Published online Feb 26, 2020. doi: 10.4330/wjc.v12.i2.91
Peer-review started: October 9, 2019
First decision: November 19, 2019
Revised: December 12, 2019
Accepted: December 23, 2019
Article in press: December 23, 2019
Published online: February 26, 2020
Processing time: 140 Days and 10.4 Hours
Myocardial bridging (MB) is increasingly recognized to stimulate atherogenesis, which may contribute to an acute coronary syndrome. Stenting the coronary segment with MB has been recognized to have an increased risk of in-stent restenosis, stent fracture and coronary perforation. The safety and efficacy of stenting the culprit lesion with overlaying MB in ST elevation myocardial infarction (STEMI) as primary reperfusion therapy has not been established.
We reported a patient who presented with inferior STEMI with a culprit lesion of an acute thrombotic occlusion in the right coronary artery and thrombolysis and thrombin inhibition in myocardial infarction 0 flow. After the stent placement during primary percutaneous coronary intervention, intravascular ultrasound revealed MB overlying the stented segment where heavy atherosclerotic plaque were present. Likely due to the combination of plaque herniation or prolapse caused by MB, as well as local increased inflammation and thrombogenicity, acute stent thrombosis occurred at this region, which led to acute stent failure. The patient required an emergent repeated cardiac catheterization and placing a second layer of stent to enhance the radial strength and reduce the inter-strut space.
Plaque herniation or prolapse after stenting a MB segment in STEMI is a potential etiology for acute stent failure.
Core tip: Stenting the coronary segment with myocardial bridging is known to have increased risks of in-stent restenosis, stent fracture and coronary perforation. Myocardial bridging is also increasingly recognized to be pro-atherosclerotic and potentially involved in acute coronary syndrome, including ST elevation myocardial infarction (STEMI). The safety and efficacy of stenting the culprit lesion with overlying myocardial bridging in STEMI as primary reperfusion therapy has not been established. Here we present a case where plaque herniation or prolapse occurred after stenting a culprit lesion in STEMI, where overlying myocardial bridging was recognized by post-stenting intravascular ultrasound. The plaque herniation at the stented segment with myocardial bridging contributed to acute stent thrombosis which required a second layer of stent deployment. This case highlighted that plaque herniation or plaque prolapse after stenting a segment with myocardial bridging in STEMI is a potential etiology for acute stent failure, and emphasized the important role of intravascular ultrasound in primary percutaneous coronary intervention.
- Citation: Ma J, Gustafson GM, Dai X. Plaque herniation after stenting the culprit lesion with myocardial bridging in ST elevation myocardial infarction: A case report. World J Cardiol 2020; 12(2): 91-96
- URL: https://www.wjgnet.com/1949-8462/full/v12/i2/91.htm
- DOI: https://dx.doi.org/10.4330/wjc.v12.i2.91
Acute stent thrombosis after coronary artery stent placement is a rare but serious complication in percutaneous coronary intervention (PCI). Stenting culprit lesions in acute myocardial infarction has higher risk of acute stent thrombosis than stable coronary artery disease[1]. Coronary dissection, stent mal-apposition, or inadequate antiplatelet/anticoagulation therapy have been considered as the most common causes of acute stent thrombosis, in addition to the local inflammation and thrombogenic environment. We report a patient who experienced acute stent closure after stenting the culprit lesion in the mid right coronary artery (RCA) of an inferior ST elevation myocardial infarction (STEMI). Intravascular ultrasound (IVUS) revealed myocardial bridging (MB) phenomenon overlaying the stented segment of the RCA, which likely promoted atherosclerotic plaque herniation (AKA, plaque prolapse) through the stent struts. We are suggesting that the herniated or prolapsed atherosclerotic plaque materials combined with the acute tissue inflammation contributed to the acute stent thrombosis.
Recurrence of chest pain 30 min after stent placement.
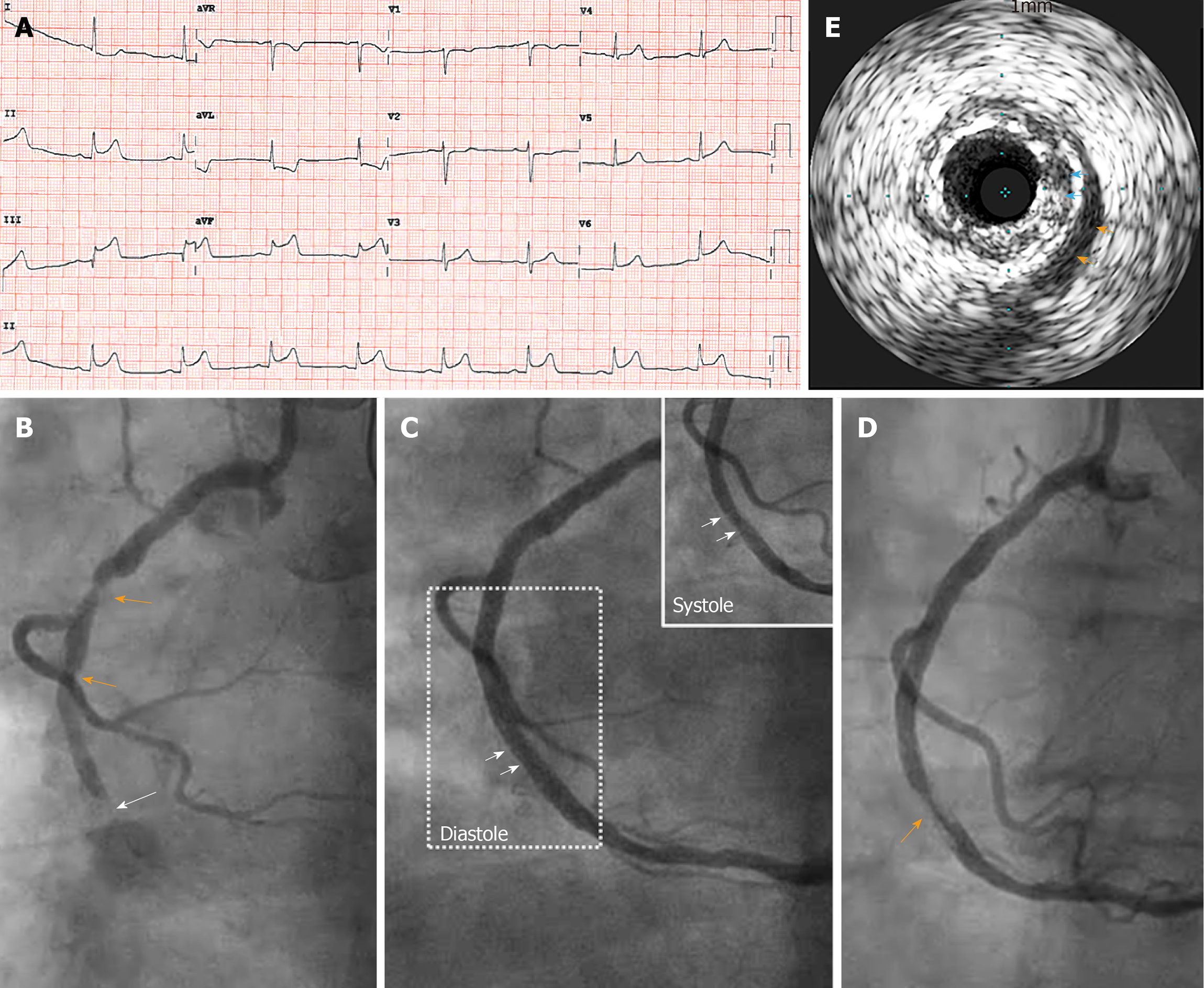
A 72-year-old woman with a history of hypertension, dyslipidemia, diabetes mellitus, and remote history of thyroid cancer status post thyroidectomy, who presented with acute onset substernal chest pain (CCS class IV angina pectoris) associated with nausea and diaphoresis. The 12-lead electrocardiogram (ECG) showed ST elevations in II, III and aVF with reciprocal ST depressions met the clinical diagnostic criteria of inferior STEMI (Figure 1A). The patient was pre-loaded with aspirin (325 mg) and P2Y12 receptor antagonist (clopidogrel 600 mg) in the emergency department, and was brought to the cardiac catheterization laboratory for emergent coronary angiography followed by reperfusion therapy by primary PCI. An acute thrombotic occlusion in the mid RCA with thrombin inhibition in myocardial infarction 0 flow was identified as the culprit lesion (Figure 1B, white arrow). Diffuse atherosclerotic disease was also seen in the proximal RCA (Figure 1B, orange arrows). Weight-based direct thrombin inhibitor (bivalirudin) infusion (0.75 mg/kg IV bolus immediately followed by 1.75 mg/kg/h IV infusion) was used for anticoagulation during the primary PCI. Primary PCI was performed with initial aspiration thrombectomy and followed by the placement of two over-lapping drug eluting stents (3.0 × 28 mm drug eluting stents deployed at 12 ATM, and 3.25 mm × 12 mm NC balloon for 18 ATM post-dilatation), which achieved 0% residual stenosis and thrombin inhibition in myocardial infarction 3 flow angiographically (Figure 1C). The patient’s symptoms and ST elevations on ECG completely resolved after this primary PCI. Pre-stenting IVUS was not performed. Serial post stenting IVUS studies were performed which revealed heavy atherosclerotic plaque burden at the stented area (Figure 1E, blue arrows). The IVUS images demonstrated that there was overlying MB with the typical IVUS appearance of echo-lucent area overlying the stented segment (the “half-moon phenomenon”) (Supplement Video 1; Figure 1E, orange arrows; Figure 1C, boxed area, arrows). IVUS also confirmed fully expanded and well apposed stent. With the recognition of the stent in a segment with overlying MB, there was some concern and discussion, but no further prophylactic action was taken. At the conclusion of the primary PCI, bivalirudin infusion was discontinued. The patient was subsequently transferred to the coronary care unit (CCU) for further post-STEMI monitoring.
Shortly after her arrival to the coronary care unit (30 min after the discontinuation of bivalirudin infusion), the patient experienced recurrent chest pain. Again, the pain was substernal, 9-10 out of 10 in pain scale. It was associated with nausea and diaphoresis. The patient appeared to be in significant distress although, vital signs were stable. She had mild shortness of breath associated with the chest pain, denied of palpitations, and any neurological symptoms. She did not have hypotension, nor evidence of tamponade on bedside transthoracic echocardiography. Other review of systems were negative except detailed above.
Immediate bedside 12-lead ECG revealed the re-appearance of the inferior leads ST elevations, which was previously confirmed to have resolved after stenting the mid RCA. An acute stent failure/thrombosis leading to acute occlusion of the stented RCA was suspected. The patient returned to the CCL immediately for repeat coronary angiography and primary PCI.
Hypertension, hyperlipidemia, diabetes mellitus, thyroid cancer status post thyroidectomy on thyroid hormone replacement therapy.
Vital signs: Temperature 36.9 °C, heart rate 58 bpm, respiratory rate 15 per minute, BP 105/68 mmHg, SpO2: 93% on room air. The patient appeared in mild distress, skin was mildly moisture suggestive for mild diaphoresis. Alert awake oriented × 3. There was no significant evidence of jugular vein distention, mild crackles in the bilateral lung bases without wheezing. There was no significant murmur audible. The right femoral access site for the previous emergent catheterization was clear without evidence of hematoma. Peripheral pulses were intact bilaterally. Other physical examinations were unremarkable.
Labs were drawn at her initial emergency department presentation with returned results by the time when she returned to the CCL with recurrent chest pain. Her complete blood count results were white blood cell, 9.16 K/µL, Hb 11.2 g/dL; Hct 34.1%, platelet 160 K/µL; her basic chemistry panel showed: Na+ 131 mEq/L, K+ 4.1 mEq/L, Cl- 95 mEq/L, HCO3- 24 mmol/L, blood urea nitrogen 18.5 mg/dL, Mg2+ 2.3 mEq/L, Troponin 3.48 ng/mL. Her liver function panel were within normal range.
12-leads ECG: Sinus bradycardia at 53 beat per minutes, with ST segment elevations > 0.1 mV in II, III and aVF and reciprocal ST depressions in leads I and aVL.
Transthoracic echocardiography (post-PCI): Mild segmental left ventricular systolic dysfunction, estimated EF 50%. The basal to mid inferior wall is hypokinetic. The inferolateral wall appears mildly hypokinetic. Normal right ventricle size and function. No significant valvular abnormalities. No evidence of pericardial effusion. No evidence of pulmonary hypertension.
Chest X-ray (post-PCI): Increased interstitial markings throughout both lungs, which may represent pulmonary edema. No pleural effusions. No pneumothorax.
Acute stent thrombosis due to plaque herniation after stenting a STEMI culprit lesion with overlying MB.
With the presumed diagnosis of acute stent thrombosis which led to the recurrent presentation of inferior STEMI, the patient was brought back to the CCL emergently for a repeat reperfusion therapy. Repeat angiography confirmed a filling defect with subtotal occlusion in the mid portion of the stented segment (Figure 1D, orange arrow), where overlying MB had been recognized on IVUS in the earlier study. MB induced plaque herniation was thought to be the likely contributing factor for the acute thrombotic closure, where the stent had been well expanded and apposed on IVUS at the end of previous procedure. In order to enhance radial strength and reduce the inter-strut spaces to minimize plaque herniation through the stented MB segment, a second layer of DES was deployed with additional post dilatations. Repeat IVUS study confirmed an improved stent expansion and luminal gain without evidence of acute plaque herniation. Since the acute stent thrombosis occurred only 30 min from the discontinuation of the bivalirudin (less than one half-life), and the P2Y12 inhibitor (clopidogrel) was loaded approximately 2 h earlier, we considered that an in-effective dual antiplatelet therapy was less likely to be the major contributor to the event. Nevertheless, the patient’s P2Y12 antagonist was changed to ticagrelor to enhance antiplatelet therapy.
The patient did well after the second procedure, and was discharged to home after 3 d of uneventful hospitalization. A pre-discharge echocardiography showed mild segmental left ventricular systolic dysfunction (EF 50%) with hypokinesis of the basal-to-mid inferior and inferolateral walls. She was doing well at 30-d follow up.
MB is a coronary anomaly in which a segment of epicardial coronary artery coursing under a “bridge” of overlying myocardium[2]. This phenomenon was initially discovered in autopsy. Coronary artery angiography, coronary computer tomographic angiography (CCTA) and IVUS are now commonly used imaging modalities to identity MB. In general population, the prevalence of MB reported by autopsy series was roughly 25%, similar by CCTA and lower in coronary angiography series (0.5%-12%)[2]. Angiographically, MB is most commonly seen in the mid segment of left anterior descending artery, followed by left circumflex and least common in RCA. However, some CCTA and autopsy series found similar involvement of left circumflex and RCA in MB[3-6]. The overlying myocardial fibers often lead to vessel compression during systole in cardiac cycle. Depending on the depth of MB, location of the MB, with or without myocardial hypertrophy, as well as systemic and coronary hemodynamic status, MB may result in clinical symptoms such as angina, myocardial ischemia or even ventricular arrhythmias. The first line of therapy of stable symptomatic MB remains medical treatment with beta-blockers and non-dihydropyridine calcium-channel blockers, while nitrates provides less consistent effects.
Stenting a coronary artery segment with overlying MB is associated with an increased risk of in-stent restenosis, stent fracture and coronary perforation. Therefore, stenting the coronary artery with MB is generally not recommended for management of ischemic symptoms in stable patients. MB is also increasingly recognized to be pro-atherosclerotic, especially at the peri-bridge edges, due to endothelial dysfunction, thickened intima and turbulent flow dynamics[7-9]. Rupture of atherosclerotic plaque in the segment of MB could result in acute thrombotic occlusion and STEMI presentation[10]. Primary PCI with stent placement remains the treatment of choice in the acute STEMI setting with or without the recognition of MB by angiography or IVUS. How often an overlying MB is involved in STEMI culprit lesion is not known. In our case, the MB overlying the stented segment involved in the culprit lesion of STEMI was recognized by post stenting IVUS during the initial primary PCI. Although concerns were raised during the initial primary PCI after the recognition of MB, and options such as empirically deploying a second layer of stent was discussed, there was no clinical evidence to guide this prophylactic use of second layer of stent against acute stent failure in this setting. In retrospect, potential adverse consequences after stenting coronary segment with overlying MB, such as acute plaque herniation, recoiling, and re-thrombosis had been underestimated. Plaque herniation or prolapse after stenting occurs up to 25% of stent placement in acute MI by IVUS imaging[11,12]. It is associated with worse clinical outcome including stent thrombosis[13]. Like the case presented here, in the setting of acute STEMI with the culprit lesion in a coronary artery segment with an overlying MB and heavy atherosclerotic plaque burden, stenting of this lesion resulted an increased risk of plaque herniation through the stent struts. With the combination of local inflammation, and the thrombogenic nature of the disrupted atherosclerotic plaque, acute stent thrombosis and closure occurred which required repeat revascularization. It’s a perfect storm. In our case, placing a second layer of stent to improve radial strength and also reduce the space between struts especially in open-cell designed stents was performed to minimize plaque herniation.
IVUS is an important imaging modality to identify MB. On IVUS, the tunneled segment of coronary artery underneath the overlying myocardium demonstrates systolic vessel compression and highly specific echo-lucent “half-moon” appearance as shown in Figure 1E (orange arrows). The use of IVUS in primary PCI is a valuable technique to provide information to recognize overlying MB and plaque herniation, which can promote the consideration of approaches of prophylaxis of acute stent failure after stenting MB segment[14,15]. We now felt strongly that pre-stenting IVUS may provide potential MB information before stent placement, which may change clinical decision making. When MB phenomenon identified by post-stenting IVUS, then empirically deploying an additional layer of stent may be advantageous after the recognition of MB and significant plaque burden and herniation in the stent on IVUS. This case further emphasizes the value of IVUS (pre- and post- stenting) during primary PCI.
The risk related to MB in the STEMI culprit lesion was under-recognized. Stenting the culprit lesion with overlying MB may result in plaque herniation, leading to acute stent failure. IVUS study in primary PCI is an useful tool for detecting MB. Empirical treatment with double layered stents may be necessary to prevent acute stent failure.
The authors also thank the patient for allowing us to provide medical service to her and use the clinical materials to publish the case report. Authors also thank all the nurses, technicians and cardiology fellows of in cardiac catheterization laboratory and CCU for their professional contribution and help in caring for this patient.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Micheu MM, Sato A, Tanabe S, Ueda H S-Editor: Yan JP L-Editor: A E-Editor: Xing YX
| 1. | Dangas GD, Claessen BE, Mehran R, Xu K, Fahy M, Parise H, Henriques JP, Ohman EM, White HD, Stone GW. Development and validation of a stent thrombosis risk score in patients with acute coronary syndromes. JACC Cardiovasc Interv. 2012;5:1097-1105. [PubMed] |
| 2. | Lee MS, Chen CH. Myocardial Bridging: An Up-to-Date Review. J Invasive Cardiol. 2015;27:521-528. [PubMed] |
| 3. | Kulkarni M, Sodani A, Rosita, Puranik C, Sullere S, Saha B. Right myocardial bridge on CT coronary angiography. J Assoc Physicians India. 2004;52:661-662. [PubMed] |
| 4. | Rychter K, Salanitri J, Edelman RR. Multifocal coronary artery myocardial bridging involving the right coronary and left anterior descending arteries detected by ECG-gated 64 slice multidetector CT coronary angiography. Int J Cardiovasc Imaging. 2006;22:713-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Nguyen TH, Burnside PR, Dieter RS, Nanjundappa A. Right coronary artery distribution of myocardial bridging: an unusual case presenting with ST-Elevation myocardial infarction. Tex Heart Inst J. 2007;34:489-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Polacek P, Kralove H. Relation of myocardial bridges and loops on the coronary arteries to coronary occulsions. Am Heart J. 1961;61:44-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 150] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Tarantini G, Migliore F, Cademartiri F, Fraccaro C, Iliceto S. Left Anterior Descending Artery Myocardial Bridging: A Clinical Approach. J Am Coll Cardiol. 2016;68:2887-2899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 138] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 8. | Corban MT, Hung OY, Eshtehardi P, Rasoul-Arzrumly E, McDaniel M, Mekonnen G, Timmins LH, Lutz J, Guyton RA, Samady H. Myocardial bridging: contemporary understanding of pathophysiology with implications for diagnostic and therapeutic strategies. J Am Coll Cardiol. 2014;63:2346-2355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 208] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 9. | Yuan SM. Myocardial Bridging. Braz J Cardiovasc Surg. 2016;31:60-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Akdemir R, Gunduz H, Emiroglu Y, Uyan C. Myocardial bridging as a cause of acute myocardial infarction: a case report. BMC Cardiovasc Disord. 2002;2:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Hong YJ, Jeong MH, Ahn Y, Sim DS, Chung JW, Cho JS, Yoon NS, Yoon HJ, Moon JY, Kim KH, Park HW, Kim JH, Cho JG, Park JC, Kang JC. Plaque prolapse after stent implantation in patients with acute myocardial infarction: an intravascular ultrasound analysis. JACC Cardiovasc Imaging. 2008;1:489-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Futamatsu H, Sabaté M, Angiolillo DJ, Jimenez-Quevedo P, Corros C, Morikawa-Futamatsu K, Alfonso F, Jiang J, Cervinka P, Hernandez-Antolin R, Macaya C, Bass TA, Costa MA. Characterization of plaque prolapse after drug-eluting stent implantation in diabetic patients: a three-dimensional volumetric intravascular ultrasound outcome study. J Am Coll Cardiol. 2006;48:1139-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, Airoldi F, Chieffo A, Montorfano M, Carlino M, Michev I, Corvaja N, Briguori C, Gerckens U, Grube E, Colombo A. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293:2126-2130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2402] [Cited by in RCA: 2331] [Article Influence: 116.6] [Reference Citation Analysis (0)] |
| 14. | Tomasevic M, Dikic M, Ostojic M. Stenting a myocardial bridge: a wrong decision in STEMI? Acta Cardiol. 2011;66:89-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Becher T, Baumann S, Huseynov A, Behnes M, Borggrefe M, Akin I. Coronary artery perforation in a patient with STEMI and a myocardial bridge: an increased risk for coronary artery perforation? Cardiovasc Revasc Med. 2015;16:246-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |