Published online Oct 26, 2020. doi: 10.4330/wjc.v12.i10.475
Peer-review started: April 18, 2020
First decision: July 25, 2020
Revised: August 12, 2020
Accepted: October 5, 2020
Article in press: October 5, 2020
Published online: October 26, 2020
Processing time: 188 Days and 6 Hours
The aim of this study was to review the most recent literature on the safety of electronic cigarettes (ECs) in the context of cardiovascular disease and in the context as a tool for smoking cessation and recreational purposes. The format of this review begins with relevant research from the basic sciences and follows through with a pertinent review of clinical trials. Daily use of ECs has implications in myocardial infarction (MI) with an odds ratio of 1.70 compared to healthy, nonsmokers and even worse risk for MI with dual use of combustible cigarettes together with EC with an odds ratio of 4.62. Studies measuring cardiac function with echocardiography reported both systolic and diastolic dysfunction along with reduced ejection fractions. Platelet aggregation, endothelial function, and hemodynamics during pregnancy were all but some of the pernicious cardiovascular implications of EC exposure. Though more studies need to be done on the topic of EC use and cardiovascular disease, the majority of studies considered in this review concluded some level of harm albeit in some instances less than that of traditional combustible cigarettes. ECs are toxic to human beings and their harmful effects cannot be overlooked. There is some favorable evidence of efficacy in smoking cessation though mixed with concern of chronic EC use. It will take decades to collect data for chronic EC use on long term sequelae, such as lung cancer. Though more and more reports of acute lung injury and hospitalizations related to EC use have been reported. Due to undergoing investigations of possible harm and life threatening complications of EC use, we cannot recommend ECs as safer or a more efficacious method of smoking cessation to traditional nicotine replacement therapies. A notable consideration for much of the literature reviewed are that standardization of EC use is difficult as device generation and battery voltage, frequency of use, and contents of EC liquid are just some of the vast complicating factors that limit the ability to effectively compare data.
Core Tip: Electronic cigarette (EC) use is rapidly expanding to cigarette-native users including adolescents with little known available studies on long term use in both respiratory and cardiovascular systems. As most recent studies have focused on respiratory implications of EC use, there is much research to be done on cardiovascular ramifications. This literature review focuses on the most recent publications relating to EC use and oxidant formation, thrombogenesis, and myocardial infarctions, and examines the safety profile of ECs in smoking cessation and recreational use.
- Citation: Vajdi B, Tuktamyshov R. Electronic cigarettes — myocardial infarction, hemodynamic compromise during pregnancy, and systolic and diastolic dysfunction: Minireview. World J Cardiol 2020; 12(10): 475-483
- URL: https://www.wjgnet.com/1949-8462/full/v12/i10/475.htm
- DOI: https://dx.doi.org/10.4330/wjc.v12.i10.475
Electronic cigarettes (ECs, E-cigarettes, E-cigs, personal vaporizers, vape pens) are recreational devices used to vaporize a solvent mixture to inhale as a cigarette alternative. As the rate of tobacco cigarette (TC) use has dropped in the United States relative to the rest of the world, industries have heavily marketed the use of ECs as the “safer” alternative. According to the surgeon general, smoking rates had reached historic lows in 2014[1]. The CDC reported that from 2017 to 2018 EC use (defined as at least 1 d of use in 30 d) amongst high school students and middle school students had increased 78% and 48%, respectively[2]. Approximately 20% of current high school students say that they have tried ECs likely because they feel they are safe[3]. Many of the claims advocating ECs as healthier alternatives to combustible cigarettes are sponsored by profit-driven tobacco companies whose assertions in the determination of EC safety profile needs to be studied.
Their rise to popularity can be attributed to a variety of factors including but not limited to unsubstantiated claims of higher safety profile compared to traditional combustion cigarettes, arguable smoking cessation efficacy, and limited market regulations. As large tobacco companies continue to enter the market, concerns about the short term and long term safety of ECs continues to trend as a popular topic[4,5].
EC devices vary in design but all share essential hardware basics consisting of a rechargeable lithium battery, a vaporization chamber, and an interchangeable liquid cartridge. Formulations of EC liquids include both tobacco-based and nontobacco mixtures. Of importance to our study, nicotine delivery to the human body is affected by various factors, such as the type of device used, voltage of battery, resistance, and chemical composition of solvent. Thus, studies on first-generation ECs reported delivery of low concentrations of nicotine to the bloodstream unlike newer generation devices equipped with high-capacity batteries[4-6]. One study by Farsalinos et al[8] showed a 35% to 72% increase in nicotine delivery with newer generations of ECs relative to first-generation devices.
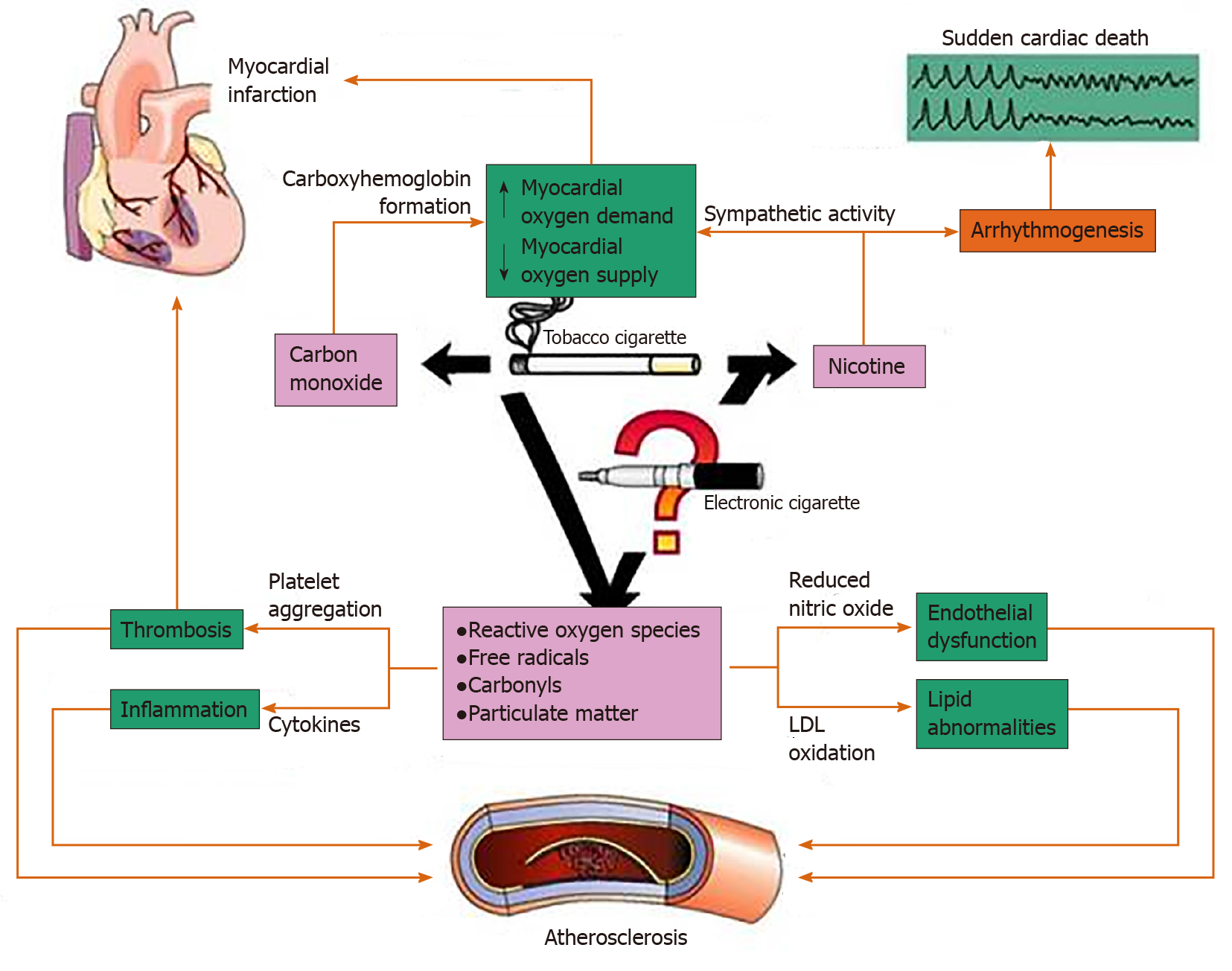
ECs introduce new complexities to studying the side effect profile that prior combustible cigarette use did not entail. The broad variability of ECs arises from the various nicotine concentrations present in e-liquids, various volumes of e-liquids per product, different carrier compounds, additives, flavors, and battery voltages[4,5]. Figure 1 shows the main effects of TC smoke on the cardiovascular system[9]. Many of these effects could be relevant to ECs as well.
Given that cardiovascular disease accounts for 30% of smoking-related deaths[4] and the recent explosion of the use of ECs, it is important to review the current literature relating to ECs and the cardiovascular system.
A joint study[10] from Charles Drew University and University of California, Los Angeles used apolipoprotein-E knockout mice to study cardiovascular effects of ECs on the heart. These mice were elected as they have been used to study the cardiovascular effects of conventional cigarettes. Mice were grouped and exposed to either aerosolized saline (used as a baseline control), ECs sans nicotine (0%), or ECs with 2.4% nicotine for 12 wk. One of the aims of the study was to use the 0% nicotine group to determine the relationship, if any, of propylene glycol and glycerol on the formation of free radicals in a heat dependent manner. The study used the commercially available E-cig brand bluCig PLUS and a specialized EC delivery system for the mice similar to human ECs. The 0% nicotine and 2.4% nicotine groups were exposed to bluCig solvents, specifically the Gold Leaf Tobacco (0% nicotine) and Classic Tobacco (2.4% nicotine), which varied only in nicotine content. This study used echocardiography and reported that mice exposed to ECs with 2.4% nicotine had decreased left ventricular fractional shortening and ejection fraction compared with 0% nicotine ECs, though they found no differences in the saline, 0%, and 2.4% groups in the following parameters: peak early diastolic, E/A ratio, and atrial filling velocity. University of California, Los Angeles researchers looked at RNA sequence changes of tissue in the left ventricle of mice in the 2.4% and 0% and aerosolized nicotine groups using an Agilent 2100 Bioanalyzer. Of the 24054 genes analyzed, 109 of them showed at least a 1.5 fold variability (48 upregulated, 61 downregulated) with a P value of < 0.05 in the 2.4% aerosolized nicotine group relative to no changes seen in the saline group. The 2.4% subgroup again was associated with expression of inflammatory molecules Col5a3, TNFRSF12A, and selectin E.
Ventricular transcriptomatic analysis revealed changes in genes associated with metabolism, circadian rhythm, and inflammation on mice exposed to ECs with 2.4% nicotine while transmission electron microscopy showed cardiomyocytes with structural irregularities suggestive of cardiomyopathy. In the same group of mice, researchers saw increased oxidative stress, mitochondrial DNA mutations, and atherosclerotic lesions compared with saline aerosol-treated mice.
A large cross-sectional study from George Washington University published in 2018 was one of the first of its kind to report that daily EC use increased the odds of having a myocardial infarction (MI) with an odds ratio of 1.79, which was relatively less than associated TC smoking (odds ratio: 2.72) though still of significant concern[7]. Study data was collected from 2014 to 2016 by the National Health Interview Survey by random selection of United States households and subsequent in-person interviews of adults over the age of 18. The study found that daily dual use of EC and traditional combustible cigarettes had significantly higher risk for MI relative to never smokers (odds ratio: 2.72 × 1.70 = 4.62). The limitations of this study were that the study was a self-reporting survey posing concerns for recall bias, and equally as important there was no differentiation between solvent nicotine presence or concentration, type of device used, and a clearly defined meaning of “daily use.”
Farsalinos et al[8] recently published a study shedding light on the little known effects of EC use and cardiovascular health. They conducted a study on 36 healthy persons who heavily smoked TC and compared them to 40 healthy, ex-TC smokers now using ECs at a comparable rate for 1 mo. Echocardiographic examinations were performed before and after using the EC device with “medium-strength” nicotine concentration. The study used echocardiography to measure acute changes in cardiac function and found no change in left ventricular function after EC use but did find changes in diastolic function after just one EC use[8]. Echocardiography studies focused on mitral flow diastolic velocities, deceleration time, isovolumetric relaxation time, and corrected heart rate[8]. Diastolic velocities were specifically measured by averaging the lateral, septal, anterior, and inferior insertion sites of the mitral leaflets. Some limitations of this study were a small sample size of 76 subjects and a lack of unhealthy participants. Further, the scope of the study was limited to acute changes and little can be said about the chronic effects of EC use from this experiment.
A cross-sectional study published in the American Journal of Preventive medicine had significant data regarding the association between EC use and myocardial infarction. Alzahrani et al[7] stratified EC and TC users as never before, former, some days, or daily users taking into account demographics and pre-existing health conditions such as hypertension, dyslipidemia, and diabetes. The study population was based on the 2014 and 2016 National Health Interview Survey with participants aged ≥ 18 years from randomly sampled United States households. Significantly, data unequivocally suggested an increased risk for MI with an odds ratio of 1.7 in both adjusted and unadjusted groups of daily EC users compared with subjects that had never used ECs before when all confounding variables were accounted for such as previous history of cigarette use, gender, etc. Moreover, participants who were dual users of ECs and combustible cigarettes had a total odds of 4.62 of having had an MI compared to those who had never smoked and never used ECs. This suggests a synergistic pernicious effect that dual usage has on cardiovascular health. In summary this study concluded that daily EC use was associated with a higher risk of MI after confounding factors are accounted for including traditional combustible cigarettes and other risk factors.
We summarized the major studies about effects of ECs on cardiac functional changes and associated risks of MI in Table 1.
| Name of the study | Type of the study | Results | Comments |
| Chronic intermittent electronic cigarette exposure induces cardiac dysfunction and atherosclerosis in apolipoprotein-E knockout mice | Animal model randomized control study with intervention | ECs with 2.4% nicotine had decreased left ventricular ejection fraction and had increased atherosclerotic lesions compared to ECs without nicotine and saline groups. Mice exposed to 2.4% nicotine vapor had increased atherosclerotic lesions on aorta as well | Mice model with limited implication to humans |
| Association between electronic cigarette use and myocardial infarction | Cross-sectional; study was based on self-reporting surveys | Increased odds ratio (1.79) of having MI with ECs and even higher odds ratio (4.62) with dual use of ECs and combustible cigarettes relative to never smokers | Number of surveys in 2014 was 36697 and 33028 in 2016 |
| Acute effects of using an electronic nicotine-delivery device (electronic cigarette) on myocardial function: Comparison with the effects of regular cigarettes | Interventional, case-control | Electronic cigarettes caused delayed myocardial relaxation, no effect on systolic function | Small study with total of 76 subjects; the study does not predict long term effects |
| Association between electronic cigarette use and myocardial infarction | Cross-sectional through surveys | Daily EC use increased the odds of having MI (OR = 1.79) while daily TC smoking had a higher correlation of MI (OR = 2.72) | Large surveys: 36697 in 2014 and 33028 in 2016 |
TCs have long been known to be associated with platelet aggregation and thrombogenesis[5,7,9,11]. One in vivo study evaluated platelet function while under incubation with ECs, TCs, and pure nicotine extracts and showed that platelet aggregation and adhesion receptors were upregulated in relation to nicotine concentration in a dose dependent manner[5,9]. Two types of ECs were used: All-in-one devices, 1.2% or 1.8% nicotine by volume (Njoy and OneJoy, respectively); and reloadable cartridge devices, 0, 12, and 18 mg of nicotine (eGo, OKC Vapes, and Desert Sands Flavor, respectively). Researchers mimicked puffing times of 5 min (time to smoke traditional cigarette) during their extraction of EC vapors. Extracts were stored in HEPES buffer and later exposed to healthy platelets. Results showed activation of platelet CD41 (GP11b) after exposure to both combustible tobacco smoke extracts and EC vapor extracts compared to controls of no smoke exposure. gC1qR, cC1qR, CD42b, GPIBa, and CD62P (P-selectin) also showed significant upregulation, and researchers also found an increase of platelet aggregation rate[5]. Interestingly, pure nicotine seemed to exacerbate platelet dysfunction in a dose-dependent manner. The study also measured deposition of complement products (C1q, C3b, C4d, and C5b-9) on the surface of platelets but was unable to show any correlation with exposure to any of the extracts. In the same study researchers at Stony Brook University showed a similar response in humans and by measuring CD40L and P-selectin platelet activation markers. After TC and EC use, an increase in activity in both of the aforementioned was observed. Another recently published study in the Journal of the American Heart Association reported a rapid increase in the number of circulating endothelial progenitor cells after EC use by examining the in vitro effects of adhesion and aggregation in healthy human volunteers[14].
Aldehydes such as formaldehyde and acrolein have long been suspected to play roles in human toxicology[9,11,12]. Acrolein is found in high concentrations in automobile exhaust, industrial waste, and tobacco smoke, which are generally thought to be produced by burning of fossil fuels exogenously and endogenously by lipid peroxidation[12]. Gaseous acrolein is one of the suspected agents in EC smoke that contributes to increased thrombogenesis, while another agent in EC smoke, formaldehyde, was seen to increase total platelet count in mice[9,11]. Sithu et al[12] showed that both acute and chronic acrolein levels correlated with increased platelet-fibrinogen formation, ADP-induced platelet aggregation, induction of platelet-leukocyte aggregates, and PF4 Levels.
One significant study from Osei et al[13] at Johns Hopkins focused on dual usage of ECs and TCs and the subsequent effects on the cardiovascular system. This was a cross-sectional telephone survey study that consisted of 449092 participants of whom 15863 (3.5%) were current EC users, 2.9% were dual users (EC + TC), and 10% of the participants had cardiovascular disease. This was a self-reported study where cardiovascular disease was defined as a composite of coronary heart disease, myocardial infarction, or stroke. Remarkably, dual smokers had a 36% higher odds of cardiovascular disease (odds ratio: 1.36) along with reports of premature cardiovascular disease in women in comparison to traditional cigarette smokers who had never used ECs[13].
A study from Stanford led by Lee et al[3] used stem-cell-derived endothelial cells to study the effects of EC use. The study included the flavors of fruit, tobacco, sweet tobacco with caramel and vanilla, sweet butterscotch, cinnamon, and menthol and nicotine concentrations of 0, 6, and 18 milligrams per milliliter. Of major significance, they found that the cells lost viability even in the absence of nicotine. Results varied from the different flavors with the cinnamon flavor having the highest adverse reactions, including decreased cell viability, increased reactive oxygen species (ROS) levels, caspase 3/7 activity, and low-density lipoprotein uptake, activation of oxidative stress-related pathway, and impaired tube formation and migration, which confirmed their hypothesis of endothelial dysfunction. Further they found activation of macrophages, which caused downstream activation of interleukin-1B and increased ROS. Lee et al[3] found that nicotine had a dose-dependent effect on many of the measured parameters including cytotoxicity, ROS generation, and apoptotic activities.
An interesting review article on vascular calcification and ECs comes from a recent publication in the Journal for the American Heart Association that looked at human, rat, and cell culture studies. First, a review on the definitions of atherosclerosis vs arteriosclerosis that this article focused on: Atherosclerosis is characterized by accrual of lipids, penetration of macrophages, and fibrotic lesions within plaques with ensuing rupture and thrombus formation; and arteriosclerosis is transformation of the arteriole wall by either hardening, thickening, or loss of elasticity. Of significance, this scholarly review of literature concluded that nicotine-containing vaping facilitates and perhaps induces osteogenic transdifferentiation and calcification of the tunica media wall of vascular smooth muscle cells by means of inflammation, endothelial dysfunction, and production of ROS. This process is believed to be a key event in the calcification of the tunica media of the vessel wall. Nicotine facilitates transdifferentiation of vascular smooth muscle cells by activation of three major pathways: NF-κB; macrophages and monocytes; and subsequently TNFα, interleukin-6 and VCAM-1. These all lead to an inflammatory state in the vasculature that can cause both acute cardiovascular compromise and chronic degradation of contractile properties of the vascular smooth muscle cells[14].
As concerns arise regarding the lack of regulation on EC products, it is important to recall that the flavoring additives alone can be toxic to humans. While combustible cigarettes are limited to menthol for flavoring, ECs remain unregulated with a wide range of flavorings available on the market. Fetterman et al[19] used freshly isolated endothelial cells from nonsmoking, healthy participants and measured nitric oxide production and vasodilation at baseline and after exposure to heated flavorings. The flavoring additives were heated to a temperature of 37 C using a tank device similar in function to an EC device and subsequently exposed to the endothelial cells for 90 min. The flavorings vanillin, cinnamaldehyde, eugenol, acetylpyridine, and menthol impaired nitric oxide production and increased expression of proinflammatory mediators and interleukin-6, suggesting that these flavors are injurious to the endothelium.
In a previously referenced article[10], mice were exposed to an EC delivery system mimicking real use in a laboratory setting to measure the formation of atherosclerotic lesion formation. They used oil red O staining and contrasted that with a hematoxylin and fast green stain and found that rats exposed to 2.4% nicotine EC vapor had increased lesions at the aortic root as quantified by Image-Pro Plus in comparison to saline treated mice. To be exact, saline treated mice had lesions that measured 53.6 ± 7.5 × 105 μm2 vs a much larger lesion in 2.4% treated mice of 103.9 ± 12.9 × 105 μm2 with a P value of less than 0.01. More information on the methods of this particular experiment is mentioned above.
One opposing view regarding cardiovascular effects of ECs comes from Cossio et al[15]. In a small study consisting of sixteen young, healthy tobacco product naïve participants, they measured acute responses to “vaping trials” using flavored ECs with either 0% or 5.4% nicotine for 6 min. The vaping protocol was a total of 6 min in length consisting of 4 s inhalations every 20 s, which corresponded to 18 puffs in total. Subjects were monitored during their trial to ensure they were puffing at a standardized rate. Measurements were done at baseline, immediately post, 1 h, and 2 h post-EC exposure. There were no noteworthy changes in heart rate, systolic and diastolic blood pressure, endothelial function (via flow-mediated dilation), or arterial stiffness (via cardio-ankle vascular index) during the trials. The study is limited in that the reported data represented acute changes to only one bout of EC use and does not account for multiple bouts per day, which is characteristic of many EC users.
We summarized the major studies about the effects of ECs on arteriosclerosis and endothelial damage in Table 2.
| Name of the study | Type of the study | Results | Comments |
| Modeling cardiovascular risks of ECs with human-induced pluripotent stem cell-derived endothelial cells | Randomized interventional on human endothelial cells; cells were exposed to EC flavoring products with and without nicotine | Flavoring e-liquids caused endothelial dysfunction even without nicotine; nicotine had a dose-dependent effect on cytotoxicity, reactive oxygen species generation, and apoptotic activities | In vitro study with limited implications |
| Flavorings in tobacco products induce endothelial cell dysfunction | Intervention study on human endothelial cells obtained from smokers and nonsmokers | The flavorings vanillin, cinnamaldehyde, eugenol, acetylpyridine, and menthol impaired nitric oxide production and increased expression of proinflammatory mediators and interleukin-6 | Small study; the endothelial cells obtained by biopsy from 3 groups of 6 to 9 subjects |
| Vascular effects of a single bout of electronic cigarette use | Interventional nonrandomized study on healthy volunteers | There were no significant changes in heart rate, systolic and diastolic blood pressure, endothelial function (via flow-mediated dilation), and arterial stiffness (via cardio-ankle vascular index) throughout the experiments | Small study on 16 volunteers; the study was limited to acute changes post smoking one bout of ECs; flow mediated dilation and cardio-ankle vascular index may not be sensitive enough |
The potential use of ECs in the area of smoking cessation therapy must be weighed against potential subsequent chronic use of ECs along with EC specific toxicities that remain to be studied extensively. As of September 24, 2019, 46 state health agencies have reported 805 patients with cases of lung injury associated with use of EC products to the Centers for Disease Control and Prevention and per the same report from the Centers for Disease Control and Prevention, 12 EC-related deaths have been confirmed in 10 states[24]. The efficacy of ECs in cessation of smoking TCs continues to be a debated topic with mixed results. Safety profile and superiority of ECs in relation to nicotine replacement products (NRTs) in smoking cessation are two areas of interest on the issue. A recent study published in the New England Journal of Medicine in February gives some insight into this topic.
Hajek et al[16] designed a two-group randomized, controlled trial with 886 participants to evaluate ECs as a means of smoking cessation relative to traditional NRTs. Smokers were divided into two treatment groups: EC users and NRTs. Subjects were treated for 3 mo with their respective cessation method and followed up in 1 year with a biochemical test (expired carbon-monoxide levels), and these values were compared to their baseline carbon-monoxide levels prior to initiation of treatment. EC users were given “starter packs” consisting of second generation refillable ECs with 18 mg per milliliter of nicotine though participants were free to purchase their own preferred brand and flavoring during the duration of the study. The NRT group was given their preferred choice of patch, gum, lozenge, nasal spray, inhalator, mouth spray, mouth strip, and microtab with the option of interchanging one for another. Both groups received weekly behavioral support for at least 4 wk from local clinicians. The abstinence rate at the 1-year mark was 18% in the EC group and 9.9% in the NRT group (relative risk, 1.83; 95% confidence interval: 1.30 to 2.58; P < 0.001). However, it is important to note that of those that were able to abstain from smoking at the year mark, EC users were more likely to be continually using their EC at the 52 wk mark than NRTs (80% vs 9%, respectively)[16]. The superiority of ECs to NRTs in smoking cessation is in contrast to the Cochrane study in 2016, which had inconclusive results of potency of ECs to NRTs in smoking cessation[6].
A recent review published in August 2019 by Worku et al[17] concluded that the few studies showing success in the role of ECs in smoking cessation were offset by the fact that the most common EC user was a dual user of both ECs and traditional combustible cigarettes and the increased susceptibility of users, particularly youth, to future use of cigarettes[18]. Youth are particular targets of the EC campaigns as the appeal for traditional cigarettes has lessened in recent years. Therefore, companies have aimed to revive the smoking industry by marketing the devices as trendy and modern. A University of California, Los Angeles review found three studies indicating that high school students who were EC users had increased interest relative to never-before-smokers to initiate use of cigarettes supporting the notion that ECs are a gateway to cigarettes and other combustible tobacco products such as hookah[20].
Youth are not the only subgroup that are especially at risk of potential harms. Pregnant females are also a population of concern. A survey study published in 2015 surveyed 184 persons, 68 of whom were 18-20-years-old, 55 were 21-30-years-old, and 61 were 31-plus-years-old. The study found that all ages perceive ECs as significantly less pernicious than combustible cigarettes including relating to lung cancer, harm during pregnancy, and addictive properties[21]. Multiple studies suggest that the negative fetal effects of tobacco are specifically linked to nicotine, an alarming finding in the context of increased usage of ECs during pregnancy due to false safety claims[21,23].
A Texas based study in May 2019 used lab rats to study how EC aerosol exposure during fetal development jeopardized vascular hemodynamic function and attributed to growth defects[22]. The study used pregnant Sprague-Dawley rats and divided them into three groups: Controls, juice-fed, and juice plus nicotine fed and used a custom engineered vaping system to simulate the EC paradigm. Researchers compounded an inhouse e-liquid with various nicotine concentrations to use in the study with an 80:20 propylene glycol to glycerol ratio, which is similar to that found in popular e-liquid brands. The rats undertook vaping treatment for 2 h a day, 5 d days per week from gestation day (GD)5 until GD 21 and gave birth on GD 22. It is important to note that the researchers exposed the nicotine rat group to 10% nicotine throughout the duration of the study. The study utilized mass spectrometry to identify and quantify blood nicotine levels. Results were significant as they found the juice plus nicotine group had a decreased fetal weight of 46.56% and a decrease of 23.83% in crown-rump length compared to controls. Again, fetal hemodynamics were compromised as both uterine and umbilical artery blood flow between the control group and nicotine group were significantly decreased (uterine artery 49.50%, P = 0.05; umbilical artery 65.33%, P = 0.01). These values were measured using a Doppler ultrasound and measured peak-to-peak times for three waveforms to pass. Although this study brings interesting insight into gestational hemodynamic compromise in rats, we are unable to draw any definitive implications regarding human gestation for obvious ethical concerns. Given the data presented in this study, we strongly admonish the use of EC during any stage of human gestation.
ECs remain a threat to cardiovascular health as studies thus far unanimously support the notion that ECs pose at least some level of risk. Misleading marketing geared towards youth and a general misconception about the safety profile of ECs are of particular concern as these products remain generally unregulated and unstudied. Atherosclerosis, free radical formation, diastolic functional changes, RNA and mDNA sequence changes, and incidence of MI are only some of the changes implicated thus far in ongoing research on the topic of ECs. Further tangential vascular concerns include fetal compromise due to significant umbilical and uterine artery blood flow restriction from EC use in rat models. Though a minority of studies show EC as posing less of a threat to cardiovascular health in relation to traditional combustible cigarettes, there is a need for more studies, particularly ones that focus on chronic use. The vast majority of literature reviewed concluded some level of harm, and multiple studies showed a significant synergistic risk when smokers concomitantly used ECs with combustible cigarettes, which greatly increased the risk for MI relative to either used individually. This literature review concludes at least some level of risk to endothelial and platelet function as well as compromised hemodynamics during rat-model gestation. After thorough review of the current literature, we are unable to endorse ECs as a safe alternative to smoking cessation especially in the context of cardiovascular disease and ongoing reports of acute lung injury and lethal consequences with EC use.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: American Board of Internal Medicine.
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Robles NR, Serhiyenko VA S-Editor: Gao CC L-Editor: Filipodia P-Editor: Wu YXJ
| 1. | National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2014. [PubMed] |
| 2. | Cullen KA, Ambrose BK, Gentzke AS, Apelberg BJ, Jamal A, King BA. Notes from the Field: Use of Electronic Cigarettes and Any Tobacco Product Among Middle and High School Students - United States, 2011-2018. MMWR Morb Mortal Wkly Rep. 2018;67:1276-1277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 479] [Cited by in RCA: 570] [Article Influence: 81.4] [Reference Citation Analysis (0)] |
| 3. | Lee WH, Ong SG, Zhou Y, Tian L, Bae HR, Baker N, Whitlatch A, Mohammadi L, Guo H, Nadeau KC, Springer ML, Schick SF, Bhatnagar A, Wu JC. Modeling Cardiovascular Risks of E-Cigarettes With Human-Induced Pluripotent Stem Cell-Derived Endothelial Cells. J Am Coll Cardiol. 2019;73:2722-2737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 4. | Qasim H, Karim ZA, Rivera JO, Khasawneh FT, Alshbool FZ. Impact of Electronic Cigarettes on the Cardiovascular System. J Am Heart Assoc. 2017;6:e006353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 140] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 5. | Hom S, Chen L, Wang T, Ghebrehiwet B, Yin W, Rubenstein DA. Platelet activation, adhesion, inflammation, and aggregation potential are altered in the presence of electronic cigarette extracts of variable nicotine concentrations. Platelets. 2016;27:694-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 6. | Hartmann-Boyce J, McRobbie H, Bullen C, Begh R, Stead LF, Hajek P. Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev. 2016;9:CD010216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 294] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 7. | Alzahrani T, Pena I, Temesgen N, Glantz SA. Association Between Electronic Cigarette Use and Myocardial Infarction. Am J Prev Med. 2018;55:455-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 242] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 8. | Farsalinos KE, Tsiapras D, Kyrzopoulos S, Savvopoulou M, Voudris V. Acute effects of using an electronic nicotine-delivery device (electronic cigarette) on myocardial function: Comparison with the effects of regular cigarettes. BMC Cardiovasc Disord. 2014;14:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 9. | MacDonald A, Middlekauff HR. Electronic cigarettes and cardiovascular health: what do we know so far? Vasc Health Risk Manag. 2019;15:159-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 10. | Espinoza-Derout J, Hasan KM, Shao XM, Jordan MC, Sims C, Lee DL, Sinha S, Simmons Z, Mtume N, Liu Y, Roos KP, Sinha-Hikim AP, Friedman TC. Chronic intermittent electronic cigarette exposure induces cardiac dysfunction and atherosclerosis in apolipoprotein-E knockout mice. Am J Physiol Heart Circ Physiol. 2019;317:H445-H459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 11. | Zhang Y, Liu X, McHale C, Li R, Zhang L, Wu Y, Ye X, Yang X, Ding S. Bone marrow injury induced via oxidative stress in mice by inhalation exposure to formaldehyde. PLoS One. 2013;8:e74974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Sithu SD, Srivastava S, Siddiqui MA, Vladykovskaya E, Riggs DW, Conklin DJ, Haberzettl P, O'Toole TE, Bhatnagar A, D'Souza SE. Exposure to acrolein by inhalation causes platelet activation. Toxicol Appl Pharmacol. 2010;248:100-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Osei AD, Mirbolouk M, Orimoloye OA, Dzaye O, Uddin SMI, Benjamin EJ, Hall ME, DeFilippis AP, Stokes A, Bhatnagar A, Nasir K, Blaha MJ. Association Between E-Cigarette Use and Cardiovascular Disease Among Never and Current Combustible-Cigarette Smokers. Am J Med. 2019;132:949-954.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 152] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 14. | Antoniewicz L, Brynedal A, Hedman L, Lundbäck M, Bosson JA. Acute Effects of Electronic Cigarette Inhalation on the Vasculature and the Conducting Airways. Cardiovasc Toxicol. 2019;19:441-450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 15. | Cossio R, Cerra ZA, Tanaka H. Vascular effects of a single bout of electronic cigarette use. Clin Exp Pharmacol Physiol. 2020;47:3-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Hajek P, Phillips-Waller A, Przulj D, Pesola F, Myers Smith K, Bisal N, Li J, Parrott S, Sasieni P, Dawkins L, Ross L, Goniewicz M, Wu Q, McRobbie HJ. A Randomized Trial of E-Cigarettes vs Nicotine-Replacement Therapy. N Engl J Med. 2019;380:629-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 986] [Cited by in RCA: 961] [Article Influence: 160.2] [Reference Citation Analysis (0)] |
| 17. | Worku D, Worku E. A narrative review evaluating the safety and efficacy of e-cigarettes as a newly marketed smoking cessation tool. SAGE Open Med. 2019;7:2050312119871405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Barrington-Trimis JL, Berhane K, Unger JB, Cruz TB, Urman R, Chou CP, Howland S, Wang K, Pentz MA, Gilreath TD, Huh J, Leventhal AM, Samet JM, McConnell R. The E-cigarette Social Environment, E-cigarette Use, and Susceptibility to Cigarette Smoking. J Adolesc Health. 2016;59:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 19. | Fetterman JL, Weisbrod RM, Feng B, Bastin R, Tuttle ST, Holbrook M, Baker G, Robertson RM, Conklin DJ, Bhatnagar A, Hamburg NM. Flavorings in Tobacco Products Induce Endothelial Cell Dysfunction. Arterioscler Thromb Vasc Biol. 2018;38:1607-1615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 20. | Levy DT, Warner KE, Cummings KM, Hammond D, Kuo C, Fong GT, Thrasher JF, Goniewicz ML, Borland R. Examining the relationship of vaping to smoking initiation among US youth and young adults: a reality check. Tob Control. 2019;28:629-635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 154] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 21. | Baeza-Loya S, Viswanath H, Carter A, Molfese DL, Velasquez KM, Baldwin PR, Thompson-Lake DG, Sharp C, Fowler JC, De La Garza R 2nd, Salas R. Perceptions about e-cigarette safety may lead to e-smoking during pregnancy. Bull Menninger Clin. 2014;78:243-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 22. | Orzabal MR, Lunde-Young ER, Ramirez JI, Howe SYF, Naik VD, Lee J, Heaps CL, Threadgill DW, Ramadoss J. Chronic exposure to e-cig aerosols during early development causes vascular dysfunction and offspring growth deficits. Transl Res. 2019;207:70-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 23. | Ernst M, Moolchan ET, Robinson ML. Behavioral and neural consequences of prenatal exposure to nicotine. J Am Acad Child Adolesc Psychiatry. 2001;40:630-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 408] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 24. | Perrine CG, Pickens CM, Boehmer TK, King BA, Jones CM, DeSisto CL, Duca LM, Lekiachvili A, Kenemer B, Shamout M, Landen MG, Lynfield R, Ghinai I, Heinzerling A, Lewis N, Pray IW, Tanz LJ, Patel A, Briss PA; Lung Injury Response Epidemiology/Surveillance Group. Characteristics of a Multistate Outbreak of Lung Injury Associated with E-cigarette Use, or Vaping - United States, 2019. MMWR Morb Mortal Wkly Rep. 2019;68:860-864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 137] [Article Influence: 22.8] [Reference Citation Analysis (0)] |