Published online Apr 26, 2019. doi: 10.4330/wjc.v11.i4.126
Peer-review started: January 8, 2019
First decision: January 21, 2019
Revised: February 11, 2019
Accepted: March 27, 2019
Article in press: March 28, 2019
Published online: April 26, 2019
Processing time: 112 Days and 0 Hours
A few randomized clinical trials (RCT) and their meta-analyses have found patent foramen ovale closure (PFOC) to be beneficial in prevention of stroke compared to medical therapy. Whether the benefit is extended across all groups of patients remains unclear.
To evaluate the efficacy and safety of PFOC vs medical therapy in different groups of patients presenting with stroke, we performed this meta-analysis of RCTs.
Electronic search of PubMed, EMBASE, Cochrane Central, CINAHL and ProQuest Central and manual search were performed from inception through September 2018 for RCTs. Ischemic stroke (IS), transient ischemic attack (TIA), a composite of IS, TIA and systemic embolism (SE), mortality, major bleeding, atrial fibrillation (AF) and procedural complications were the major outcomes. Random-effects model was used to perform analyses.
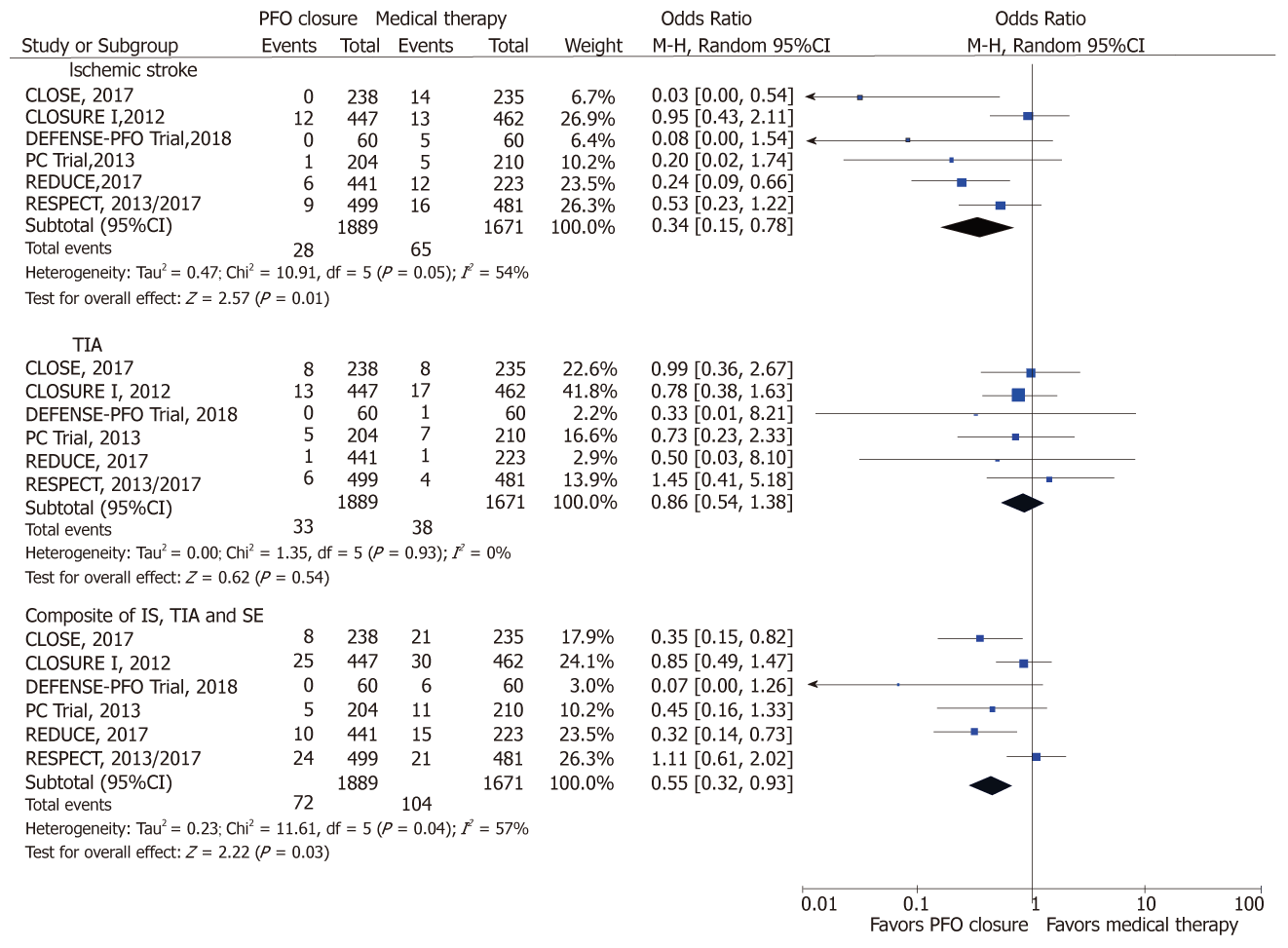
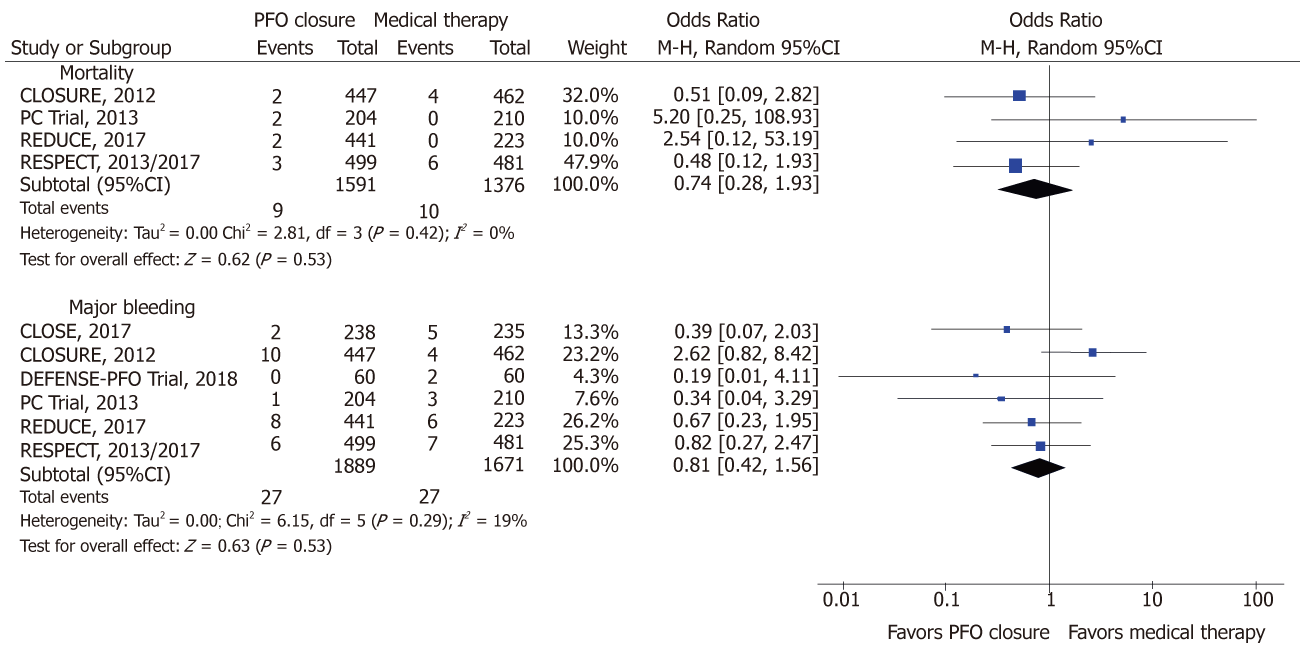
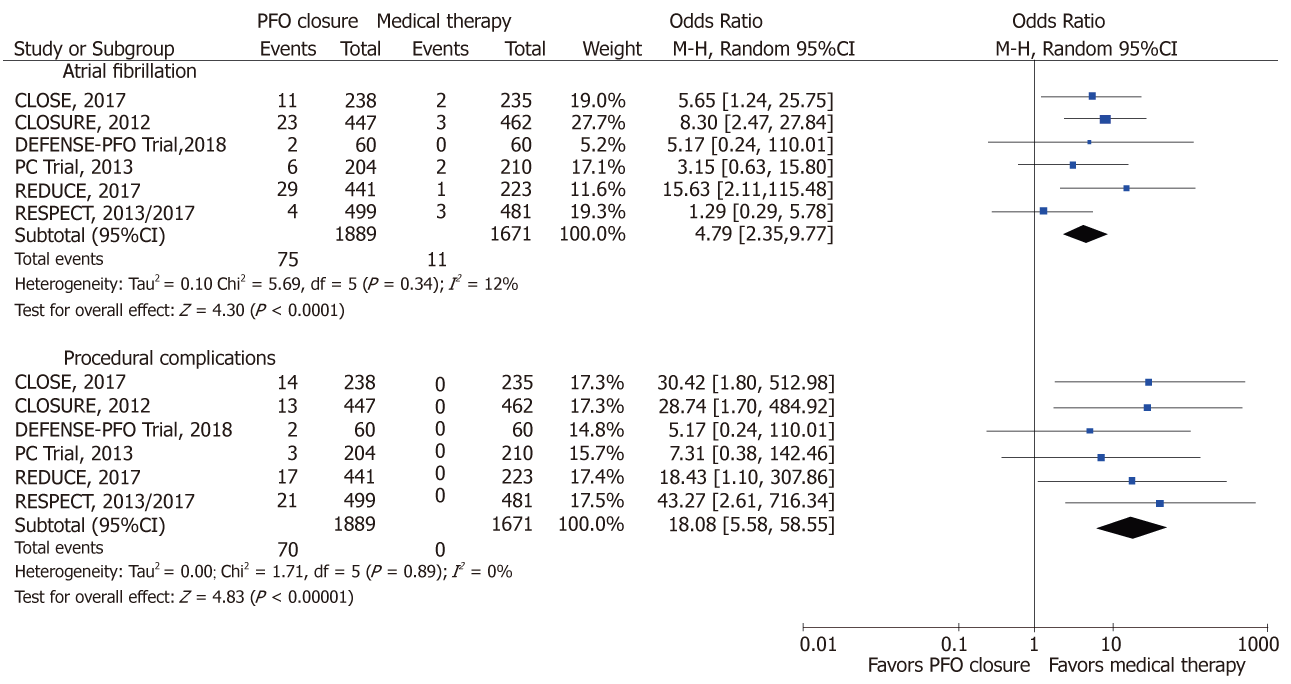
Meta-analysis of 6 RCTs including 3560 patients showed that the PFOC, compared to medical therapy reduced the risk of IS [odds ratio: 0.34; 95% confidence interval: 0.15-0.78; P = 0.01] and the composite of IS, TIA and SE [0.55 (0.32-0.93); P = 0.02] and increased the AF risk [4.79 (2.35-9.77); P < 0.0001]. No statistical difference was observed in the risk of TIA [0.86 (0.54-1.38); P = 0.54], mortality [0.74 (0.28-1.93); P = 0.53] and major bleeding [0.81 (0.42-1.56); P = 0.53] between two strategies. Subgroup analyses showed that compared to medical therapy, PFOC reduced the risk of stroke in persons who were males, ≤ 45 years of age and had large shunt or atrial septal aneurysm.
In certain groups of patients presenting with stroke, PFOC is beneficial in preventing future stroke compared to medical therapy.
Core tip: Closure of patent foramen ovale closure (PFOC) is a treatment modality for patients with stroke. To evaluate the efficacy and safety of PFOC vs medical therapy in different groups of patients presenting with stroke, we performed this meta-analysis of randomized trials following standard techniques. It showed that PFOC, compared to medical therapy reduced the risk of ischemic stroke and the composite outcome of stroke, transient ischemic attack (TIA) and systemic thromboembolism but no difference was observed in the risk of TIA, mortality and major bleeding. PFOC increased the risk of atrial fibrillation. Subgroup analyses showed that PFOC reduced the risk of stroke in males.
- Citation: Dahal K, Yousuf A, Watti H, Liang B, Sharma S, Rijal J, Katikaneni P, Modi K, Tandon N, Azrin M, Lee J. Who benefits from percutaneous closure of patent foramen ovale vs medical therapy for stroke prevention? In-depth and updated meta-analysis of randomized trials. World J Cardiol 2019; 11(4): 126-136
- URL: https://www.wjgnet.com/1949-8462/full/v11/i4/126.htm
- DOI: https://dx.doi.org/10.4330/wjc.v11.i4.126
Every year more than 10 million people suffer from stroke in the world, two thirds of which are ischemic in etiology[1]. Up to 32% of patients with ischemic stroke (IS) are cryptogenic in origin[2] and 43% of cryptogenic stroke patients have patent foramen ovale (PFO)[3]. Many people who are living with a PFO are asymptomatic, until they experience the symptoms of a cryptogenic stroke. While the current guidelines recommend medical therapy to prevent future strokes in such patients, percutaneous closure of PFO is an alternative that has been shown to reduce future strokes in several randomized trials and their meta-analyses[4-8].
While previously published randomized clinical trials (RCTs) were inconclusive[9-11] to show a benefit, their meta-analyses did show a statistically clear benefit of PFO closure over medical therapy[12,13]. Recently three additional trials, and a prolonged follow-up results of a previously published RCT have been published and reported more evidence of a reduction in recurrent stroke after PFO closure[6-8,14]. Nevertheless, it is unclear which group of patients benefit from PFO closure compared to medical therapy and how PFO closure compares with anticoagulation therapy. In that regard, to perform an updated meta-analysis on this evolving topic of interest and to evaluate the different patient groups who will benefit from PFO closure compared to medical therapy, we performed a meta-analysis.
We performed and reported this meta-analysis according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines[15]. We searched electronic databases of MEDLINE, EMBASE, Cochrane Central Register of Clinical Trials and CINAHL with no language restriction from inception through September 2018 using the search terms: “patent foramen ovale” OR “PFO” AND “closure” AND “stroke” OR “transient ischemic attack” OR “TIA” with restriction to randomized design. Two investigators (KD and AY) independently performed the database search and agreed on final study selection. In addition, a manual search was performed by reviewing the references of randomized trials and meta-analyses.
Randomized trials comparing patent foramen ovale closure and medical therapy in adult patients (≥ 18 years) with stroke were selected for meta-analysis. Studies were excluded if they were meeting abstracts, single arm or non-randomized studies and were performed for different disease states.
Two investigators (BL and AY) extracted data from the selected studies in duplicate using standardized data-extraction form and obtained data on study characteristics (study design, patient selection, inclusion and exclusion criteria, follow-up duration, number of patients, type of PFO device and medical therapy and outcomes), patient characteristics (age, sex, race, co-morbidities including diabetes mellitus, hypertension, hyperlipidemia and body mass index, and medication use), and crude events on mortality, recurrent stroke, transient ischemic attack (TIA), systemic embolism (SE), major bleeding and procedural complications at follow-up.
Recurrent IS, TIA, SE, a composite of IS, TIA and SE, major bleeding, mortality and procedural complications including atrial fibrillation (AF) risk were the major outcomes.
We calculated odds ratio (OR) with 95% confidence interval (CI) using random-effects model from the individual studies using the total number of events and patients. The quality of studies was assessed with Cochrane Collaboration’s Bias Assessment Tools[16]. Study heterogeneity was evaluated with Cochran’s Q and I2 index and significant heterogeneity (I2 > 50%) was further explored with sensitivity analyses. We planned pre-specified subgroup analyses based on age, gender, presence of atrial septal aneurysm (ASA) and PFO size (as defined the individual papers). We performed statistical analyses with Review Manager (RevMan 5.3, Cochrane Collaboration, Nordic Cochrane Center, Copenhagen, Denmark).
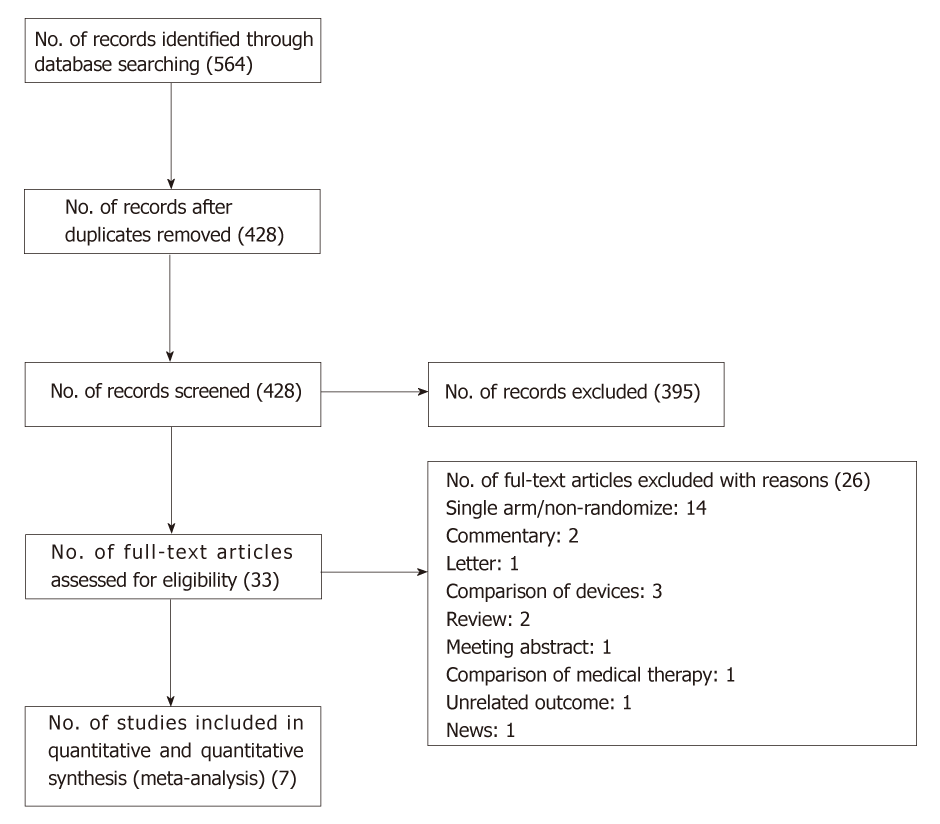
The flow diagram of study selection is shown in Figure 1. Electronic search of five databases (PubMed, EMBASE, CENTRAL, CINAHL and ProQuest Central) retrieved a total of 564 publications. After removal of 136 duplicates, we screened 428 citations for eligibility and extracted 33 publications for full text review. Finally, we had a total of seven publications from six randomized trials for qualitative and quantitative analysis. One study resulted in two publications reporting findings at different duration of follow-up.
The individual study characteristics, patient characteristics, and procedural outcomes and complications of the included studies are shown respectively in Tables 1, 2 and 3. There were 3560 total patients (1889 in patent foramen ovale closure (PFOC) arm and 1671 in medical therapy arm). Studies included only patients under 60 years of age. Follow-up duration was between 2 and 5.9 years. The medical therapy arm in two of these trials (REDUCE and CLOSE) consisted of anti-platelet therapy only[6,8], whereas the other four trials (RESPECT, CLOSURE I, PC and DEFENSE PFO) permitted the use of anti-platelet therapy, anticoagulation or both in the medical-therapy group at the discretion of the investigating physicians[7,9,10,14].
| Study name,year | Country of origin | Study design | Indication of PFOC | Total Patients (PFOC + medical therapy), n | Medical therapy | Type of device | Follow-up, in years (mean) |
| CLOSE[6], 2017 | France and Germany | Multicenter, randomized, open-label, superiority trial | Recent stroke due to PFO with atrial septal aneurysm or substantial right-to-left intra-atrial shunt | 6631 (238 + 235) | Antiplatelet therapy (aspirin + clopidogrel)1 | 11 different devices | 5.4 PFOC, 5.2 AC-AP |
| CLOSURE I[11], 2012 | United States and Canada | Multicenter, randomized, open-label trial | Stroke or TIA within 6 mo | 909 (447 + 462) | Warfarin, aspirin or both | STARFlex device | 2 |
| PC Trial[9], 2013 | Europe, Canada, Brazil, and Australia | Multicenter, randomized, superiority trial | Stroke, TIA or systemic thromboembolism | 414 (204 + 210) | Aspirin+ ticlopidine/clopidogrel | Amplatzer PFO occluder | 4.1 PFOC, 4.0 AC/AP |
| REDUCE[8], 2017 | Europe and United States | Multinational, prospective, randomized, controlled, open-label trial | Stroke within 180 d | 664 (441 + 223) | Aspirin, aspirin + dipyridamole, or clopidogrel | Helex or Cardioform Septal Occluder | 3.2 |
| RESPECT[7], 2017 | United States and Canada | Multicenter, randomized, open-label, controlled clinical trial | Stroke within 270 d | 980 (499 + 481) | Aspirin + clopidogrel | Amplatzer PFO occluder | 5.9 |
| DEFENCE PFO[14], 2018 | South Korea | Multicenter, randomized, open-label, superiority | Ischemic stroke in past 6 mos | 120 (60 + 60) | Aspirin, aspirin + clopidogrel, aspirin + cilostazol, or warfarin | Amplatzer PFO occluder | 2.8 |
PFO closure, compared to medical therapy reduced the risk of IS (OR: 0.34; 95%CI: 0.15-0.78, P = 0.01; I2 = 54%) and the composite outcome of IS, TIA and systemic thromboembolism [0.44 (0.22-0.90); P = 0.02; I2 = 76%] (Figure 2). With PFO closure, no difference was observed in the risk of TIA [0.55 (0.32-0.93); P = 0.02], mortality [0.74 (0.28-1.93); P = 0.53; I2 = 0%] and major bleeding [0.81 (0.42-1.56); P = 0.53; I2 = 19%] (Figure 3). PFO closure increased the risk of AF [4.79 (2.35-9.77); P < 0.0001; I2 = 12%] compared to medical therapy (Figure 4). The risk of procedural complications was [18.08 (5.58-58.55); P <0.00001; I2 = 0%].
Procedural success ranged from 88.3%-99.6%, and PFO closure was successful in 88.6%-100% patients (Table 3). The risk of AF ranged from 1.4%-6.6% across different devices. The risk of AF seemed to numerically lower in patients who received Amplatzer PFO Occluder (1.4%-3.3%) compared to other devices (4.6%-6.6%).
| Study name, year | Total patients PFOC | Type of Device | Success of device implantation | Success of PFO closure | Procedural complications | Atrial fibrillation/ flutter in PFOC, n (%) | Timing of Afib/flutter | Recurrence of Afib/flutter at f/u |
| CLOSE[6], 2017 | 238 | 11 different devices | 234/235 (99.6) | 202/228 (88.6) | 14/238 (5.9) | 11 (4.6) | 10/11 within a month | None |
| CLOSURE I[11], 2012 | 447 | STARFlex device | 362/405 (89.4) | 315/366 (86.1) | 13/402 (3.2) | 23 (5.7) | 14/23 within a month | 6 persistent |
| PC Trial[9], 2013 | 204 | Amplatzer PFO occluder | 188/196 (95.9) | 142/148 (95.9) | 3/204 (1.5) | 6 (2.9) | Timing not defined | 1 persistent |
| REDUCE[8], 2017 | 441 | Helex or Cardioform Septal Occluder | 408/413 (98.8) | 408/413 (98.8) | 11/441 (2.5) | 29 (6.6) | 24 within 45 d | Not defined |
| RESPECT[7], 2017 | 499 | Amplatzer PFO occluder | 462/464 (99.1) | NR | 25/499 (5.0) | 7 (1.4) | Periprocedural period | NR |
| Defense Trial PFO[14], 2018 | 60 | Amplatzer PFO occluder | 53/60 (88.3) | 53/53 (100) | 2/60 (3.3) | 2 (3.3) | 1 periprocedural | NR |
Several sensitivity analyses were planned a-priori. Since CLOSURE I Trial used Starflex closure device, which the manufacturer has stopped producing, we performed analysis after excluding that study. The overall results did not change. An analysis restricted to the studies with at least 3 years or more follow-up (after exclusion of CLOSURE I and DEFENCE PFO Trials) did not change the overall results.
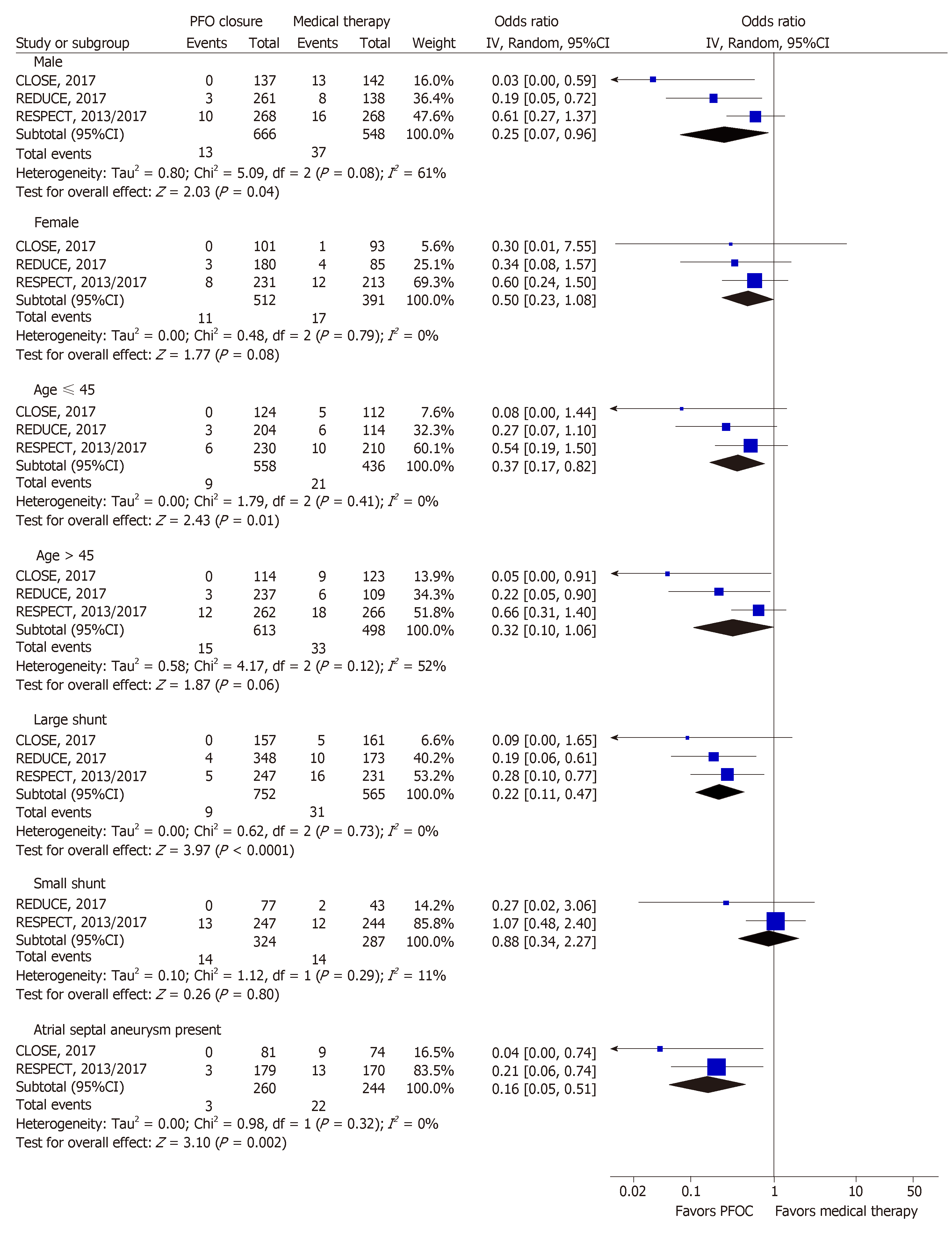
Several subgroup analyses were planned a-priori. PFO closure, compared to medical therapy resulted in a reduction in the risk of stroke (Figure 5) in patients who were male [0.25; 0.07-0.96; P = 0.04; I2 = 61%], ≤ 45 years of age [0.37; 0.17-0.82; P = 0.01; I2 = 0%] and had large shunt [0.22; 0.11-0.47; P < 0.0001; I2 = 0%] or ASA [0.16; 0.05-0.51; P = 0.002; I2 = 0%]. Compared to medical therapy, PFO closure showed a reduction in stroke risk in females [0.50; 0.23-1.08; P = 0.08; I2 = 0%] and patients > 45 years of age [0.32; 0.10-1.06; P = 0.06; I2 = 52%]; however, it did not reach statistical significance. In patients with small shunt, there was no statistical difference in the stroke outcomes [0.88; 0.34-2.27; P = 0.8; I2 = 11%].
All the randomized studies showed bias for non-blinding of the participants and the outcomes per Cochrane collaboration’s bias tools. Publication bias was not tested due to small number of studies for meaningful assessment of publication bias.
The major findings of our meta-analysis were the reduced risk of recurrent IS and the composite outcome of stroke, TIA, and systemic thromboembolism with PFO closure compared with the medical therapy in patients who presented with stroke. Interestingly, subgroup analyses showed such benefits in persons who were males, ≤ 45 years of age and had large shunt or ASA. In females and persons > 45 years of age, there was a strong trend towards reduction in stroke risk, but it did not reach statistical significance.
Despite all three former PFO trials showing a lack of benefit from PFO closure in reducing the risk of recurrent stroke, meta-analyses and pooled analysis of individual participant data from these three trials showed a significant risk reduction of recurrent IS with PFO closure compared with medical therapy[12,17]. The reported risk of recurrent stroke in these studies was small and less than anticipated, which indicated the need for a larger sample and longer follow-ups to increase the possibility of detecting a significant difference in reducing the risk of recurrent stroke. Three recently published trials and a long-term follow-up of a previously published trial demonstrated that among patients with PFO and cryptogenic IS, PFO closure combined with medical therapy was associated with significantly lower risk of recurrent stroke compared with medical therapy alone. Subsequently, a few meta-analyses have been published comparing PFOC with medical therapy, that have consistently shown a reduction in stroke with PFOC[4,5]. Our meta-analysis adds substantially by performing subgroup analyses in an attempt to define which groups of patients clearly benefit from PFO closure. In addition, we performed an in-depth analysis on the increased risk of AF and the role of anticoagulation as medical therapy. It is interesting to note that our meta-analysis clearly showed benefit in males who are 45 years of age or younger with large shunt or ASA. These are the patient groups who are at increased risk of stroke. In females and patients > 45 years of age, it did not show statistical significance, which could largely be an issue of sample size.
Medical therapy arm across the studies were not the same, which made it hard to make a definite statement regarding a medical therapy regimen when compared to PFOC. In the RESPECT trial, anti-platelet agents constituted 74.8% of the medical therapy arm but in the CLOSURE and PC trials, the percentage of patients prescribed antiplatelet vs oral anticoagulation was not reported. The subgroup analyses comparing PFO closure vs oral anticoagulation in the RESPECT trial did not show advantage in reduction of stroke, whereas similar analysis in CLOSURE Trial did not show advantage of PFOC vs medical therapy in reduction of primary end-point, which was a composite of death, stroke, TIA or SE. No such outcomes were reported in the PC trial. In the CLOSE trial, the only trial to compare the anti-platelet agents to oral anticoagulants, the patients in the anti-platelet arm could receive aspirin or aspirin with clopidogrel or with extended release dipyridamole and patients in the oral anticoagulants arm could take either vitamin K antagonists (93%) or direct oral anticoagulants (7%). There were numerically fewer recurrent strokes in the anticoagulation group compared to the antiplatelet group in the intention-to-treat cohort; however, the trial was not powered to detect a difference in such a comparison. Therefore, there is a need for randomized trials with large study population powered to compare the PFO closure to anticoagulation therapy, and anticoagulation to anti-platelet therapy to address the efficacy and safety of anticoagulation therapy compared to antiplatelet therapy and PFO closure.
There was a significant increase in the risk of AF in the PFO closure group compared to the medical therapy group in our meta-analysis, a finding that was reported in several individual trials (CLOSE, REDUCE, CLOSURE-I) and observational studies that used different devices[18,19]. Most of the cases occurred within 30-45 d of the procedure and the majority were transient without recurrence at long-term follow-up (Table 1). This finding suggests that the PFO closure itself could increase the risk for developing AF. However, the significance and clinical relevance of AF associated with PFO closure and the subsequent risk of stroke remains unclear and warrants additional investigations.
The studies were not blinded, which is always a problem in the procedural trials. The medical therapy arms were heterogenous, which limits our ability to make a definite statement regarding specific medical treatment. The subgroup analyses should be interpreted with caution as not all studies reported those outcomes, which reduced the number of individual patients for analysis. However, this meta-analysis is strengthened by in-depth analysis on the type of patients who may benefit from PFO closure compared to medical therapy.
In conclusion, patients with PFO and cryptogenic stroke benefit from percutaneous closure more so in certain population. Further research is needed to assess how the increased periprocedural AF from PFO closure impacts these patients and how does PFO closure compare with anticoagulation in head-to-head trials.
A few randomized clinical trials (RCT) and their meta-analyses have found patent foramen ovale closure (PFOC) to be beneficial in prevention of stroke compared to medical therapy.
Whether the benefit is extended across all groups of patients remains unclear.
To evaluate the efficacy and safety of PFOC vs medical therapy in different groups of patients presenting with stroke, we performed this meta-analysis of RCTs.
Following standard technique, a meta-analysis of randomized clinical trials was performed. Random-effects model was used to analyze summary results.
PFO closure is beneficial in preventing stroke in patients with stroke and a PFO. In certain population, the benefits are clear.
This study showed that PFO closure is beneficial in patients with PFO and stroke. It was beneficial in patients who were male, younger than 45, had atrial septal aneurysm and had a large shunt.
Future research should compare anticoagulation vs PFO closure and establish whether PFO closure can be useful in all group of patients.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Greenway SC, Vermeersch P S-Editor: Ji FF L-Editor: A E-Editor:Wu YXJ
| 1. | Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, Fang MC, Fisher M, Furie KL, Heck DV, Johnston SC, Kasner SE, Kittner SJ, Mitchell PH, Rich MW, Richardson D, Schwamm LH, Wilson JA; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160-2236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2779] [Cited by in RCA: 2966] [Article Influence: 269.6] [Reference Citation Analysis (0)] |
| 2. | Yaghi S, Bernstein RA, Passman R, Okin PM, Furie KL. Cryptogenic Stroke: Research and Practice. Circ Res. 2017;120:527-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 141] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 3. | Handke M, Harloff A, Olschewski M, Hetzel A, Geibel A. Patent foramen ovale and cryptogenic stroke in older patients. N Engl J Med. 2007;357:2262-2268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 410] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 4. | Smer A, Salih M, Mahfood Haddad T, Guddeti R, Saadi A, Saurav A, Belbase R, Ayan M, Traina M, Alla V, Del Core M. Meta-analysis of Randomized Controlled Trials on Patent Foramen Ovale Closure Versus Medical Therapy for Secondary Prevention of Cryptogenic Stroke. Am J Cardiol. 2018;121:1393-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | De Rosa S, Sievert H, Sabatino J, Polimeni A, Sorrentino S, Indolfi C. Percutaneous Closure Versus Medical Treatment in Stroke Patients With Patent Foramen Ovale: A Systematic Review and Meta-analysis. Ann Intern Med. 2018;168:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Mas JL, Derumeaux G, Guillon B, Massardier E, Hosseini H, Mechtouff L, Arquizan C, Béjot Y, Vuillier F, Detante O, Guidoux C, Canaple S, Vaduva C, Dequatre-Ponchelle N, Sibon I, Garnier P, Ferrier A, Timsit S, Robinet-Borgomano E, Sablot D, Lacour JC, Zuber M, Favrole P, Pinel JF, Apoil M, Reiner P, Lefebvre C, Guérin P, Piot C, Rossi R, Dubois-Randé JL, Eicher JC, Meneveau N, Lusson JR, Bertrand B, Schleich JM, Godart F, Thambo JB, Leborgne L, Michel P, Pierard L, Turc G, Barthelet M, Charles-Nelson A, Weimar C, Moulin T, Juliard JM, Chatellier G; CLOSE Investigators. Patent Foramen Ovale Closure or Anticoagulation vs. Antiplatelets after Stroke. N Engl J Med. 2017;377:1011-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 755] [Article Influence: 94.4] [Reference Citation Analysis (0)] |
| 7. | Saver JL, Carroll JD, Thaler DE, Smalling RW, MacDonald LA, Marks DS, Tirschwell DL; RESPECT Investigators. Long-Term Outcomes of Patent Foramen Ovale Closure or Medical Therapy after Stroke. N Engl J Med. 2017;377:1022-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 707] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 8. | Søndergaard L, Kasner SE, Rhodes JF, Andersen G, Iversen HK, Nielsen-Kudsk JE, Settergren M, Sjöstrand C, Roine RO, Hildick-Smith D, Spence JD, Thomassen L; Gore REDUCE Clinical Study Investigators. Patent Foramen Ovale Closure or Antiplatelet Therapy for Cryptogenic Stroke. N Engl J Med. 2017;377:1033-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 757] [Article Influence: 94.6] [Reference Citation Analysis (0)] |
| 9. | Meier B, Kalesan B, Mattle HP, Khattab AA, Hildick-Smith D, Dudek D, Andersen G, Ibrahim R, Schuler G, Walton AS, Wahl A, Windecker S, Jüni P; PC Trial Investigators. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368:1083-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 630] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 10. | Carroll JD, Saver JL, Thaler DE, Smalling RW, Berry S, MacDonald LA, Marks DS, Tirschwell DL; RESPECT Investigators. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 682] [Cited by in RCA: 667] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 11. | Furlan AJ, Reisman M, Massaro J, Mauri L, Adams H, Albers GW, Felberg R, Herrmann H, Kar S, Landzberg M, Raizner A, Wechsler L; CLOSURE I Investigators. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 730] [Cited by in RCA: 708] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 12. | Rengifo-Moreno P, Palacios IF, Junpaparp P, Witzke CF, Morris DL, Romero-Corral A. Patent foramen ovale transcatheter closure vs. medical therapy on recurrent vascular events: a systematic review and meta-analysis of randomized controlled trials. Eur Heart J. 2013;34:3342-3352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 13. | Pandit A, Aryal MR, Pandit AA, Jalota L, Kantharajpur S, Hakim FA, Lee HR. Amplatzer PFO occluder device may prevent recurrent stroke in patients with patent foramen ovale and cryptogenic stroke: a meta-analysis of randomised trials. Heart Lung Circ. 2014;23:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Lee PH, Song JK, Kim JS, Heo R, Lee S, Kim DH, Song JM, Kang DH, Kwon SU, Kang DW, Lee D, Kwon HS, Yun SC, Sun BJ, Park JH, Lee JH, Jeong HS, Song HJ, Kim J, Park SJ. Cryptogenic Stroke and High-Risk Patent Foramen Ovale: The DEFENSE-PFO Trial. J Am Coll Cardiol. 2018;71:2335-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 375] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 15. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 46954] [Article Influence: 2934.6] [Reference Citation Analysis (0)] |
| 16. | Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. 2011; Available from: http://www.njrcentre.org.uk/. |
| 17. | Kent DM, Dahabreh IJ, Ruthazer R, Furlan AJ, Reisman M, Carroll JD, Saver JL, Smalling RW, Jüni P, Mattle HP, Meier B, Thaler DE. Device Closure of Patent Foramen Ovale After Stroke: Pooled Analysis of Completed Randomized Trials. J Am Coll Cardiol. 2016;67:907-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 157] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 18. | Sievert H, Wunderlich N, Reiffenstein I, Ruygrok P, Grube E, Buellesfeld L, Meier B, Schofer J, Muller D, Jones RK, Gillam L. Initial clinical experience with the Coherex FlatStent™ and FlatStent™ EF PFO closure system for in-tunnel PFO closure: results of the Coherex-EU study. Catheter Cardiovasc Interv. 2014;83:1135-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Burow A, Schwerzmann M, Wallmann D, Tanner H, Sakata T, Windecker S, Meier B, Delacrétaz E. Atrial fibrillation following device closure of patent foramen ovale. Cardiology. 2008;111:47-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |