Published online Dec 26, 2020. doi: 10.4330/wjc.v12.i12.634
Peer-review started: September 9, 2020
First decision: October 5, 2020
Revised: October 10, 2020
Accepted: November 11, 2020
Article in press: November 11, 2020
Published online: December 26, 2020
Processing time: 94 Days and 7.2 Hours
Heparin-induced thrombocytopenia (HIT) is a rare complication of heparin therapy, and is characterized by arteriovenous thrombosis and bleeding events. The incidence of HIT after percutaneous coronary intervention (PCI) in patients with myocardial infarction complicated with renal failure is rarely reported.
We report a 73-year-old man with acute myocardial infarction and renal failure who underwent hemodialysis and PCI, and developed a progressive decline in platelets and subcutaneous hemorrhage of both upper limbs after heparin treatment. In addition to a gradual decrease in platelets, the patient’s 4T's score was 7, and HIT antibody was positive, confirming the diagnosis of HIT.
Patients receiving heparin combined with antiplatelet therapy should be monitored closely, especially for their platelet count. In the case of thrombo-cytopenia, HIT should be highly suspected. When the diagnosis of HIT is confirmed, timely individualized treatment should be delivered.
Core Tip: Heparin-induced thrombocytopenia (HIT) is a rare complication of heparin therapy. Its pathogenesis includes thrombotic events that can rarely affect the coronary arteries. We report a 73-year-old man who presented with extensive lower extremities deep venous thrombosis. After being treated with heparin, he developed ST-elevation myocardial infarction secondary to acute thrombus formation. The patient’s platelets dropped within 6 d, and heparin-platelet factor 4 immunoglobulin G antibody and serotonin release assay were positive, confirming the diagnosis of HIT. HIT is associated with an increased risk for coronary thrombosis and ischaemia. HIT can cause coronary complications usually in previously disrupted coronary vessels and bypass grafts.
- Citation: Wang J, Deng SB, She Q. Heparin-induced thrombocytopenia in renal insufficiency undergoing dialysis and percutaneous coronary intervention after acute myocardial infarction: A case report. World J Cardiol 2020; 12(12): 634-641
- URL: https://www.wjgnet.com/1949-8462/full/v12/i12/634.htm
- DOI: https://dx.doi.org/10.4330/wjc.v12.i12.634
Heparin-induced thrombocytopenia (HIT) refers to thrombocytopenia occurs following therapy with heparins including unfractionated heparin (UFH) and heparin derivatives, such as low-molecular weight heparin (LMWH). During HIT, immune antibodies mediated by platelets play an important role, and cause a reduction in platelet count. This can lead to arteriovenous thrombosis and bleeding events, and may even cause death in severe cases[1,2]. With an increasing number of patients undergoing dialysis or coronary intervention treatment, HIT has gradually gained more and more attention. Timely detection and treatment significantly improve the prognosis of these patients. We report a case of HIT in a patient with chronic renal insufficiency and acute myocardial infarction (AMI) following percutaneous coronary intervention (PCI).
A 73-year-old man was admitted to a regional hospital because of AMI occurring 1 wk before admission to our hospital.
During his stay in the regional hospital, a coronary angiogram (CAG) was obtained, in which 3000 units of UFH was administered, and CAG revealed the presence of trunk and 3-vessel disease. The patient was transferred to our department (November 7, 2018) for further treatment.
The patient had a history of chronic kidney disease for 5 years and hypertension for 4 years. He received hemodialysis treatment four times before being transferring to our hospital.
This patient had no specific personal and family history.
On initial presentation to our hospital, he was hemodynamically stable with a heart rate of 76 bpm, and blood pressure of 156/74 mmHg. A small amount of moist rales was heard at the bottom of both lungs on auscultation. His heart boundary size was critical on percussion and rhythm was regular on auscultation. A grade-III systolic murmur was heard in the aortic valve area.
Laboratory studies were significant for a cardiac troponin T level of 7.670 μg/L (normal 0.000-0.100 μg/L), pro-brain natriuretic peptide of 46034 pg/mL (normal 0.00-125.00 pg/mL), creatinine of 580.5 μmmol/L, glomerular filtration rate (GFR) of 7.6 mL/L, hemoglobin of 90 g/L (normal 130-175 g/L), and platelet (PLT) count of 121 × 109/L (normal 100-300 × 109/L).
