Published online Mar 26, 2020. doi: 10.4330/wjc.v12.i3.97
Peer-review started: December 18, 2019
First decision: January 6, 2020
Revised: January 8, 2020
Accepted: January 28, 2020
Article in press: January 28, 2020
Published online: March 26, 2020
Processing time: 89 Days and 13 Hours
Cardiovascular disease is the leading cause of death in patients with Turner syndrome (TS), and cardiovascular surgery is frequently required for management of these patients. TS is associated with medical comorbidities than can complicate the care of this patient population.
To describe the cardiovascular surgical outcomes of patients with TS.
A retrospective case series was compiled of 51 consecutive TS patients who had at least one cardiovascular surgery at Mayo Clinic Rochester from 1977-2017. The baseline clinical data of these patients were reviewed including demographics, medical comorbidities, congenital heart disease history, and medications. Echocardiographic reports were analyzed in detail. Operative reports and surgical hospital courses were reviewed. Long-term mortality was determined using medical records and the Social Security Death Index. Survival analysis was performed with the Kaplan Meier method.
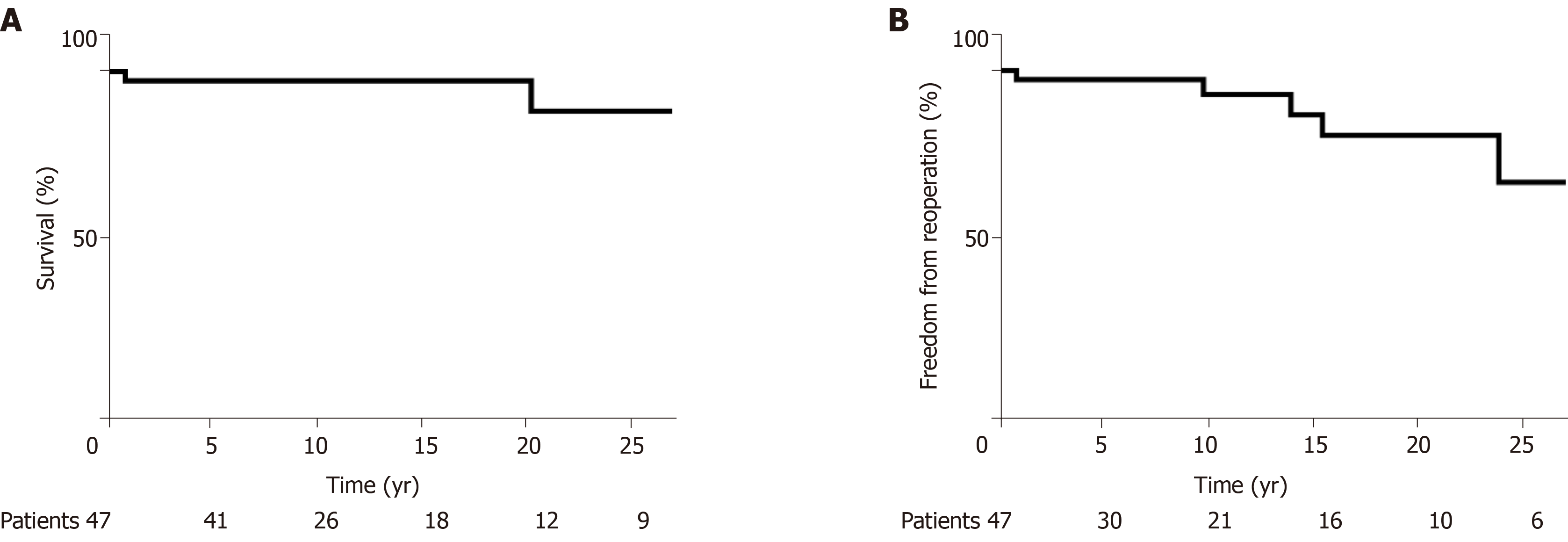
The cohort comprised 51 TS patients, average age at the time of surgery at Mayo Clinic was 28 (8-41) years, and 23 (45%) patients were under the age of 18. At the time of first Mayo Clinic surgery, 18 (35%) patients had previously undergone cardiac surgery at another institution. The most common procedures were repair of aortic coarctation in 14 (28%) patients, aortic valve replacement in 6 (12%) patients, and composite aortic root/ascending aorta replacement in 7 (14%) patients, with 7 patients undergoing repair of more than one lesion. Aortic dissection required operative intervention in 5 patients. After initial Mayo Clinic surgery, subsequent operations were required in 6 (13%) patients. Average hospital length of stay was 6 ± 2 d. There were 4 (8%) early surgical deaths. Freedom from death was 97% and 89% at 10 and 20 years, and the freedom from reoperation was 93% and 81% at 10 and 20 years.
Cardiovascular surgery is associated with 8% early mortality given the medical complexity of TS patients. Those who survive to dismissal have good survival. Later cardiovascular reoperations are not rare.
Core tip: Patients with Turner syndrome have relatively high early mortality after cardiovascular surgery, likely related to their high burden of medical comorbidities including congenital heart disease and vascular disease. Those patients that survive to hospital dismissal have good long-term survival. Many patients require multiple cardiovascular surgeries over their lifetime.
- Citation: Fuchs MM, Attenhofer Jost CH, Said SM, Hagler DJ, Connolly HM, Dearani JA, Egbe AC. Cardiovascular surgery in Turner syndrome - early outcome and long-term follow-up. World J Cardiol 2020; 12(3): 97-106
- URL: https://www.wjgnet.com/1949-8462/full/v12/i3/97.htm
- DOI: https://dx.doi.org/10.4330/wjc.v12.i3.97
Turner syndrome (TS) is a sex chromosome disorder caused by partial or complete loss of an X chromosome, and it is the most common sex chromosome disorder in women[1] with a prevalence of approximately 1/2000 live births[2]. Cardiovascular disease, both congenital and acquired, is common in TS[3], and has been recognized as a primary cause of increased mortality in this population[1,2]. The syndrome was described separately by Ullrich[4] in 1930, and by Turner[5] in 1938. Since the initial description more than 8 decades ago, many studies have reported comorbidities including vascular disease, autoimmune conditions, and renal and genitourinary anomalies in patients with TS[1-3,6,7]. Many TS patients require cardiovascular surgery and the high level of medical complexity in this cohort can confer an increased risk of complications. There are limited data about cardiovascular surgical outcomes in patients with TS. We present a single center retrospective review of patients undergoing cardiovascular surgery at Mayo Clinic, describing surgical procedures and outcomes.
We identified all patients with a diagnosis of TS who had cardiovascular surgery at Mayo Clinic Rochester from January 1, 1977 to December 31, 2017. The Mayo Clinic institutional review board approved this study and waived informed consent for patients that provided research authorization. Hardcopies of medical records (1977 to 1994) and electronic health records (1994 to 2017) were reviewed by two of the investigators (Attenhofer Jost CH and Fuchs MM). In addition to a detailed review of the surgical records, clinical notes and imaging records were also analyzed. Echocardiographic measurements of the aortic size were collected from reports and verified with image review, where available. Aortic size index and Turner-specific Z scores were calculated for each sinus of Valsalva and mid-ascending aortic measurement.
The purpose of the study was to describe surgical outcomes in terms of types of surgical procedure, reoperation, and early and late mortality. Early surgical mortality was defined as death prior to hospital dismissal or within 30 d from the time of surgery. Late surgical mortality was defined as death after 30 d from the time of surgery. We used data from the medical records and Social Security Death Index to determine late surgical mortality.
We reported categorical variables as percentages, and continuous variables as mean ± SD or median (interquartile range) for skewed data. Survival was assessed using Kaplan Meier method and we used the time of first cardiovascular surgery at Mayo Clinic as the “time zero” or the beginning of “at risk” period. We performed all statistical analyses with JMP software (version 13.0; SAS Institute Inc, Cary NC), and a P < 0.05 was considered statistically significant.
There were 281 patients with TS cared for at Mayo Clinic Rochester from January 1, 1977 to December 31, 2017, and 51 (18%) patients had at least one cardiovascular operation at Mayo Clinic Rochester within the study period. The age at the time of first cardiovascular operation at Mayo Clinic was 28 (8-41) years, and 23 (45%) patients were under the age of 18 years at the time of surgery. The baseline clinical and echocardiographic data of the patients are shown in Table 1, Table 2 and Table 3.
| Characteristics | Number |
| Age at the first surgery at Mayo years | 28 (8-41) |
| Height, cm | 154 ± 31 |
| Weight, kg | 58 ± 20 |
| Body surface area, m2 | 1.56 ± 0.46 |
| Body mass index, kg/m2 | 27 ± 8 |
| Comorbidities | |
| Hypertension | 26 (51%) |
| Hyperlipidemia | 21 (42%) |
| Current or prior smoker | 5 (10%) |
| Diabetes mellitus | 9 (18%) |
| Sleep apnea | 7 (14%) |
| Hypothyroidism | 29 (57%) |
| Atrial fibrillation | 7 (14%) |
| Coronary artery disease | 9 (18%) |
| Prior stroke Medications | 4 (8%) |
| Medications | |
| Diuretics | 12 (15%) |
| Beta and/or calcium channel blockers | 28 (36%) |
| RAAS antagonist | 26 (33%) |
| Warfarin | 6 (6%) |
| Aspirin | 37 (47%) |
| Statin | 21 (27%) |
| Levothyroxine | 27 (35%) |
| Estrogen | 43 (55%) |
| Growth hormone (prior or current) | 12 (25%) |
| Associated congenital heart disease | n (%) |
| Bicuspid aortic valve | 33 (65%) |
| Coarctation of aorta | 31 (61%) |
| Persistent left superior vena cava | 7 (14%) |
| Anomalous pulmonary vein | 2 (4%) |
| Atrial septal defect | 5 (10%) |
| Ventricular septal defect | 4 (8%) |
| Patent ductus arteriosus | 5 (10%) |
| Partial atrioventricular canal defect | 1 (2%) |
| Unroofed coronary sinus | 1 (2%) |
| Others | 6 (12%) |
| Echocardiographic and aortic measurements | mean ± SD/n (%) |
| Left ventricular ejection fraction, % | 54 ± 5 |
| Left ventricular end-diastolic dimension, mm | 45 ± 7 |
| Left ventricular end-systolic dimension, mm | 32 ± 4 |
| Left ventricular mass index, g/m2 | 106 ± 24 |
| Aortic valve annulus, mm | 20 ± 2 |
| Aortic valve annulus, Z score | 0.6 (-0.3-1.1) |
| Aortic root/sinus of Valsalva, mm | 33 ± 4 |
| Aortic root/sinus of Valsalva, Turner-specific Z score | 1.7 (0.7-2.9) |
| Aortic root/sinus of Valsalva, Turner-specific Z score > 2 | 21 (54%) |
| Aortic size index, cm/m2 | 2.5 ± 0.4 |
| Aortic size index, ≥ 2.5 cm/m2 | 20 (51%) |
| Mid ascending aorta, mm | 32 ± 4 |
| Mid ascending aorta, Turner-specific Z score | 1.2 (0.6-3.0) |
| Mid ascending aorta, Turner-specific Z score > 2 | 22 (56%) |
| Aortic size index, cm/m2 | 2.2 ± 0.3 |
| Aortic size index, ≥ 2.5 cm/m2 | 22 (56%) |
Of the 51 patients included in this study, 18 (35%) had 20 cardiovascular surgical procedures before their first surgery at Mayo Clinic (Table 4). Table 5 shows the first cardiovascular surgical procedures performed at Mayo Clinic, and 37 (72%) of these procedures were sternotomies while 14 (28%) were lateral thoracotomies. The most common procedures were aortic coarctation repair in 14 (28%) patients followed by aortic valve replacement in 6 (12%) patients and composite aortic root/ascending aorta replacement in 7 (14%) patients, with 7 patients (14%) undergoing repair of more than one lesion. The mean cardiopulmonary bypass time was 167 ± 49 min and aortic cross clamp time was 98 ± 34 min.
| Prior cardiovascular procedures | n (%) |
| Coarctation of aorta repair | 11 (22%) |
| Aortic valvuloplasty | 2 (4%) |
| Aortic valve replacement | 1 (2%) |
| Atrial septal defect closure | 1 (2%) |
| Ventricular septal defect closure | 1 (2%) |
| Pulmonary artery banding | 1 (2%) |
| Mitral valve replacement | 1 (2%) |
| Mitral valve repair | 1 (2%) |
| Repair of type B aortic dissection | 1 (2%) |
| First surgery type | n (%) |
| Coarctation of aorta repair | 14 (27%) |
| End-to-end anastomosis | 8 (16%) |
| Subclavian flap repair | 2 (4%) |
| Patch aortoplasty | 1 (2%) |
| Ascending-to-descending aorta bypass | 1 (2%) |
| Interposition graft | 2 (4%) |
| Aortic valvotomy | 2 |
| Aortic valve replacement | 6 (12%) |
| Aortic valve/root/ascending aorta replacement | 7 (14%) |
| Valve sparing root/ascending aorta replacement | 3 (6%) |
| Coronary artery bypass grafting | 5 (10%) |
| Descending aorta replacement | 2 (4%) |
| Pulmonary valve replacement | 2 (2%) |
| Atrial septal defect closure | 5 (10%) |
| Warden procedure | 1 (2%) |
| Ventricular defect closure | 3 (6%) |
| Tricuspid valve repair | 1 (2%) |
| Mitral valve repair | 1 (2%) |
| Mitral valve replacement | 1 (2%) |
| Patent ductus arteriosus ligation | 5 (10%) |
| Closure of ostium of coronary sinus | 1 (2%) |
| Partial atrioventricular canal defect | 1 (2%) |
There were 3 cases of Type A aortic dissection, 2 of the patients (age 41 and 42 years) underwent composite aortic root/ascending aorta replacement, and the third patient (age 67 years) with sub-acute dissection underwent aortic valve sparing root/ascending aorta replacement. At the time of dissection, maximum aortic dimension ranged from 47 to 66 mm, with aortic size index 3.6-4.3 cm/m2 and Turner-specific Z score 6.3-8.9. There were 2 cases of type B dissection. A 33-year old presented with acute type B dissection and underwent emergent descending thoracic aorta replacement with 28 mm graft 1 wk after presentation due to failed medical management and subsequent aortic rupture. A 40-year old presented with acute type B dissection and underwent delayed descending thoracic aorta replacement with 26 mm graft 6 mo after presentation. All five patients with aortic dissection had a prior diagnosis of hypertension, four had bicuspid aortic valve, and two had aortic coarctation. There were 13 aortic valve replacements (11 mechanical and 2 bioprosthetic valves) in patients with mean body surface area 1.63 m2, and the mean size of the prostheses was 20 ± 1 mm. Two patients had pulmonary valve replacement using bioprostheses (25 and 27 mm) and one patient had mitral valve replacement using a mechanical prosthesis (25 mm) due to mitral stenosis related to a parachute mitral valve.
The average hospital stay was 6 ± 2 d and there were 4 (8%) early surgical deaths. All early surgical deaths occurred after the year 2000. Details of the perioperative deaths include: Patient 1 was a 42-year old with severe aortic stenosis and Type A dissection who underwent composite root/ascending aorta replacement but died of tamponade and cardiogenic shock on postoperative day 2. Patient 2 was a 17-year old who had third time redo sternotomy for aortic valve replacement, pulmonary valve replacement, and tricuspid and mitral valve repair, and required hemodynamic support using extracorporeal membrane oxygenation, dying on postoperative day 13 from multi-organ failure. Patient 3 was a 70-year old who underwent aortic valve replacement and coronary artery bypass grafting and died on postoperative day 8 from bowel ischemia and sepsis. Patient 4 was a 31-year old who presented with acute myocardial infarction secondary to spontaneous coronary artery dissection complicated by cardiogenic shock, and was transferred to Mayo Clinic after an unsuccessful percutaneous coronary intervention at a different hospital. Intraoperative examination demonstrated complete dissection of the left anterior descending artery with extensive thrombus, and there was no suitable target for coronary artery bypass grafting. The patient required hemodynamic support using extracorporeal membrane oxygenation and died on postoperative day 24 from multi-organ failure.
There were 47 patients that survived to hospital dismissal and of these 47 patients, 4 (9%) died and 6 (13%) underwent reoperation during a follow-up of 11 (2-18) years. Of the 4 late deaths, one patient who underwent repair of an aneurysmal distal aortic arch 30 years prior developed a 61 mm aneurysm of an aberrant right subclavian artery, underwent subclavian artery repair, and died on postoperative day 2 of cardiac arrest. A second patient with prior aortic coarctation repair and coronary disease treated with percutaneous coronary intervention died at age 72 at an outside hospital of acute myocardial infarction after cystectomy. Additionally, one patient died of lung carcinoma, and one patient died at age 62 after developing gastrointestinal bleeding related to cirrhosis and esophageal varices. The indications for reoperation in the cohort were aortic re-coarctation that was treated with extra-anatomic bypass from ascending to descending aorta (n = 1), aortic stenosis that was treated with surgical aortic valvotomy (n = 1), aortic bioprosthesis dysfunction treated with mechanical aortic valve re-replacement (n = 2), a combination of aortic prosthesis dysfunction and pulmonary regurgitation that was treated with Konno procedure and pulmonary valve replacement (n = 1), and graft repair of left subclavian artery aneurysm (n = 1). The freedom from death was 97% and 89% at 10 and 20 years, and the freedom from reoperation was 93% and 81% at 10 and 20 years, respectively (Figure 1).
We present the outcomes of patients with TS undergoing cardiovascular surgery at Mayo Clinic. Our findings support the known significance of cardiovascular disease in this population, with 18% of TS patients at our institution undergoing cardiovascular surgery, nearly half under the age of 18 years. Coarctation repair and aortic valve replacement (frequently combined with aortic root replacement) were the most common interventions, demonstrating the prevalence of aortic valve and aortic disease in this population. Surgery for aortic dissection was required in 5 patients. Early mortality was 8%, which is significantly higher than expected. Long-term outcome in survivors was good.
The overall burden of congenital heart disease in the TS population has been estimated anywhere from 22% to 70%, with bicuspid aortic valve and coarctation of the aorta the most commonly identified lesions[8]. Accordingly, coarctation repair was the most commonly performed operation in our cohort, and was also the most common operation to have been performed before the patients presented to our institution. Aortic valve replacement was the second most common intervention.
Mortality after cardiovascular surgery in TS is largely undefined. One prior study has investigated surgical outcomes in a mixed cohort of adults and children with TS, and cites a post-operative mortality rate of 8.6%, which was similar to mortality of 7.2% (P NS) in a non-TS control group undergoing the same procedures[9]. Notably, the majority of deaths in both groups in that study occurred in children under 1 year of age[9]. The 30-d mortality rate in our study of 8% is comparable to the results of Madriago et al[9], though in our study all early deaths occurred in adolescents or adults. Additionally, all early surgical deaths in our cohort occurred in the modern era. An additional recent multi-center study investigated outcomes of cardiovascular surgery in children with TS compared to children without TS[10]. Similar to Madriago et al[9], mortality was not higher in the group of TS children, though they were noted to be smaller and have a higher rate of chylothorax after surgery[10]. The investigators conclude that TS does not confer additional operative risk in this pediatric population.
Indeed, it would seem that operative risk of cardiovascular surgery is higher for adults with TS compared to children. A prior Mayo Clinic study of patients undergoing isolated aortic coarctation repair at a mean age of 17 years demonstrated a 30-d mortality of 2.4%[11], substantially lower than our cohort of exclusively TS patients. The higher than expected mortality in our patient cohort is likely related to medical complexity: acute aortic dissection, redo sternotomies, and relatively advanced age with multiple cardiovascular risk factors. As the pediatric data demonstrates, it may be incorrect to conclude that all TS patients should expect elevated mortality risk when undergoing cardiovascular surgery. Rather, the inherent comorbidities that are associated with TS, cardiovascular and otherwise, can confer increased cardiovascular surgical risk when present in a single patient, especially at advancing age. Although the current study does not directly compare surgical outcomes in TS patients to outcomes of non-TS patients due to absence of a control group, it does demonstrate long term survival of 89% at 20 years for the patients that survived to hospital dismissal. This information is important for preoperative counseling in the TS population. Of note, many patients had already undergone an operation in childhood prior to their current presentation increasing the risk. The late deaths and reoperations demonstrated in this study further emphasize the cardiovascular morbidity in this population and the need for life-long surveillance.
Not only was congenital heart disease prevalent in this cohort, but many patients required serial operations. Prior to Mayo Clinic presentation, 18 (35%) patients had already undergone one operation, and in the follow up period 6 (13%) patients required re-operation. Very little information is available to compare reoperation rates for TS and non-TS patients with congenital heart disease. Considering aortic coarctation, a study of all patients with isolated aortic coarctation undergoing surgical intervention at Mayo Clinic 1946-2005 demonstrated only an 11% reoperation rate at 30 years[11]. While a direct comparison of reoperation rates cannot be made between the two studies, our cohort of TS patients required reoperation at much higher rates, suggesting that congenital heart disease in the TS population may be associated with additional morbidity compared to the non-TS population.
The etiology of aortic aneurysm and dissection in TS is understood to be multifactorial, related to structural and hemodynamic factors as well as underlying abnormality of the aortic tissue. Most TS patients with aortic dissection have comorbidities that predispose to dissection. A review of TS patients with aortic dissection revealed that the vast majority of cases (89%) occurred in a patient with high blood pressure, congenital heart disease (primarily bicuspid aortic valve and/or aortic coarctation), or both conditions[12]. Our study supports these findings, with greater than half of the patients having hypertension and nearly two-thirds with aortic coarctation or bicuspid aortic valve. Additionally, all five patients with aortic dissection had at least one of those risk factors. There have been, however, a small number of TS patients described in the literature who suffer aortic dissection without an identifiable cause, and TS in isolation has been identified as an independent risk factor for aortic aneurysm[13]. Analysis of vascular function in adults with TS reveals an arteriopathy with greater intima media thickness and enlargement of conduit arteries over controls[14]. Estrogen deficiency may contribute to arteriopathy, with greater intima media thickness also noted in 46, XX women with primary amenorrhea, however arterial enlargement is likely unique to TS patients[14]. Magnetic resonance imaging of TS patients supports these findings, with statistically significant correlations between TS and enlargement of the aortic branch vessels[15]. Indeed, one of our patients died after repair of a denovo aberrant right subclavian artery aneurysm. Notably, vasculopathy has also been appreciated in children with TS[16] before estrogen deficiency or adult-onset comorbidities are expected. Thus, despite ongoing research into the specific molecular signaling pathways for arteriopathy in TS, these patients have both inherent and acquired risk factors for aortic dissection.
Aortic dissection occurred primarily in the fifth decade of life in our cohort, much earlier than would be expected for women in the general population[17]. TS patients have been observed to suffer aortic dissection at both a younger age and a smaller aortic dimension than the general population[17]. In one series, subjects with ascending aortic dissection had ascending aortic dimensions of 37-48 mm[18], sizes that would not likely cause alarm in the general population. While patients in our study had larger ascending aortic dimensions at the time of dissection compared to those cited above, this may be related to the early era in which some of these events occurred. No ascending aortic dissection occurred in our cohort after 2004, suggesting that improved recognition of aortic aneurysms in this population has allowed us to replace the aorta prior to dissection. In addition to provider’s awareness of aortic dissection risk in this population, recognition of normal aortic dimensions in patients with TS has helped to identify those with pathologic aortic dilation, and to determine appropriate thresholds for elective aortic intervention. Aortic size index (defined as aortic dimension indexed to body surface area) has been studied in thoracic aortic aneurysms as a means of stratifying patient risk[19]. While an aortic size index of 2 cm/m2 is the 95th percentile for healthy female control subjects, in patients with TS this same value represents abnormal dilation of the aorta. TS patients with an aortic size index of 2.5 cm/m2 have the greatest risk of aortic dissection, and hence this calculation is important for risk stratification[18]. In our surgical cohort, the average aortic size indices of the sinus of Valsalva and mid-ascending aorta were 2.5 cm/m2 and 2.2 cm/m2, respectively, demonstrating that this was a population at high risk of dissection, and in fact 10 patients ultimately underwent root/ascending aorta replacement. In addition to aortic size index, Turner-specific Z scores have also been devised, allowing for further risk stratification, especially in very short-statured adults and in children[17,20].
This is a single center retrospective study and the conclusions are subject to the inherent bias associated with the study design. Echocardiography was not available preoperatively for all patients, limiting observations regarding preoperative aortic dimensions. Echocardiographic measurements of aortic dimension were obtained from reports, though measurements were personally verified where possible. The small sample size and the absence of a matched non-TS surgical control group made it impossible to provide procedure-specific mortality risk, or to compare overall surgical risk to the non-TS population.
In conclusion, cardiovascular surgery was associated with 8% early mortality in TS patients, and most of these operations were performed due to disease of the aortic valve and thoracic aorta. The patients who survived to hospital dismissal had good long-term survival, although the frequency of reoperations in this cohort underscores the need for lifelong cardiovascular follow-up. The current study provides important information for preoperative counseling of TS patients who are being evaluated for cardiovascular surgery.
Turner syndrome (TS) is a sex chromosome disorder involving partial or complete loss of the X chromosome. Multiorgan involvement has been observed, with cardiovascular disease recognized as a principle cause of mortality. Congenital and acquired cardiovascular disease, including aortic aneurysms, aortic dissection, and coronary artery disease, occur with increased frequency in this population, and cardiovascular surgery is not uncommonly required. The surgical outcomes of population have not been well characterized.
The topic of the study is the outcomes of cardiovascular surgery in TS. Due to the relatively small size of this patient population, outcomes of cardiovascular surgery have not been well described. As patients with TS live longer, and their cardiovascular disease is better recognized, understanding how to optimally manage these patients in the perioperative period is important to ensure the best possible surgical outcomes.
The objectives of this study were to describe the types of cardiovascular surgery these patients require and to characterize their operative morbidity and mortality. By identifying surgical mortality rate and long-term survival, we have highlighted the importance of ongoing focus on this cohort of patients. Further research is needed to improve early surgical outcomes and minimize reoperation.
A retrospective case series was assembled of patients undergoing cardiovascular surgery at Mayo Clinic from 1977-2017. Data pertaining to the patients’ baseline demographics, congenital heart disease history, medical comorbidities, and baseline echocardiographic imaging was compiled. Their operative course and medical follow up were then reviewed to establish survival rates using the Kaplan Meier method.
Fifty-one patients had at least one cardiovascular surgery at Mayo Clinic during the study period. Almost half of these patients were under the age of 18, and a third had already undergone a cardiovascular surgery at a prior institution. Aortic surgeries were most commonly performed, including aortic coarctation repair and composite aortic root/ascending aortic replacement. Aortic valve replacement was also common. After the initial Mayo Clinic surgery, 6 patients required a subsequent cardiovascular surgery. The early post-operative mortality rate was 8%. Freedom from death was 97% at 10 years and 89% at 20 years. After 20 years, 81% of patients remained free of reoperation.
We demonstrate that TS patients have a relatively high early post-operative mortality rate, and propose that this is related to the greater level of medical complexity present in this patient population. In patients that survive to hospital dismissal, however, long-term survival is good despite the prevalence of medical comorbidities. Many patients require reoperation in their lifetime. These findings suggest that more research is needed into the perioperative management of TS patients, so that their early mortality and reoperation rates are minimized.
Future research should be directed at perioperative management of TS patients, as well as pre-anesthesia medical examination to identify any confounding factors that may raise an individual patient’s operative risk.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Erkut B, Moschovi MA S-Editor: Dou Y L-Editor: A E-Editor: Xing YX
| 1. | Schoemaker MJ, Swerdlow AJ, Higgins CD, Wright AF, Jacobs PA; United Kingdom Clinical Cytogenetics Group. Mortality in women with turner syndrome in Great Britain: a national cohort study. J Clin Endocrinol Metab. 2008;93:4735-4742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 221] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 2. | Stochholm K, Juul S, Juel K, Naeraa RW, Gravholt CH. Prevalence, incidence, diagnostic delay, and mortality in Turner syndrome. J Clin Endocrinol Metab. 2006;91:3897-3902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 430] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 3. | Gravholt CH, Juul S, Naeraa RW, Hansen J. Morbidity in Turner syndrome. J Clin Epidemiol. 1998;51:147-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 355] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 4. | Ullrich O. Uber typische Kombinationsbilder multipler Abartungen. Eur J Pediatr. 1930;49:271. [DOI] [Full Text] |
| 5. | Turner HH. A Syndrome of Infantilism, Congenital Webbed Neck, and Cubitus Valgus. Endocrinology. 1938;23:566-574. [RCA] [DOI] [Full Text] [Cited by in Crossref: 814] [Cited by in RCA: 617] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 6. | Price WH, Clayton JF, Collyer S, De Mey R, Wilson J. Mortality ratios, life expectancy, and causes of death in patients with Turner's syndrome. J Epidemiol Community Health. 1986;40:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 102] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Levitsky LL, Luria AH, Hayes FJ, Lin AE. Turner syndrome: update on biology and management across the life span. Curr Opin Endocrinol Diabetes Obes. 2015;22:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Mortensen KH, Andersen NH, Gravholt CH. Cardiovascular phenotype in Turner syndrome--integrating cardiology, genetics, and endocrinology. Endocr Rev. 2012;33:677-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 152] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 9. | Madriago E, Nguyen T, McFerson M, Larson EV, Airhart N, Moller JH, Silberbach M. Frequency and outcomes of cardiac operations and catheter interventions in Turner syndrome. Am J Cardiol. 2012;110:580-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Chew JD, Hill KD, Jacobs ML, Jacobs JP, Killen SAS, Godown J, Wallace AS, Thibault D, Chiswell K, Bichell DP, Soslow JH. Congenital Heart Surgery Outcomes in Turner Syndrome: The Society of Thoracic Surgeons Database Analysis. Ann Thorac Surg. 2019;108:1430-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Brown ML, Burkhart HM, Connolly HM, Dearani JA, Cetta F, Li Z, Oliver WC, Warnes CA, Schaff HV. Coarctation of the aorta: lifelong surveillance is mandatory following surgical repair. J Am Coll Cardiol. 2013;62:1020-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 211] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 12. | Carlson M, Silberbach M. Dissection of the aorta in Turner syndrome: two cases and review of 85 cases in the literature. J Med Genet. 2007;44:745-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 148] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 13. | Lopez L, Arheart KL, Colan SD, Stein NS, Lopez-Mitnik G, Lin AE, Reller MD, Ventura R, Silberbach M. Turner syndrome is an independent risk factor for aortic dilation in the young. Pediatrics. 2008;121:e1622-e1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Ostberg JE, Donald AE, Halcox JP, Storry C, McCarthy C, Conway GS. Vasculopathy in Turner syndrome: arterial dilatation and intimal thickening without endothelial dysfunction. J Clin Endocrinol Metab. 2005;90:5161-5166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Mortensen KH, Hjerrild BE, Andersen NH, Sørensen KE, Hørlyck A, Pedersen EM, Lundorf E, Christiansen JS, Gravholt CH. Abnormalities of the major intrathoracic arteries in Turner syndrome as revealed by magnetic resonance imaging. Cardiol Young. 2010;20:191-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Lawson SA, Urbina EM, Gutmark-Little I, Khoury PR, Gao Z, Backeljauw PF. Vasculopathy in the young Turner syndrome population. J Clin Endocrinol Metab. 2014;99:E2039-E2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Gravholt CH, Andersen NH, Conway GS, Dekkers OM, Geffner ME, Klein KO, Lin AE, Mauras N, Quigley CA, Rubin K, Sandberg DE, Sas TCJ, Silberbach M, Söderström-Anttila V, Stochholm K, van Alfen-van derVelden JA, Woelfle J, Backeljauw PF; International Turner Syndrome Consensus Group. Clinical practice guidelines for the care of girls and women with Turner syndrome: proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. Eur J Endocrinol. 2017;177:G1-G70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 664] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 18. | Matura LA, Ho VB, Rosing DR, Bondy CA. Aortic dilatation and dissection in Turner syndrome. Circulation. 2007;116:1663-1670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 234] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 19. | Davies RR, Gallo A, Coady MA, Tellides G, Botta DM, Burke B, Coe MP, Kopf GS, Elefteriades JA. Novel measurement of relative aortic size predicts rupture of thoracic aortic aneurysms. Ann Thorac Surg. 2006;81:169-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 379] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 20. | Quezada E, Lapidus J, Shaughnessy R, Chen Z, Silberbach M. Aortic dimensions in Turner syndrome. Am J Med Genet A. 2015;167A:2527-2532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |