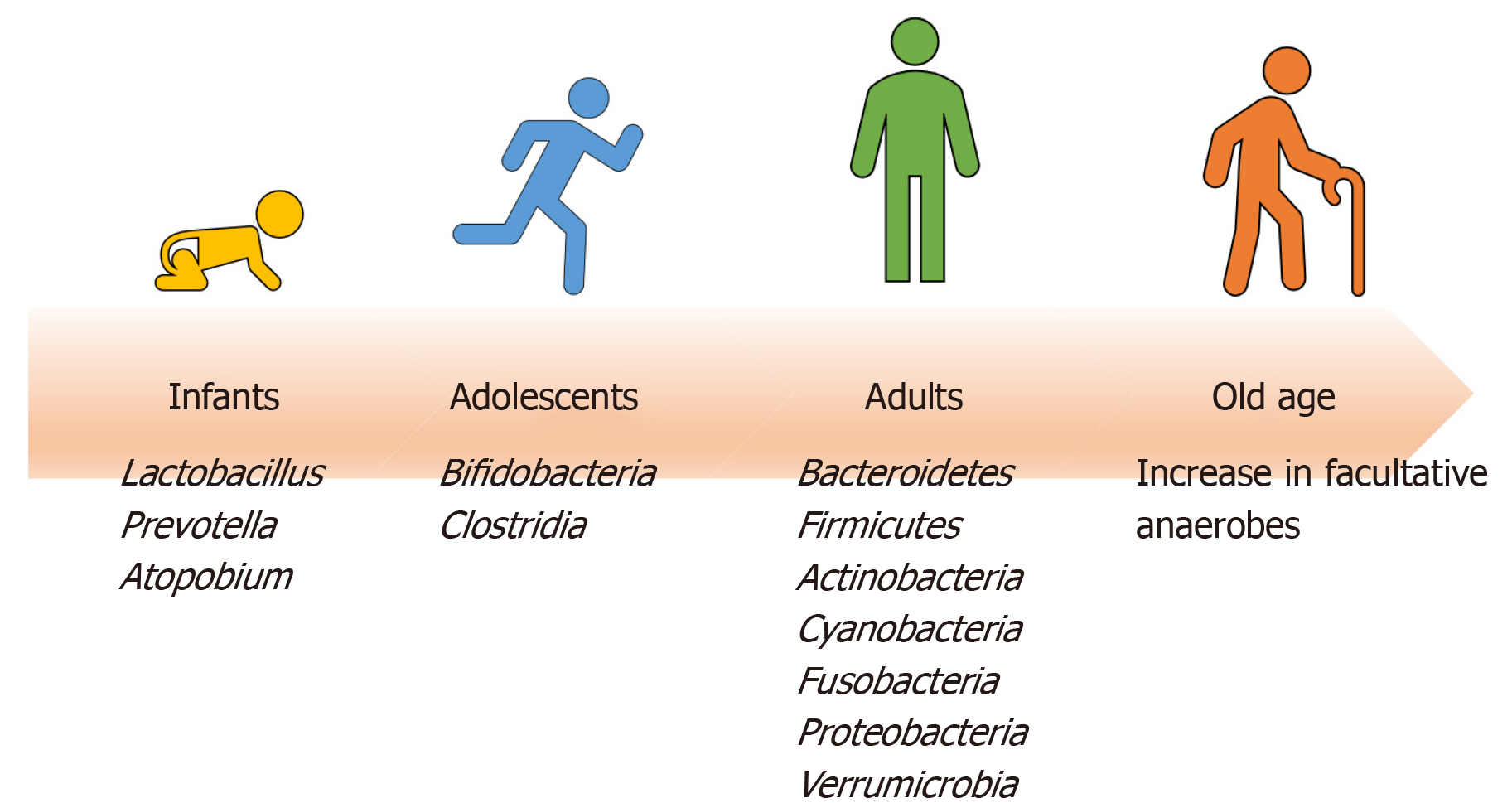
Starting from birth, multiple factors (both intrinsic and extrinsic) affect the development of human gut microbiota pool including host genome, geography and lifestyle factors (e.g. diet, disease, antibiotic exposure, etc.) (Figure 1)[7]. In the perinatal life, maternal flora, delivery method, breastfeeding, and weaning off breastmilk affects the development of microbiome. Notably, the gut microbiota of infants delivered vaginally consists of Lactobacillus, Prevotella, and Atopobium, whereas babies delivered by caesarean section predominantly carry maternal skin microflora in their guts, consisting mainly of Staphylococcus[8]. As the infant matures, the dominant aerobic microbiome diversifies to form an anaerobic environment, as evidenced by a high abundance of Bifidobacteria and Clostridia in adolescents compared to adults[9]. Interestingly, the metabolic environment of the gut changes as the microbiota evolves with age. The composition of core gut microbiota has been shown to be essentially stable throughout adulthood[9]. Changes occur with old age in accordance with the decline of physiological functions (Figure 2). As the immune system declines, an increase in facultative anaerobes, a shift in the ratio of Bacteroidetes to Firmicutes phyla, and a marked decrease in Bifidobacteria have been noted[9].
The gut microbiome plays an important function in both healthy and diseased individuals. It protects the host from epithelial cell injury and enteropathogens, regulates fat metabolism, affects the absorption of various nutrients and optimizes digestion[10,11]. The immune system is continuously modified by the introduction of components of the microbiome through the leaks in the intestinal wall. This interaction shapes the immune system, which in turn also changes the gut microbiota[7,12].
Intestinal mucosal epithelial barrier, which protects the internal milieu from the hostile external environment, is maintained by the formation of tight junctions (TJs, a complex made of intramembranous proteins, occludin and several molecules from claudin family of proteins) that spread between the epithelial cells, thus creating a semi-permeable seal[13]. Lipopolysaccharides (LPS, an endotoxin) is a component of Gram-negative bacterial cell wall and is a known inducer of the inflammatory response. LPS, via toll-like receptors (TLRs) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway, induces expression of inflammatory mediators and activates the innate immune system[14]. Higher levels of bloodstream endotoxins (especially > 50 pg/mL) have been associated with a threefold increased risk of atherosclerosis[15]. Gut microbiota is a large source of LPS, and under normal conditions with a functional intestinal barrier, it causes no harm and lower levels of LPS have been detected in healthy subjects[16,17]. In a diseased state, this barrier loses its protective function leading to increased intestinal permeability, especially to the locally produced LPS by the gut bacteria. Earlier, it was thought that leaky gut develops because of specific pathological conditions, but more recently, several studies have indicated a causal role of leaky gut rather than a consequence of the pathologic conditions[18-20]. In order to understand the role of gut microbiota in CVD, we have first to understand the factors contributing to the leaky gut syndrome.
Dyslipidemia is a known risk factor for CVD. High-energy diet and excessive fat intake are associated with significantly increased levels of LPS in blood[21,22]. Two pathways are proposed to be involved in the increased LPS with such diets - direct and indirect. In the direct pathway, food high in fat content causes an increased accumulation of chylomicrons increasing the local intercellular pressure contributing to loosening of the tight junctions. The loosening of tight junctions allows a generous influx of larger molecules such as LPS[23,24]. In the indirect pathway, the dietary fat stimulates mast cell activation in the intestinal mucosa with subsequent release of histamine and other inflammatory mediators known to increase intestinal permeability[25]. Similar to a high-fat diet, high carbohydrate intake can also lead to increased intestinal permeability and endotoxins levels[26]. With the expansion of industrial food processing, the human gut is increasingly exposed to new food additives such as nanoparticles, emulsifiers, organic solvents, and microbial transglutaminases. These products compromise the integrity of the intestinal barrier and expose the immune system to a number of foreign particles[27].
Genetic susceptibility has been implicated in several autoimmune intestinal diseases that may contribute to the leaky gut such as celiac disease and autoimmune enteropathy[28]. Zonulin is a physiological modulator of TJs and is activated by intestinal mucosa-microbiota interactions. Zonulin regulates antigen trafficking, and its upregulation in genetically susceptible individuals can lead to inflammatory and autoimmune processes[29]. Autoimmune disorders have been seen as a consequence of increased intestinal wall permeability; however, the reverse (i.e. autoimmune disorder causing increased intestinal wall permeability) has also been suggested in animal studies[30].
Other endogenous factors include the role of alterations in the enteric nervous system, and conditions compromising intestinal integrity. The enteric nervous system is a collection of neurons in the gastrointestinal tract, which functions independently from the central nervous system secreting various neurotransmitters including serotonin and histamine. In murine models, the downregulation of serotonin reuptake transporter has been associated with increased proinflammatory bowel response, increased intestinal permeability and increased fructose-induced endotoxin translocation leading to liver steatosis[31-33]. Studies have reported intestinal insult like major abdominal surgeries, shock and trauma compromises intestinal integrity as a cause or as a consequence of systemic inflammation[34].
The integrity of the intestinal barrier is also prone to many pathogen microorganisms and toxins. Helicobacter pylori can cause interruption of TJs by delivering cytotoxin-associated gene A, which results in loss of polarity of epithelial cells[35,36]. Enteropathogenic Escherichia coli secretes EspM and NIeA proteins which can induce TJ mislocalization[37,38]. Clostridium difficile toxin A increases paracellular permeability and translocation of zonula occludens-1 protein leading to degradation of filamentous actin[39]. TJ disruption was also implicated in cases of infection with Vibrio parahaemolyticus and Salmonella enterica[40,41].
Chronic stress and alcohol consumption can also affect the gut microbiome. Studies suggest a key role of corticotropin-releasing factor (CRF) and its receptors (CRFR1 and CRFR2) in the pathophysiological mechanism of development of the leaky gut[42,43]. Acetaldehyde, a product of alcohol metabolism, promotes phosphorylation of tight junction proteins in the intestinal epithelium causing direct damage in addition to indirect damage by an increase in nitric oxide which damages microtubules[44]. Alcohol also alters the composition of gut microbiota with an increase in Gram-negative bacteria[44].
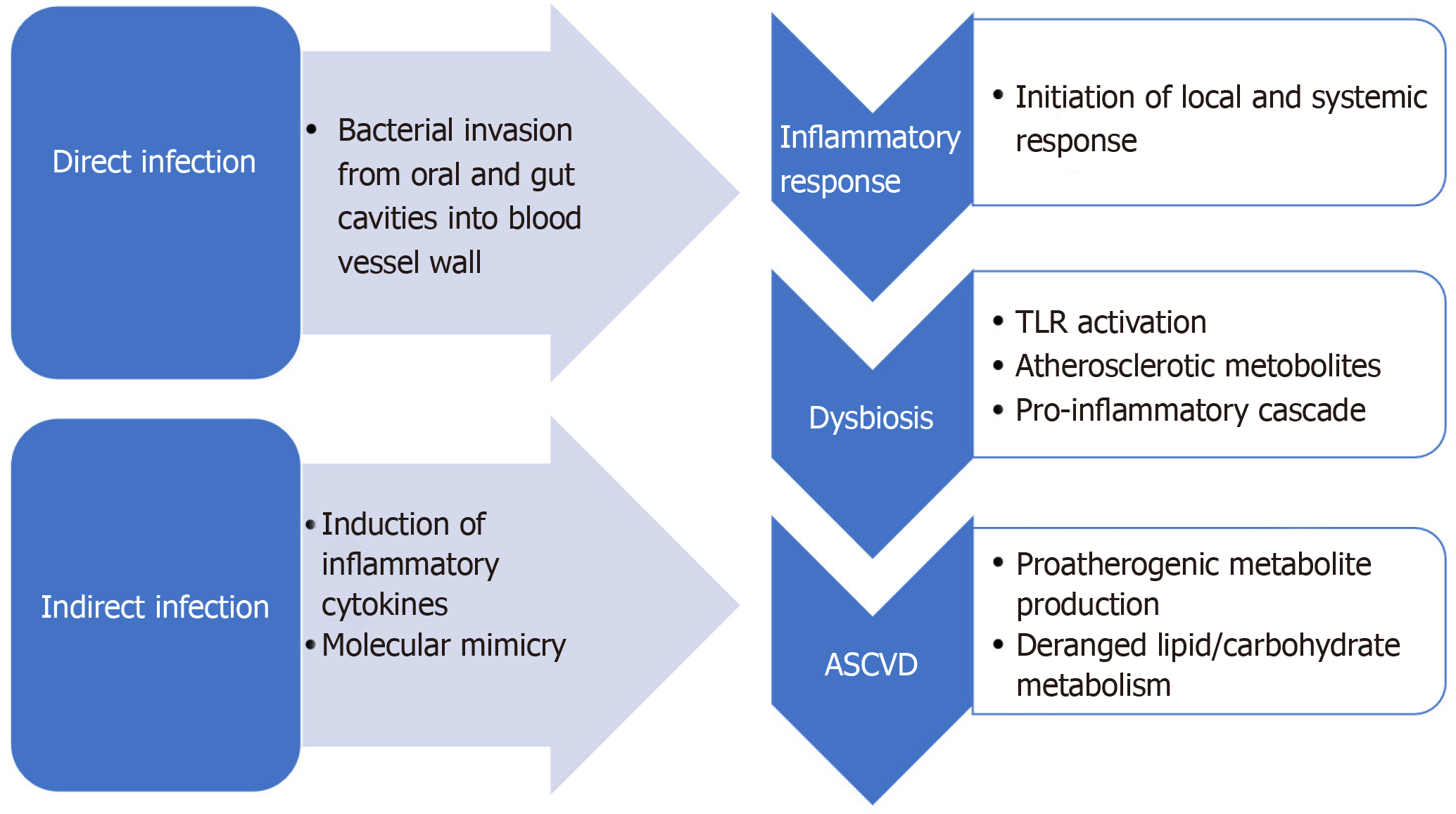
Atherosclerosis is an inflammatory disease with a growing body of evidence supporting a potential autoimmune background[45]. Infection is one of the major contributors to inflammation in the body and is a proposed mechanism of atherosclerosis. A large number of microorganisms such as Chlamydophila pneumoniae, Porphyromonas gingivalis, Helicobacter pylori, Influenza A virus, Hepatitis C virus, cytomegalovirus, and human immunodeficiency virus have been associated with an increased risk of cardiovascular diseases[46]. Infections contribute towards atherosclerosis via two predominant mechanisms: direct infection of the blood vessel wall (making it prone to plaque formation), or indirectly with an infection at a distant site by promoting proinflammatory mediators from a systemic immune response affect plaque growth (Figure 3)[47]. Additionally, dysbiosis also contributes to the production of atherosclerotic metabolites in the gut like trimethylamine N-oxide (TMAO) and can alter bile acid metabolism[48]. In this section, we will discuss the role and the evidence for each of the proposed mechanisms.
Over 50 species of bacterial DNA have been observed in atherosclerotic plaques[49]. Proteobacteria phylum (Chryseomonas and Helicobacter genera) is found to be most abundant in atherosclerotic plaques[49]. Firmicutes phylum (Anaeroglobus, Clostridium, Eubacterium, Lactobacillales and Roseburia genera) is predominantly found in the oral and gut cavity and is also present in atherosclerotic plaques[49]. Other bacteria that have been shown to be altered in the gut among patients with atherosclerotic cardiovascular disease includes Lactobacillales, Collinsella (stenotic atherosclerotic plaques in the carotid artery leading to cerebrovascular events), Enterobacteriaceae and Streptococcus spp (Table 1)[50,51]. In fact, it has been suggested that gut microbiota, especially Bacteroides, Clostridium and Lactobacillales could be considered as diagnostic markers in patients suffering from coronary artery disease[52].
| Microorganisms associated with cardiovascular disease |
| C. pneumoniae |
| P. gingivalis |
| H. pylori |
| Lactobacillales |
| Influenza A |
| Cytomegalovirus |
| Human immunodeficiency virus |
| Enterobacteriaceae |
| Streptococcus parasanguinis |
| Collinsella |
| Veillonella |
| Aggregatibacter |
| Firmicutes |
| Bacteroidetes |
| Actinobacteria |
| Fusobacteria |
| Proteobacteria |
| Candidate division TM7 single-cell isolate TM7c |
| Spirochaetes |
| SR1 |
| Tenericutes |
| Deinococcus-Thermus |
| Gemmatimonadetes |
| Chloroflexi |
| Neisseria polysaccharea |
| Neisseria subflava |
| Waddlia chondrophila |
| Prevotella |
| Beggiatoa sp. P5 |
| Alloprevotella rava |
| Megasphaera micronuciformis |
| Acidovorax sp. CF316 |
| Atopobium parvulum |
| Solobacterium moorei |
| Clostridium difficile |
Microorganisms, through inflammatory cytokine production and stimulation of acute-phase reactants, contribute to the development of atherosclerosis by further adding to the chronic inflammation within the atheromatous plaques[46]. In murine models, the use of antibiotics has shown an alteration in the gut microbiome, which affects carbohydrate and lipid metabolism. Initial studies investigating the role of pathogens in the development of atherosclerotic plaques had accounted for single microorganisms and not the overall microbiome, more recently it is being recognized that the aggregate number of microorganisms which an individual is colonized or infected with correlates more with atherogenesis, a concept referred to as "pathogen burden" or "infectious burden"[53].
Another possible mechanism for increased inflammation is cross-reactivity or molecular mimicry between self-antigens and bacterial antigens like heat-shock proteins and oxidized low-density lipoproteins[54]. Human heat-shock protein 60 (hHSP60) is expressed on the arterial endothelium in response to stress such as acute hypertension, hypercholesterolemia and in reperfusion injury. Also, a major antigenic component of bacteria during infection is the bacterial heat-shock protein 60s (HSP60s). Due to the high degree of homology between human and bacterial HSP, it is suggested that the antibodies formed against bacteria can target host cells expressing hHSP60. Indeed, high titres of serum antibody to mycobacterial HSP-65 were found in subjects with coronary or carotid atherosclerosis and post-myocardial infarction state[55].
As mentioned before, dysbiosis also leads to alteration in the immune system, which causes increased inflammation and atherogenesis. TLRs have been known to play a crucial role in bacterial infection and activation of the innate immune response. Once activated by ligands such as LPS, TLR dimerizes with the interleukin-1 receptor (IL-1R) forming a complex that binds myeloid differentiation primary response protein, MyD88, leading to downstream signalling cascade ultimately activating NF-κB. This cascade results in stimulation of the synthesis of proinflammatory cytokines, chemokines and costimulatory molecules[56]. TLR’s expression is found in most cardiovascular cells like endothelial cells, cardiomyocytes, adventitial fibroblasts, and macrophages. Among TLRs, TLR4 is best understood. Studies have described activation of TLR4 by saturated fatty acids, acting as a ligand through the same downstream pathways as for LPS resulting in the production of proinflammatory cytokines and chemokines[57,58]. Additionally, saturated fatty acids contribute to the induction of the inflammation by alternating gut microbiota in favour of Gram-negative bacteria, thus, increasing LPS levels. These processes promote translocation of bacteria and endotoxins into the bloodstream from the intestinal lumen due to an increase in intestinal permeability, further adding to the activation of TLR4[59]. In the animal models with a genetic deficiency of TLR4 and MyD88 genes, reduced proinflammatory cytokines and decreased plaque lipid content and aortic atherosclerosis were observed[60]. Human studies have also shown increased expression of TLR1, TLR2 and TLR4 in atherosclerotic plaques, suggesting a potential role in pathogenesis[61].
TMAO is an intestinal microbiota metabolite of choline and phosphatidylcholine. Dietary components such as choline, phosphatidylcholine, and carnitine, found in various animal-based products and energy drinks, are metabolized by gut microbiota to trimethylamine (TMA), and then oxidized by flavin monooxidases 3 in the liver to TMAO[62,63]. Flavin monooxidases 3 is an important regulator of TMAO synthesis and is regulated by farnesoid X receptor (FXR) whose expression can be upregulated by bile acids. TMAO can lead to atherogenesis via multiple mechanisms, though the underlying pathway is not completely understood. It inhibits reverse cholesterol transport causing reduced cholesterol removal from peripheral macrophages, and also affects atheroprotective effects of high-density lipoprotein thus promotes atherosclerosis[64]. TMAO also acts on platelets and increases platelet hyperre-sponsiveness by enhancing the stimulus-dependent release of Ca2+ from intracellular Ca2+ stores leading to increased thrombotic risk[63]. The effects of TMAO have also been observed in vascular cells promoting proinflammatory protein activation such as interleukin-6, cyclooxygenase-2, intercellular adhesion molecule-1 and E-cadherin – through the NF-κB signalling pathway[65]. Tang et al[62] showed elevated TMAO levels were associated with increased risk of major adverse cardiovascular events, including death, myocardial infarction and stroke over a 3-year follow-up period involving more than 4000 human subjects. A strong correlation between TMAO levels and CVD was noted even after adjustments of traditional risk factors. Also, an increased risk was associated with a graded increase in TMAO levels with a significant risk of major adverse cardiovascular events seen in the highest quartile.
There are an increasing number of studies explaining the complex interplay between intestinal microflora, bile acids and metabolic disease. Bile acids affect cardiac function and play a significant, yet poorly understood, role in CVD[66]. Direct and indirect pathways have been proposed to explain their effects in CVD. In the direct pathway, bile acids have been shown to interact with cardiac myocytes affecting muscle contractility and electrical excitation. In the indirect pathway, bile acids play a significant role in lipid metabolism, plaque formation, endothelial vasodilation and neovascularization of injured organs[66]. Having been metabolized by intestinal microflora, bile acid metabolites affect different metabolic pathways through FXR-induced signalling[67]. FXR is an endogenous bile acid sensor, a member of the nuclear receptor family with chenodeoxycholic acid being its most potent ligand. FXR acts as a receptor-transcription factor which, after being bound by ligand, regulates promoter activity in a coordinated manner. In adult human tissues, FXR expression has been found in adrenal glands, colon, liver, small intestine, kidneys and heart whereas no expression detected in brain, lung and skeletal muscles[68]. In vitro studies have recognized the prevention of vascular inflammation and neointimal proliferation as the potential roles FXR activation in the vascular smooth muscle cells[69].
As our understanding of the gut microbiome and its role in CVD grows, the gut microbiome is emerging as a major potential target for intervention among patients with CVD for improving clinical outcomes. The currently proposed therapeutic interventions are targeted towards the restoration of the intestinal barrier and improvement of gut microbiota. In this section, we will discuss the role of dietary modification and supplementation in the gut microbiome, followed by the possible role of faecal transplantation and targeting microbial enzyme pathways for further prevention of CVD.
Fermentable oligo-, di- and monosaccharides and polyols group includes short-chain carbohydrates and sugar alcohols that have poor absorption in the small intestine due to osmotic activity and undergo rapid fermentation by gut microflora[70]. Studies have shown their potential therapeutic effects in diseases that are associated with increased intestinal permeability, such as non-celiac gluten sensitivity and irritable bowel syndrome[71-74]. These findings suggest their potential role in dyslipidaemias and atherosclerosis, though further investigations are warranted.
Whole-grain intake has been inversely associated with metabolic syndrome and mortality from CVD, independent of demographic, lifestyle and dietary factors[75]. Epidemiologic studies have also suggested a decreased risk of CVD with adequate dietary fiber intake likely through the reduction of low-density lipoprotein levels[76]. Prebiotics are fibers, mostly oligosaccharides, that are selectively fermented (mostly Lactobacilli and Bifidobacteria genera) and exert changes on both the composition and function of the gastrointestinal microflora to confer benefits upon host well-being and health[77]. Their proposed health benefits were observed in a mouse model, where a diet rich in various inulin-type fructans, was associated with a reduced burden of atherosclerosis[78].
Probiotics are live viable microorganisms (predominantly Lactobacilli and Bifidobacteria) that improve microbial balance in the gut, thus exerting positive health effects[79]. In a randomized trial, consumption of live Lactobacillus Plantarum was shown to diversify homogenous gut microbial flora and was associated with a reduction in incident CVD events[80]. Naruszewicz et al[81], in a study of 36 healthy volunteers who were active smokers showed an inverse correlation between administration of Lactobacillus Plantarum and blood pressure levels, fibrinogen levels, degree of adhesion of isolated monocytes and levels of proinflammatory cytokines suggesting its potential role in primary prevention of atherosclerosis. Reduced levels of low-density lipoprotein were noted in women with normal or moderately elevated cholesterol after ingestion of fermented milk containing Lactobacillus acidophilus and Bifidobacterium longum[82]. Another study found Akkermancia muciniphila to suppress inflammation and atherosclerotic lesion formation in the apolipoprotein E-deficient (ApoE-/-) mice. It was proposed that A. muciniphila reduce circulating endotoxins and improve the intestinal barrier by increasing the expression of TJ proteins[83]. Looking through the prism of intestinal microflora and gut permeability, probiotics appear to be promising protective agents, especially with regards to prophylaxis of atherosclerosis. Larger clinical trials with hard clinical outcomes are awaited for this approach to gain more credibility.
Anthocyanins represent a group of flavonoids that commonly found in fruits, vegetables, grains, and even red wine. They play a protective role against atherosclerosis after being transformed to various metabolites by gut microbiota[84,85]. Protocatechuic acid (PCA) is a metabolite derived from human gut microbiota metabolism of anthocyanin called cyanidin-3-O-glucoside[86]. PCA had been shown to inhibit atherosclerosis by reducing monocyte inflammation and adhesion in ApoE-/-mice[87,88]. PCA has also shown to decrease miR-10b expression in macrophages, which induces gene expression promoting reverse cholesterol transport contributing to regression of established atherosclerotic plaque in ApoE-/- mouse model[85]. Human studies are needed to show a clinical benefit of anthocyanin as a food supplement in the prevention of atherosclerosis.
Faecal microbiota transplantation (FMT) is described as the restoration of “healthy” functional gut microflora by administrating a faecal solution from a donor into the intestinal tract of the recipient. The beneficial effect of FMT for recurrent clostridium difficile infection has been proven and is now a part of the guidelines for the treatment of recurrent clostridium difficile. It has also been explored as a therapeutic intervention in several other pathologies such as irritable bowel syndrome, metabolic syndrome, neurodevelopmental disorders, autoimmune diseases, allergic diseases and chronic fatigue syndrome[89,90].
In a mouse model, gut microbial transplantation was conducted from the atherosclerosis-prone strain of mice and atherosclerosis–resistant strain of mice to apolipoprotein e null mice in which resident intestinal microbes were first suppressed with antibiotics. Mice which received FMT from atherosclerosis-prone strain demonstrated choline diet-dependent enhancement in atherosclerotic plaque burden as compared with recipients of atherosclerosis-resistant strain[91]. In another study with human subjects, allogenic FMT from lean subjects to obese subjects with metabolic syndrome leads to improved insulin sensitivity and glucose metabolism[92]. The role of FMT as a secondary or primary prevention strategy to improve CVD outcomes remains to be explored with a severe limitation of its delivery method and possible complications of exposing the host to other infections.
Aortic lesions have a positive correlation with TMAO but an inverse correlation with choline levels[93]. Inhibition of FMO gene expression has been shown to reduce TMAO levels, alteration of lipid and cholesterol metabolism, and reduction in atherosclerotic lesions[94-96]. A study by Wang et al[97] showed that 3,3-dimethyl-1-butanol, a structural analogue of choline, inhibits microbial TMA lyases resulting in reduced TMAO levels and atherosclerotic lesion development in mice. In 2018, Roberts et al[98] reported the development of choline analogues iodomethylcholine and fluoro-methyl choline which can irreversibly inactivate choline TMA lyase activity. In animal models, these potent inhibitors reduced plasma TMAO levels > 95% after a single dose, for a sustained period and without any reported toxicity. The inhibitor selectively accumulated within intestinal microbes to millimolar levels, a concentration over 1-million-fold higher than needed for a therapeutic effect[98]. These studies reveal that mechanism-based inhibition of gut microbial TMA and TMAO production reduces thrombosis potential, a critical adverse complication in heart disease. They also offer a generalizable approach for the selective nonlethal targeting of gut microbial enzymes linked to host disease limiting systemic exposure of the inhibitor in the host. Despite holding significant potential, these agents still need to undergo human testing for efficacy and safety evaluation.
Gut microbiota represents an inseparable part of the human organism and remains an area of exploration in its role in the development of various pathological conditions. So far, significant progress of acknowledging our co-habitants has been made with respect to discovering its genome, functions, composition differences across different age and cultural groups. In addition, the recognition of the leaky gut syndrome has paved the way to reveal potential pathophysiological mechanisms behind numerous associations between the gut microbiota and CVD. Several factors have been identified, exogenous and endogenous, in the leaky gut and has made gut microbiome alteration a potential therapeutic target in managing several diseases including potentially CVD. However, much needs to be explored to evaluate the translation of benefits observed predominantly in animal studies to human subjects.
Manuscript source: Invited Manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Srivastava M, Akarsu M, Kharlamov AN S-Editor: Zhou JJ L-Editor: A E-Editor: Qi LL
| 1. | Nicholson JK, Holmes E, Wilson ID. Gut microorganisms, mammalian metabolism and personalized health care. Nat Rev Microbiol. 2005;3:431-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 682] [Cited by in RCA: 655] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 2. | Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. 2015;26:26191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 514] [Cited by in RCA: 801] [Article Influence: 80.1] [Reference Citation Analysis (0)] |
| 3. | Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J; MetaHIT Consortium, Bork P, Ehrlich SD, Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9101] [Cited by in RCA: 7837] [Article Influence: 522.5] [Reference Citation Analysis (4)] |
| 4. | Joseph J, Loscalzo J. Nutri(meta)genetics and cardiovascular disease: novel concepts in the interaction of diet and genomic variation. Curr Atheroscler Rep. 2015;17:505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9292] [Cited by in RCA: 8062] [Article Influence: 620.2] [Reference Citation Analysis (2)] |
| 6. | Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449:804-810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4573] [Cited by in RCA: 3719] [Article Influence: 206.6] [Reference Citation Analysis (0)] |
| 7. | Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336:1262-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2838] [Cited by in RCA: 3234] [Article Influence: 248.8] [Reference Citation Analysis (0)] |
| 8. | Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA. 2011;108 Suppl 1:4680-4687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2090] [Cited by in RCA: 2686] [Article Influence: 179.1] [Reference Citation Analysis (0)] |
| 9. | Mariat D, Firmesse O, Levenez F, Guimarăes V, Sokol H, Doré J, Corthier G, Furet JP. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009;9:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 997] [Cited by in RCA: 1246] [Article Influence: 77.9] [Reference Citation Analysis (0)] |
| 10. | Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T, Taylor TD, Itoh K, Kikuchi J, Morita H, Hattori M, Ohno H. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1444] [Cited by in RCA: 1690] [Article Influence: 120.7] [Reference Citation Analysis (0)] |
| 11. | Sonnenburg JL, Xu J, Leip DD, Chen CH, Westover BP, Weatherford J, Buhler JD, Gordon JI. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science. 2005;307:1955-1959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 994] [Cited by in RCA: 881] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 12. | Geuking MB, Köller Y, Rupp S, McCoy KD. The interplay between the gut microbiota and the immune system. Gut Microbes. 2014;5:411-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 148] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 13. | Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 2707] [Article Influence: 169.2] [Reference Citation Analysis (0)] |
| 14. | Muzio M, Polntarutti N, Bosisio D, Prahladan MK, Mantovani A. Toll like receptor family (TLT) and signalling pathway. Eur Cytokine Netw. 2000;11:489-490. [PubMed] |
| 15. | Wiedermann CJ, Kiechl S, Dunzendorfer S, Schratzberger P, Egger G, Oberhollenzer F, Willeit J. Association of endotoxemia with carotid atherosclerosis and cardiovascular disease: prospective results from the Bruneck Study. J Am Coll Cardiol. 1999;34:1975-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 376] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 16. | Erridge C, Attina T, Spickett CM, Webb DJ. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr. 2007;86:1286-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 550] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 17. | Ghanim H, Sia CL, Upadhyay M, Korzeniewski K, Viswanathan P, Abuaysheh S, Mohanty P, Dandona P. Orange juice neutralizes the proinflammatory effect of a high-fat, high-carbohydrate meal and prevents endotoxin increase and Toll-like receptor expression. Am J Clin Nutr. 2010;91:940-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 214] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 18. | Köhler CA, Maes M, Slyepchenko A, Berk M, Solmi M, Lanctôt KL, Carvalho AF. The Gut-Brain Axis, Including the Microbiome, Leaky Gut and Bacterial Translocation: Mechanisms and Pathophysiological Role in Alzheimer's Disease. Curr Pharm Des. 2016;22:6152-6166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 161] [Article Influence: 17.9] [Reference Citation Analysis (1)] |
| 19. | Camilleri M. Leaky gut: mechanisms, measurement and clinical implications in humans. Gut. 2019;68:1516-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 664] [Article Influence: 110.7] [Reference Citation Analysis (0)] |
| 20. | Alhasson F, Das S, Seth R, Dattaroy D, Chandrashekaran V, Ryan CN, Chan LS, Testerman T, Burch J, Hofseth LJ, Horner R, Nagarkatti M, Nagarkatti P, Lasley SM, Chatterjee S. Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS One. 2017;12:e0172914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 127] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 21. | Laugerette F, Vors C, Peretti N, Michalski MC. Complex links between dietary lipids, endogenous endotoxins and metabolic inflammation. Biochimie. 2011;93:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 22. | Amar J, Burcelin R, Ruidavets JB, Cani PD, Fauvel J, Alessi MC, Chamontin B, Ferriéres J. Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr. 2008;87:1219-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 411] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 23. | Ghanim H, Abuaysheh S, Sia CL, Korzeniewski K, Chaudhuri A, Fernandez-Real JM, Dandona P. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. Diabetes Care. 2009;32:2281-2287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 361] [Cited by in RCA: 399] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 24. | Moreira AP, Texeira TF, Ferreira AB, Peluzio Mdo C, Alfenas Rde C. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br J Nutr. 2012;108:801-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 480] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 25. | Scudamore CL, Jepson MA, Hirst BH, Miller HR. The rat mucosal mast cell chymase, RMCP-II, alters epithelial cell monolayer permeability in association with altered distribution of the tight junction proteins ZO-1 and occludin. Eur J Cell Biol. 1998;75:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 78] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Zhang DM, Jiao RQ, Kong LD. High Dietary Fructose: Direct or Indirect Dangerous Factors Disturbing Tissue and Organ Functions. Nutrients. 2017;9:335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 143] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 27. | Lerner A, Matthias T. Changes in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune disease. Autoimmun Rev. 2015;14:479-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 317] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 28. | Mu Q, Kirby J, Reilly CM, Luo XM. Leaky Gut As a Danger Signal for Autoimmune Diseases. Front Immunol. 2017;8:598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 266] [Cited by in RCA: 381] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 29. | Fasano A. Zonulin, regulation of tight junctions, and autoimmune diseases. Ann N Y Acad Sci. 2012;1258:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 236] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 30. | Nouri M, Bredberg A, Weström B, Lavasani S. Intestinal barrier dysfunction develops at the onset of experimental autoimmune encephalomyelitis, and can be induced by adoptive transfer of auto-reactive T cells. PLoS One. 2014;9:e106335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 31. | Bischoff SC, Mailer R, Pabst O, Weier G, Sedlik W, Li Z, Chen JJ, Murphy DL, Gershon MD. Role of serotonin in intestinal inflammation: knockout of serotonin reuptake transporter exacerbates 2,4,6-trinitrobenzene sulfonic acid colitis in mice. Am J Physiol Gastrointest Liver Physiol. 2009;296:G685-G695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 155] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 32. | Haub S, Ritze Y, Bergheim I, Pabst O, Gershon MD, Bischoff SC. Enhancement of intestinal inflammation in mice lacking interleukin 10 by deletion of the serotonin reuptake transporter. Neurogastroenterol Motil. 2010;22:826-834, e229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Haub S, Kanuri G, Volynets V, Brune T, Bischoff SC, Bergheim I. Serotonin reuptake transporter (SERT) plays a critical role in the onset of fructose-induced hepatic steatosis in mice. Am J Physiol Gastrointest Liver Physiol. 2010;298:G335-G344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 34. | de Haan JJ, Lubbers T, Derikx JP, Relja B, Henrich D, Greve JW, Marzi I, Buurman WA. Rapid development of intestinal cell damage following severe trauma: a prospective observational cohort study. Crit Care. 2009;13:R86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 35. | Amieva MR, Vogelmann R, Covacci A, Tompkins LS, Nelson WJ, Falkow S. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science. 2003;300:1430-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 584] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 36. | Saadat I, Higashi H, Obuse C, Umeda M, Murata-Kamiya N, Saito Y, Lu H, Ohnishi N, Azuma T, Suzuki A, Ohno S, Hatakeyama M. Helicobacter pylori CagA targets PAR1/MARK kinase to disrupt epithelial cell polarity. Nature. 2007;447:330-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 396] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 37. | Simovitch M, Sason H, Cohen S, Zahavi EE, Melamed-Book N, Weiss A, Aroeti B, Rosenshine I. EspM inhibits pedestal formation by enterohaemorrhagic Escherichia coli and enteropathogenic E. coli and disrupts the architecture of a polarized epithelial monolayer. Cell Microbiol. 2010;12:489-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 38. | Thanabalasuriar A, Koutsouris A, Weflen A, Mimee M, Hecht G, Gruenheid S. The bacterial virulence factor NleA is required for the disruption of intestinal tight junctions by enteropathogenic Escherichia coli. Cell Microbiol. 2010;12:31-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 39. | Chen ML, Pothoulakis C, LaMont JT. Protein kinase C signaling regulates ZO-1 translocation and increased paracellular flux of T84 colonocytes exposed to Clostridium difficile toxin A. J Biol Chem. 2002;277:4247-4254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 120] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 40. | Yarbrough ML, Li Y, Kinch LN, Grishin NV, Ball HL, Orth K. AMPylation of Rho GTPases by Vibrio VopS disrupts effector binding and downstream signaling. Science. 2009;323:269-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 306] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 41. | Boyle EC, Brown NF, Finlay BB. Salmonella enterica serovar Typhimurium effectors SopB, SopE, SopE2 and SipA disrupt tight junction structure and function. Cell Microbiol. 2006;8:1946-1957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 140] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 42. | Rodiño-Janeiro BK, Alonso-Cotoner C, Pigrau M, Lobo B, Vicario M, Santos J. Role of Corticotropin-releasing Factor in Gastrointestinal Permeability. J Neurogastroenterol Motil. 2015;21:33-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 43. | Overman EL, Rivier JE, Moeser AJ. CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-α. PLoS One. 2012;7:e39935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 172] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 44. | Leclercq S, Matamoros S, Cani PD, Neyrinck AM, Jamar F, Stärkel P, Windey K, Tremaroli V, Bäckhed F, Verbeke K, de Timary P, Delzenne NM. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci USA. 2014;111:E4485-E4493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 701] [Article Influence: 63.7] [Reference Citation Analysis (0)] |
| 45. | Hansson GK, Jonasson L. The discovery of cellular immunity in the atherosclerotic plaque. Arterioscler Thromb Vasc Biol. 2009;29:1714-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 46. | Rosenfeld ME, Campbell LA. Pathogens and atherosclerosis: update on the potential contribution of multiple infectious organisms to the pathogenesis of atherosclerosis. Thromb Haemost. 2011;106:858-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 246] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 47. | Jonsson AL, Bäckhed F. Role of gut microbiota in atherosclerosis. Nat Rev Cardiol. 2017;14:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 430] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 48. | Bu J, Wang Z. Cross-Talk between Gut Microbiota and Heart via the Routes of Metabolite and Immunity. Gastroenterol Res Pract. 2018;2018:6458094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 49. | Koren O, Spor A, Felin J, Fåk F, Stombaugh J, Tremaroli V, Behre CJ, Knight R, Fagerberg B, Ley RE, Bäckhed F. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci USA. 2011;108 Suppl 1:4592-4598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 856] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 50. | Emoto T, Yamashita T, Sasaki N, Hirota Y, Hayashi T, So A, Kasahara K, Yodoi K, Matsumoto T, Mizoguchi T, Ogawa W, Hirata K. Analysis of Gut Microbiota in Coronary Artery Disease Patients: a Possible Link between Gut Microbiota and Coronary Artery Disease. J Atheroscler Thromb. 2016;23:908-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 213] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 51. | Jie Z, Xia H, Zhong SL, Feng Q, Li S, Liang S, Zhong H, Liu Z, Gao Y, Zhao H, Zhang D, Su Z, Fang Z, Lan Z, Li J, Xiao L, Li J, Li R, Li X, Li F, Ren H, Huang Y, Peng Y, Li G, Wen B, Dong B, Chen JY, Geng QS, Zhang ZW, Yang H, Wang J, Wang J, Zhang X, Madsen L, Brix S, Ning G, Xu X, Liu X, Hou Y, Jia H, He K, Kristiansen K. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. 2017;8:845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1169] [Cited by in RCA: 1036] [Article Influence: 129.5] [Reference Citation Analysis (0)] |
| 52. | Emoto T, Yamashita T, Kobayashi T, Sasaki N, Hirota Y, Hayashi T, So A, Kasahara K, Yodoi K, Matsumoto T, Mizoguchi T, Ogawa W, Hirata KI. Characterization of gut microbiota profiles in coronary artery disease patients using data mining analysis of terminal restriction fragment length polymorphism: gut microbiota could be a diagnostic marker of coronary artery disease. Heart Vessels. 2017;32:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 53. | Elkind MS, Luna JM, Moon YP, Boden-Albala B, Liu KM, Spitalnik S, Rundek T, Sacco RL, Paik MC. Infectious burden and carotid plaque thickness: the northern Manhattan study. Stroke. 2010;41:e117-e122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 54. | Lamb DJ, El-Sankary W, Ferns GA. Molecular mimicry in atherosclerosis: a role for heat shock proteins in immunisation. Atherosclerosis. 2003;167:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 55. | Wick G. Atherosclerosis--an autoimmune disease due to an immune reaction against heat-shock protein 60. Herz. 2000;25:87-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 56. | Satoh T, Akira S. Toll-Like Receptor Signaling and Its Inducible Proteins. Microbiol Spectr. 2016;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 197] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 57. | Wong SW, Kwon MJ, Choi AM, Kim HP, Nakahira K, Hwang DH. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J Biol Chem. 2009;284:27384-27392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 426] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 58. | Lee JY, Ye J, Gao Z, Youn HS, Lee WH, Zhao L, Sizemore N, Hwang DH. Reciprocal modulation of Toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. J Biol Chem. 2003;278:37041-37051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 402] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 59. | Rocha DM, Caldas AP, Oliveira LL, Bressan J, Hermsdorff HH. Saturated fatty acids trigger TLR4-mediated inflammatory response. Atherosclerosis. 2016;244:211-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 354] [Article Influence: 35.4] [Reference Citation Analysis (1)] |
| 60. | Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci USA. 2004;101:10679-10684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 804] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 61. | Edfeldt K, Swedenborg J, Hansson GK, Yan ZQ. Expression of toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation. 2002;105:1158-1161. [PubMed] |
| 62. | Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575-1584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2130] [Cited by in RCA: 2406] [Article Influence: 200.5] [Reference Citation Analysis (0)] |
| 63. | Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, Sartor RB, McIntyre TM, Silverstein RL, Tang WHW, DiDonato JA, Brown JM, Lusis AJ, Hazen SL. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell. 2016;165:111-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 947] [Cited by in RCA: 1376] [Article Influence: 152.9] [Reference Citation Analysis (0)] |
| 64. | Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576-585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2783] [Cited by in RCA: 3158] [Article Influence: 263.2] [Reference Citation Analysis (0)] |
| 65. | Seldin MM, Meng Y, Qi H, Zhu W, Wang Z, Hazen SL, Lusis AJ, Shih DM. Trimethylamine N-Oxide Promotes Vascular Inflammation Through Signaling of Mitogen-Activated Protein Kinase and Nuclear Factor-κB. J Am Heart Assoc. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 395] [Cited by in RCA: 653] [Article Influence: 72.6] [Reference Citation Analysis (0)] |
| 66. | Khurana S, Raufman JP, Pallone TL. Bile acids regulate cardiovascular function. Clin Transl Sci. 2011;4:210-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 67. | Wahlström A, Sayin SI, Marschall HU, Bäckhed F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016;24:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1317] [Cited by in RCA: 1850] [Article Influence: 205.6] [Reference Citation Analysis (0)] |
| 68. | Bishop-Bailey D, Walsh DT, Warner TD. Expression and activation of the farnesoid X receptor in the vasculature. Proc Natl Acad Sci USA. 2004;101:3668-3673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 187] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 69. | Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1235] [Article Influence: 77.2] [Reference Citation Analysis (0)] |
| 70. | Gibson PR, Shepherd SJ. Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach. J Gastroenterol Hepatol. 2010;25:252-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 397] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 71. | Biesiekierski JR, Peters SL, Newnham ED, Rosella O, Muir JG, Gibson PR. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology. 2013;145:320-8.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 548] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 72. | Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146:67-75.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 810] [Cited by in RCA: 829] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 73. | Altobelli E, Del Negro V, Angeletti PM, Latella G. Low-FODMAP Diet Improves Irritable Bowel Syndrome Symptoms: A Meta-Analysis. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 149] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 74. | Wang L, Alammar N, Singh R, Nanavati J, Song Y, Chaudhary R, Mullin GE. Gut Microbial Dysbiosis in the Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis of Case-Control Studies. J Acad Nutr Diet. 2020;120:565-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 75. | Sahyoun NR, Jacques PF, Zhang XL, Juan W, McKeown NM. Whole-grain intake is inversely associated with the metabolic syndrome and mortality in older adults. Am J Clin Nutr. 2006;83:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 205] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 76. | Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients. 2013;5:1417-1435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1380] [Cited by in RCA: 1241] [Article Influence: 103.4] [Reference Citation Analysis (0)] |
| 77. | Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 2004;17:259-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1563] [Cited by in RCA: 1375] [Article Influence: 105.8] [Reference Citation Analysis (1)] |
| 78. | Rault-Nania MH, Gueux E, Demougeot C, Demigné C, Rock E, Mazur A. Inulin attenuates atherosclerosis in apolipoprotein E-deficient mice. Br J Nutr. 2006;96:840-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 79. | Markowiak P, Śliżewska K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients. 2017;9:1021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1200] [Cited by in RCA: 1238] [Article Influence: 154.8] [Reference Citation Analysis (1)] |
| 80. | Karlsson C, Ahrné S, Molin G, Berggren A, Palmquist I, Fredrikson GN, Jeppsson B. Probiotic therapy to men with incipient arteriosclerosis initiates increased bacterial diversity in colon: a randomized controlled trial. Atherosclerosis. 2010;208:228-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 81. | Naruszewicz M, Johansson ML, Zapolska-Downar D, Bukowska H. Effect of Lactobacillus plantarum 299v on cardiovascular disease risk factors in smokers. Am J Clin Nutr. 2002;76:1249-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 243] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 82. | Andrade S, Borges N. Effect of fermented milk containing Lactobacillus acidophilus and Bifidobacterium longum on plasma lipids of women with normal or moderately elevated cholesterol. J Dairy Res. 2009;76:469-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 83. | Li J, Lin S, Vanhoutte PM, Woo CW, Xu A. Akkermansia Muciniphila Protects Against Atherosclerosis by Preventing Metabolic Endotoxemia-Induced Inflammation in Apoe-/- Mice. Circulation. 2016;133:2434-2446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 541] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 84. | de Pascual-Teresa S, Moreno DA, García-Viguera C. Flavanols and anthocyanins in cardiovascular health: a review of current evidence. Int J Mol Sci. 2010;11:1679-1703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 421] [Cited by in RCA: 336] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 85. | Wang D, Xia M, Yan X, Li D, Wang L, Xu Y, Jin T, Ling W. Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing miRNA-10b. Circ Res. 2012;111:967-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 235] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 86. | Vitaglione P, Donnarumma G, Napolitano A, Galvano F, Gallo A, Scalfi L, Fogliano V. Protocatechuic acid is the major human metabolite of cyanidin-glucosides. J Nutr. 2007;137:2043-2048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 363] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 87. | Wang D, Wei X, Yan X, Jin T, Ling W. Protocatechuic acid, a metabolite of anthocyanins, inhibits monocyte adhesion and reduces atherosclerosis in apolipoprotein E-deficient mice. J Agric Food Chem. 2010;58:12722-12728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 88. | Wang D, Zou T, Yang Y, Yan X, Ling W. Cyanidin-3-O-β-glucoside with the aid of its metabolite protocatechuic acid, reduces monocyte infiltration in apolipoprotein E-deficient mice. Biochem Pharmacol. 2011;82:713-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 89. | Choi HH, Cho YS. Fecal Microbiota Transplantation: Current Applications, Effectiveness, and Future Perspectives. Clin Endosc. 2016;49:257-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 188] [Article Influence: 20.9] [Reference Citation Analysis (1)] |
| 90. | Borody TJ, Khoruts A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol. 2011;9:88-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 471] [Article Influence: 33.6] [Reference Citation Analysis (1)] |
| 91. | Gregory JC, Buffa JA, Org E, Wang Z, Levison BS, Zhu W, Wagner MA, Bennett BJ, Li L, DiDonato JA, Lusis AJ, Hazen SL. Transmission of atherosclerosis susceptibility with gut microbial transplantation. J Biol Chem. 2015;290:5647-5660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 378] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 92. | Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JF, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R, Derrien M, Druesne A, Van Hylckama Vlieg JE, Bloks VW, Groen AK, Heilig HG, Zoetendal EG, Stroes ES, de Vos WM, Hoekstra JB, Nieuwdorp M. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913-6.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1881] [Cited by in RCA: 2012] [Article Influence: 154.8] [Reference Citation Analysis (0)] |
| 93. | Bennett BJ, Davis RC, Civelek M, Orozco L, Wu J, Qi H, Pan C, Packard RR, Eskin E, Yan M, Kirchgessner T, Wang Z, Li X, Gregory JC, Hazen SL, Gargalovic PS, Lusis AJ. Genetic Architecture of Atherosclerosis in Mice: A Systems Genetics Analysis of Common Inbred Strains. PLoS Genet. 2015;11:e1005711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 94. | Warrier M, Shih DM, Burrows AC, Ferguson D, Gromovsky AD, Brown AL, Marshall S, McDaniel A, Schugar RC, Wang Z, Sacks J, Rong X, Vallim TA, Chou J, Ivanova PT, Myers DS, Brown HA, Lee RG, Crooke RM, Graham MJ, Liu X, Parini P, Tontonoz P, Lusis AJ, Hazen SL, Temel RE, Brown JM. The TMAO-Generating Enzyme Flavin Monooxygenase 3 Is a Central Regulator of Cholesterol Balance. Cell Rep. 2015;10:326-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 304] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 95. | Miao J, Ling AV, Manthena PV, Gearing ME, Graham MJ, Crooke RM, Croce KJ, Esquejo RM, Clish CB; Morbid Obesity Study Group, Vicent D, Biddinger SB. Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat Commun. 2015;6:6498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 279] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 96. | Shih DM, Wang Z, Lee R, Meng Y, Che N, Charugundla S, Qi H, Wu J, Pan C, Brown JM, Vallim T, Bennett BJ, Graham M, Hazen SL, Lusis AJ. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J Lipid Res. 2015;56:22-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 251] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 97. | Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, Gu X, Huang Y, Zamanian-Daryoush M, Culley MK, DiDonato AJ, Fu X, Hazen JE, Krajcik D, DiDonato JA, Lusis AJ, Hazen SL. Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell. 2015;163:1585-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 733] [Cited by in RCA: 957] [Article Influence: 106.3] [Reference Citation Analysis (0)] |
| 98. | Roberts AB, Gu X, Buffa JA, Hurd AG, Wang Z, Zhu W, Gupta N, Skye SM, Cody DB, Levison BS, Barrington WT, Russell MW, Reed JM, Duzan A, Lang JM, Fu X, Li L, Myers AJ, Rachakonda S, DiDonato JA, Brown JM, Gogonea V, Lusis AJ, Garcia-Garcia JC, Hazen SL. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med. 2018;24:1407-1417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 380] [Cited by in RCA: 390] [Article Influence: 55.7] [Reference Citation Analysis (0)] |