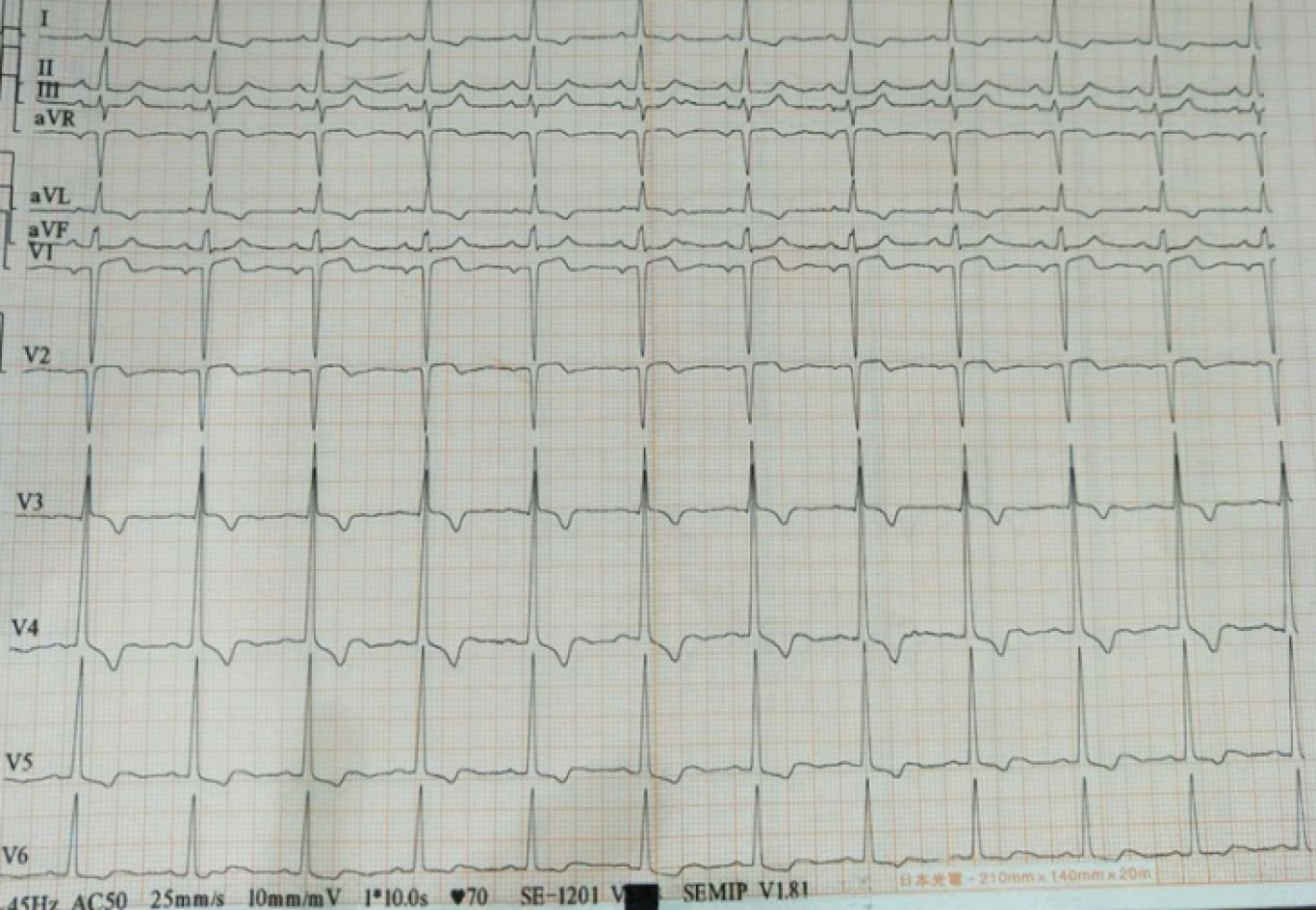
Cardiac ultrasound showed that the left atrium and left ventricle were enlarged, and the aorta and pulmonary arteries were widened. Severe aortic insufficiency with moderate stenosis, and moderate mitral valve insufficiency were also found by ultrasound. Left ventricular systolic and diastolic function was reduced. The bedside electrocardiogram (Figure 1) after admission showed sinus rhythm, T wave inversion in leads I, aVL, and V3-V6, and the QRS wave in leads V1 and V2 was QS type.
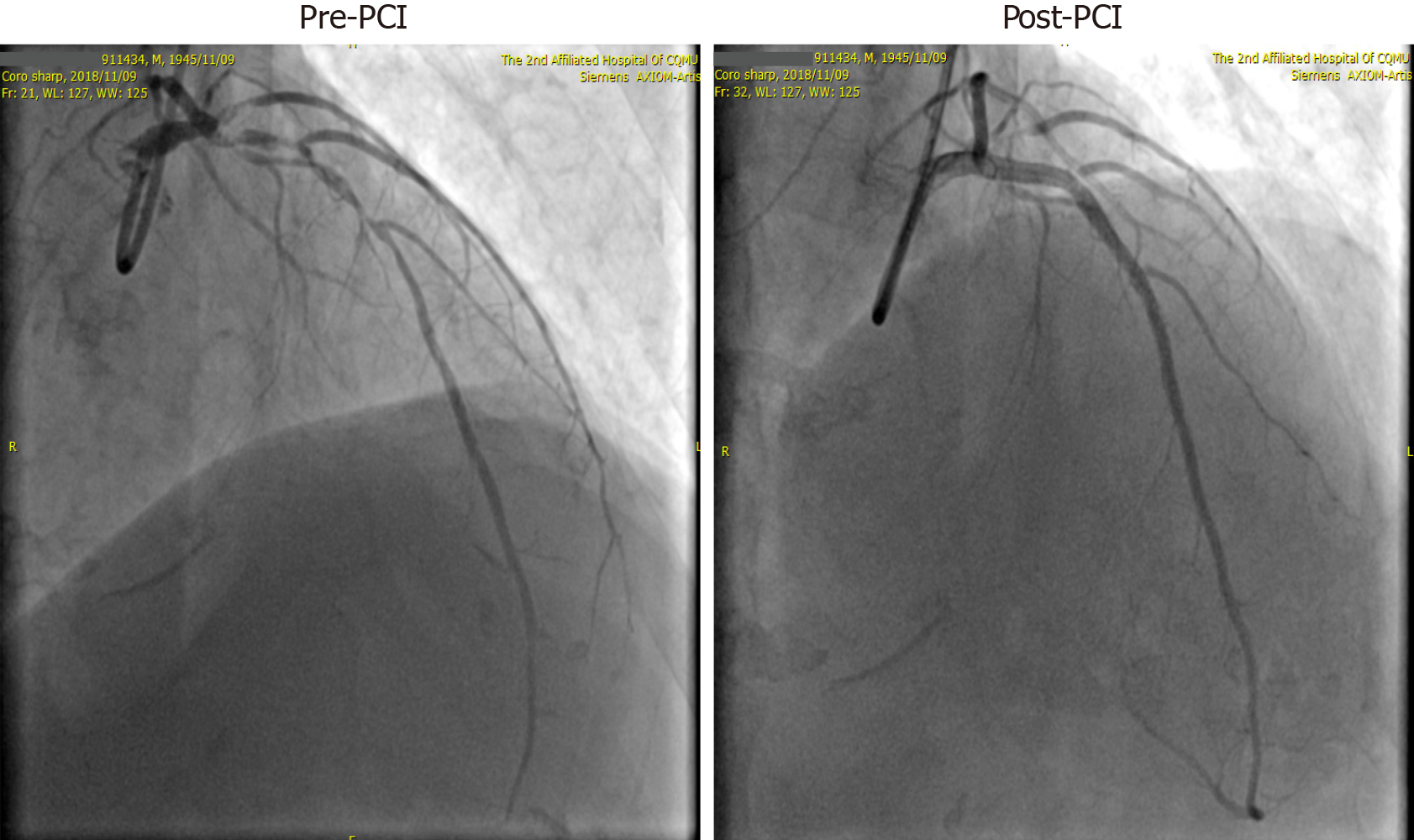
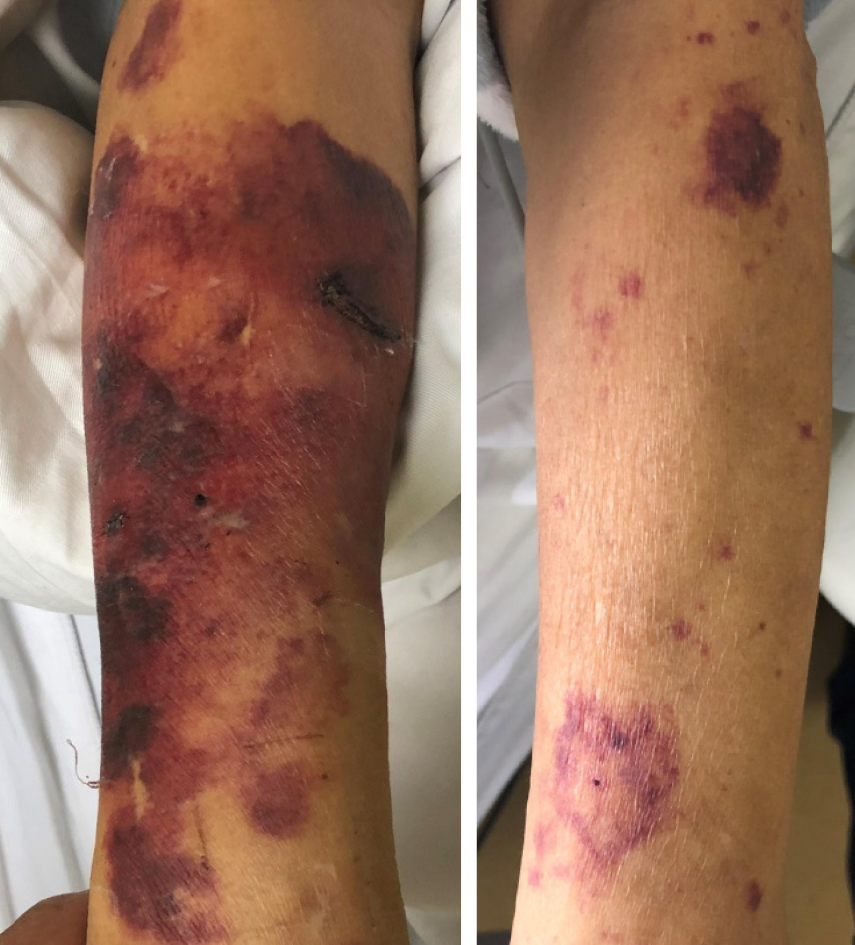
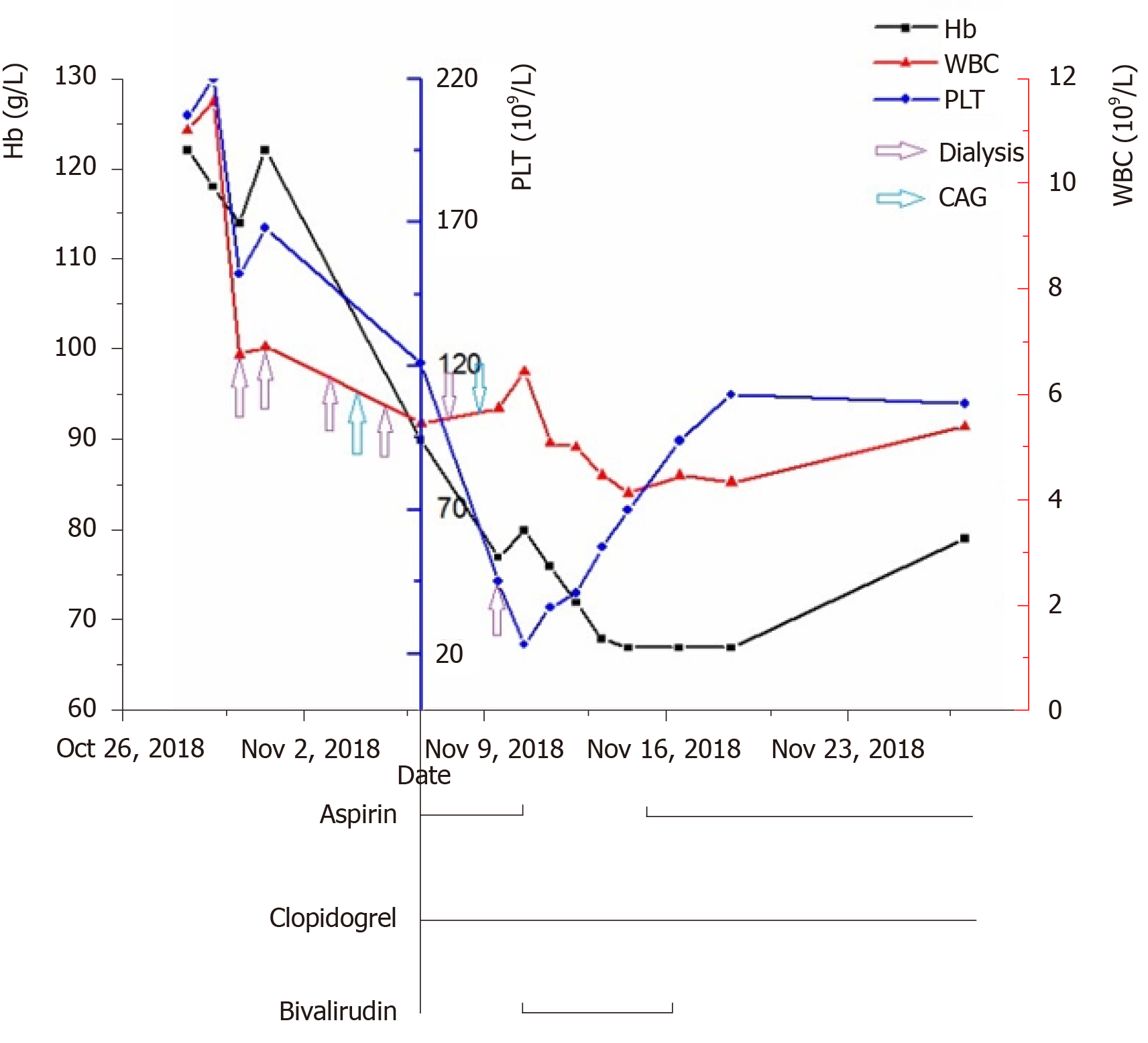
After admission, the patient was initially diagnosed with AMI, chronic renal failure, renal anemia, and hypertension. His GRACE score was 183, and CRUSADE score was 53. Thus, the ischemic risk and hemorrhagic risk were both high in this patient. He received standard treatment for secondary prevention of coronary heart disease, including dual-antiplatelet (aspirin 100 mg/d and Plavix 75 mg/d) and lipid-lowering (rosuvastatin 10 mg/d) therapies. Simultaneously, intravascular ultrasound (IVUS) examination and CAG were performed (November 9, 2018), which revealed left main coronary artery (LMT) and 3-vessel disease. Following a team discussion, the patient underwent PCI, with one stent placed in the LMT-LAD (Xience Xpedition 2.75 mm × 38 mm) and two overlapping stents placed in the mid-left circumflex (LCx) (Firehawk 2.5 mm × 13 mm) and proximal LCx (Xience Xpedition 3.5 mm × 33 mm) (Figure 2). During the operation, bivalirudin was administered for anticoagulation. The day before and after the angiogram, the patient was treated with hemodialysis, in which 8700 IU of LMWH calcium was used in total. The second day after CAG, he developed a fever with a temperature of 37.8 °C after hemodialysis, without chills, chest pain, or other discomfort. Routine blood examination was performed, which showed a hemoglobin level of 77 g/L, and a PLT count of 45 × 109/L. There was no clinical bleeding at that time. The patient’s fever resolved the following day, but both upper limbs showed signs of skin necrosis (Figure 3). His PLT count decreased to 23 × 109/L. HIT was then considered. Calculation of the 4T’s score revealed a count of 7, which indicated a high probability of HIT.
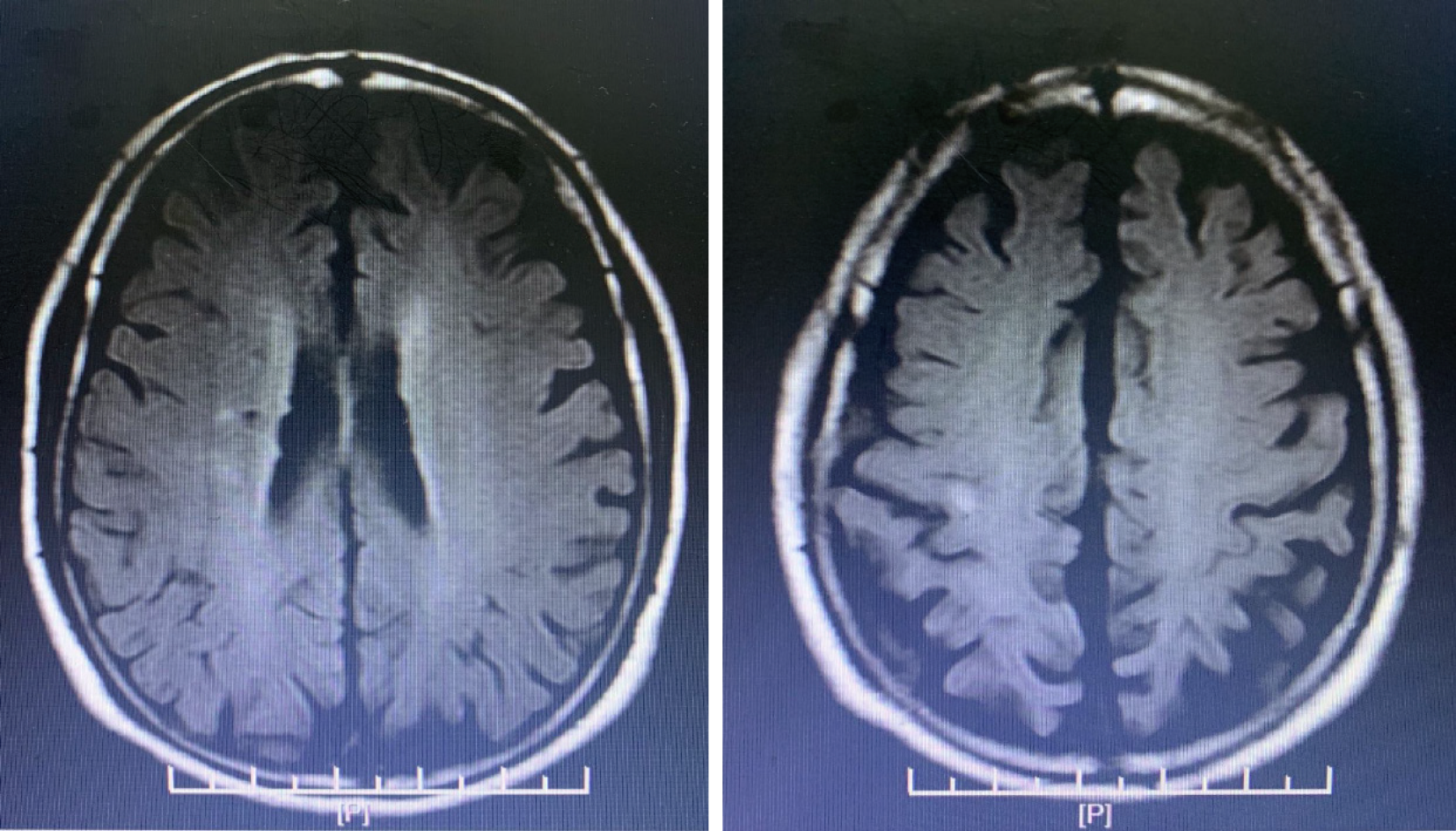
Hence, all heparin agents were stopped. At the same time, platelet factor 4 (PF4) antibody was determined. In order to prevent stent thrombosis, bivalirudin was administered (0.20 mg/kg per hour, activated clotting time approximately 200 s). Due to a high risk of bleeding, aspirin was stopped, and antiplatelet therapy with clopidogrel was continued. After 4 d, the PLT count rose to 70 × 109/L, and the PF4 antibody result was reported as 7.1 U/mL (normal 0-0.1 U/mL), which confirmed the diagnosis of HIT. Due to renal insufficiency, the use of a new oral anticoagulant and fondaparinux was limited. In addition, as the PLT count did not reach 300 × 109/L, the patient did not receive warfarin. Following treatment, the patient's condition was stable, and there were no more embolism events. Bivalirudin was discontinued on November 16, 2018, and dual-antiplatelet therapy with aspirin and clopidogrel was resumed. The next day, a sudden deviation of the patient’s mouth was observed. Brain magnetic resonance imaging confirmed acute cerebral infarction at the right ventricle and upper insular lobe (Figure 4). No special adjustments were made to the treatment regimen. His symptoms improved gradually. The PLT count recovered to 110 × 109/L on November 19, 2018.
No emboli or bleeding events occurred during the 18-mo follow-up period.
Reports of HIT in patients with renal failure undergoing dialysis and PCI after AMI are rare. According to statistics, the risk of HIT after exposure to UFH is 2.6%[3,4]. In addition to clinical manifestations and dynamic monitoring of the PLT count, the diagnosis of HIT is mainly based on the 4T's scoring system combined with HIT antibody detection and PLT function examination[5]. HIT is divided into type I and type II[1,6]. Our patient’s PLT count decreased significantly after 1 wk of heparin therapy, to 23 × 109/L at the lowest value. He presented additional symptoms of a general reaction and skin necrosis. His 4T's score was 7 points, which showed a high clinical probability of HIT. The HIT antibody test was positive, and his PLT count recovered gradually following heparin discontinuation. This patient was diagnosed with type II HIT[6,7].
When the patient’s history was traced back, his PLT count showed a progressive decline after heparin administration during the initial dialysis and CAG (Figure 5). HIT was diagnosed when the patient developed general reactions and skin necrosis with an obvious decrease in PLT count after heparin therapy after the patient received dialysis again. During diagnosis and treatment, the drugs administered and the autoimmune factor may be responsible for thrombocytopenia. In this patient, indicators of autoimmune diseases were negative, such as immunoglobulin and complement, β2 glycoprotein I antibody, platelet antibody, and other tests. Therefore, we were able to exclude thrombocytopenia caused by autoimmune factors. Medications that could have led to a decrease in PLT count in this patient included aspirin, clopidogrel, and heparins. Low-dose aspirin can reduce the production of thromboxane A2 (TXA2), thus inhibiting platelet aggregation induced by TXA2. The incidence of thrombocytopenia caused by aspirin is 0.1%. In addition, the incidence of thrombocytopenia caused by clopidogrel is approximately 0.2%, which mostly occurs within 2-3 mo after taking medication, and thrombotic thrombocytopenia purpura is more common with clopidogrel. The patient had been taking clopidogrel since the onset of his disease, and the PLT count gradually rebounded after discontinuation of heparin. Thus, decreased PLT count caused by clopidogrel was excluded. In addition, his PLT count did not decrease again when aspirin was taken orally but increased to more than 60 × 109/L; thus, thrombocytopenia caused by aspirin was also excluded.
When HIT is diagnosed or highly suspected, heparin should be discontinued immediately and an alternative anticoagulant should be given. Argatroban, a recombinant hirudin, and bivalirudin have been approved by the FDA as anticoagulants in patients with HIT who require PCI, and both bivalirudin and argatroban have been recommended by the American College of Chest Physicians (ACCP)[1,8]. This was based on multiple randomized trials (> 19000 patients without HIT) that proved the efficacy and safety of anticoagulant therapy using bivalirudin in patients undergoing PCI. Compared with UFH, bivalirudin showed a similar incidence of ischemic complications and was able to reduce the rate of bleeding events[9-14]. Although recent clinical trials have also shown that the risk of bleeding was similar to or lower than UFH, the stent thrombosis rate increased. In view of these new data, the clinical efficacy and cost-effectiveness of bivalirudin in patients not undergoing PCI have been questioned[15-17]. However, in patients with HIT, bivalirudin was still the best choice[1]. Fondaparinux, a new oral anticoagulant, and warfarin could also be used as alternative therapies, but the evidence for these agents is relatively weak. Our patient with AMI and chronic renal insufficiency developed HIT after PCI with a CRUSADE score of 53 points. Thus, the bleeding risk was high. However, considering the risk of coronary stent thrombosis and other emboli, stopping one type of antiplatelet and adding another anticoagulant at the same time was necessary. Bivalirudin was the preferred alternative anticoagulant in this patient because of his renal function. After 5 d of bivalirudin treatment, no new bleeding or thromboembolic events occurred. Bivalirudin was suspended in view of the financial situation of the patient's family. Considering the patient’s renal function, new oral anticoagulants and fondaparinux could not be used to replace bivalirudin. Warfarin was not added as the PLT count was below 300 × 109/L. After the recovery of the PLT count, dual-antiplatelet therapy was resumed, but acute cerebral infarction occurred after the withdrawal of bivalirudin. At present, there is no consensus on the course of anticoagulant therapy for HIT patients. The 2012 ACCP guidelines for HIT diagnosis and treatment do not clearly indicate the best alternative anticoagulant therapy. In 2018, the HIT treatment guidelines of the American Society of Hematology[6] proposed that alternative anticoagulation could be sustained until the PLT count returned to normal. According to the 2017 Chinese expert consensus on HIT[18], alternative anticoagulants should be used for at least 1 mo in patients with simple thrombocytopenia. For HIT patients with thrombus formation, anticoagulation therapy should continue for no less than 3 mo. The optimal course of alternative anticoagulation therapy still requires further research. Our patient was elderly and receiving dialysis for renal failure, which had an effect on the treatment process for HIT. Individualized treatment plans should be developed for special populations, in order to achieve satisfactory treatment effects.
HIT is a rare complication of heparin therapy. Due to its high mortality, it is important to diagnose this disease in a timely manner. Thrombocytopenia can be considered an early warning sign. When a patient is assessed as having a high clinical likelihood of HIT, the use of heparin should be discontinued as soon as possible and further tests should be carried out to identify or exclude HIT. With vigilance and suspicion, HIT can be diagnosed in the early stage, and individualized alternative anticoagulant therapy can reduce the mortality rate.
I would like to thank Dr. Deng SB for providing materials, advice, and inspiration. I would also like to thank The Second Affiliated Hospital of Chongqing Medical University, for providing the conditions to complete this paper, and to all those who helped me during the writing of this paper.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: MD AS S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Li JH
| 1. | Linkins LA, Dans AL, Moores LK, Bona R, Davidson BL, Schulman S, Crowther M. Treatment and prevention of heparin-induced thrombocytopenia: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e495S-e530S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 643] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 2. | Arepally GM, Ortel TL. Clinical practice. Heparin-induced thrombocytopenia. N Engl J Med. 2006;355:809-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 321] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 3. | Martel N, Lee J, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood. 2005;106:2710-2715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 554] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 4. | Girolami B, Prandoni P, Stefani PM, Tanduo C, Sabbion P, Eichler P, Ramon R, Baggio G, Fabris F, Girolami A. The incidence of heparin-induced thrombocytopenia in hospitalized medical patients treated with subcutaneous unfractionated heparin: a prospective cohort study. Blood. 2003;101:2955-2959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 191] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 5. | Almeqdadi M, Aoun J, Carrozza J. Native coronary artery thrombosis in the setting of heparin-induced thrombocytopenia: a case report. Eur Heart J Case Rep. 2018;2:yty138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Cuker A, Arepally GM, Chong BH, Cines DB, Greinacher A, Gruel Y, Linkins LA, Rodner SB, Selleng S, Warkentin TE, Wex A, Mustafa RA, Morgan RL, Santesso N. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2:3360-3392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 428] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 7. | Lovecchio F. Heparin-induced thrombocytopenia. Clin Toxicol (Phila). 2014;52:579-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Lanzarotti S, Weigelt JA. Heparin-induced thrombocytopenia. Surg Clin North Am. 2012;92:1559-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Bittl JA, Strony J, Brinker JA, Ahmed WH, Meckel CR, Chaitman BR, Maraganore J, Deutsch E, Adelman B. Treatment with bivalirudin (Hirulog) as compared with heparin during coronary angioplasty for unstable or postinfarction angina. Hirulog Angioplasty Study Investigators. N Engl J Med. 1995;333:764-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 357] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 10. | Bittl JA, Feit F. A randomized comparison of bivalirudin and heparin in patients undergoing coronary angioplasty for postinfarction angina. Hirulog Angioplasty Study Investigators. Am J Cardiol. 1998;82:43P-49P. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Lincoff AM, Bittl JA, Harrington RA, Feit F, Kleiman NS, Jackman JD, Sarembock IJ, Cohen DJ, Spriggs D, Ebrahimi R, Keren G, Carr J, Cohen EA, Betriu A, Desmet W, Kereiakes DJ, Rutsch W, Wilcox RG, de Feyter PJ, Vahanian A, Topol EJ; REPLACE-2 Investigators. Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA. 2003;289:853-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 919] [Cited by in RCA: 849] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 12. | Lincoff AM, Bittl JA, Kleiman NS, Sarembock IJ, Jackman JD, Mehta S, Tannenbaum MA, Niederman AL, Bachinsky WB, Tift-Mann J 3rd, Parker HG, Kereiakes DJ, Harrington RA, Feit F, Maierson ES, Chew DP, Topol EJ; REPLACE-1 Investigators. Comparison of bivalirudin vs heparin during percutaneous coronary intervention (the Randomized Evaluation of PCI Linking Angiomax to Reduced Clinical Events [REPLACE]-1 trial). Am J Cardiol. 2004;93:1092-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 158] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Gurm HS, Bhatt DL. Thrombin, an ideal target for pharmacological inhibition: a review of direct thrombin inhibitors. Am Heart J. 2005;149:S43-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Lee MS, Liao H, Yang T, Dhoot J, Tobis J, Fonarow G, Mahmud E. Comparison of bivalirudin vs heparin plus glycoprotein IIb/IIIa inhibitors in patients undergoing an invasive strategy: a meta-analysis of randomized clinical trials. Int J Cardiol. 2011;152:369-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Stone GW, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Kornowski R, Hartmann F, Gersh BJ, Pocock SJ, Dangas G, Wong SC, Kirtane AJ, Parise H, Mehran R; HORIZONS-AMI Trial Investigators. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med. 2008;358:2218-2230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1450] [Cited by in RCA: 1359] [Article Influence: 79.9] [Reference Citation Analysis (0)] |
| 16. | Steg PG, van 't Hof A, Hamm CW, Clemmensen P, Lapostolle F, Coste P, Ten Berg J, Van Grunsven P, Eggink GJ, Nibbe L, Zeymer U, Campo dell' Orto M, Nef H, Steinmetz J, Soulat L, Huber K, Deliargyris EN, Bernstein D, Schuette D, Prats J, Clayton T, Pocock S, Hamon M, Goldstein P; EUROMAX Investigators. Bivalirudin started during emergency transport for primary PCI. N Engl J Med. 2013;369:2207-2217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 387] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 17. | Shahzad A, Kemp I, Mars C, Wilson K, Roome C, Cooper R, Andron M, Appleby C, Fisher M, Khand A, Kunadian B, Mills JD, Morris JL, Morrison WL, Munir S, Palmer ND, Perry RA, Ramsdale DR, Velavan P, Stables RH; HEAT-PPCI trial investigators. Unfractionated heparin vs bivalirudin in primary percutaneous coronary intervention (HEAT-PPCI): an open-label, single centre, randomised controlled trial. Lancet. 2014;384:1849-1858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 413] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 18. | Committee on thrombosis prevention and treatment, Internal Medicine Branch of Cardiovascular Diseases, Chinese Physicians’ Association, Xu JT, Li WM, Men JL, Zhao X, Li Y. Chinese expert consensus on heparin-induced thrombocytopenia. Zhonghua Yixue Zazhi. 2018;98:408-417. [DOI] [Full Text] |