Published online Dec 26, 2019. doi: 10.4330/wjc.v11.i12.305
Peer-review started: March 15, 2019
First decision: June 6, 2019
Revised: September 17, 2019
Accepted: October 27, 2019
Article in press: October 27, 2019
Published online: December 26, 2019
Processing time: 282 Days and 10 Hours
Acute coronary syndromes constitute a variety of myocardial injury presentations that include a subset of patients presenting with myocardial infarction with non-obstructive coronary arteries (MINOCA). This acute coronary syndrome differs from type 1 myocardial infarction (MI) regarding patient characteristics, presentation, physiopathology, management, treatment, and prognosis. Two-thirds of MINOCA subjects present ST-segment elevation; MINOCA patients are younger, are more often female and tend to have fewer cardiovascular risk factors. Moreover, MINOCA is a working diagnosis, and defining the aetiologic mechanism is relevant because it affects patient care and prognosis. In the absence of relevant coronary artery disease, myocardial ischaemia might be triggered by an acute event in epicardial coronary arteries, coronary microcirculation, or both. Epicardial causes of MINOCA include coronary plaque disruption, coronary dissection, and coronary spasm. Microvascular MINOCA mechanisms involve microvascular coronary spasm, takotsubo syndrome (TTS), myocarditis, and coronary thromboembolism. Coronary angiography with non-significant coronary stenosis and left ventriculography are first-line tests in the differential study of MINOCA patients. The diagnostic arsenal includes invasive and non-invasive techniques. Medical history and echocardiography can help indicate vasospasm or thrombosis, if one finite coronary territory is affected, or specify TTS if apical ballooning is present. Intravascular ultrasound, optical coherence tomography, and provocative testing are encouraged. Cardiac magnetic resonance is a cornerstone in myocarditis diagnosis. MINOCA is not a benign diagnosis, and its polymorphic forms differ in prognosis. MINOCA care varies across centres, and future multi-centre clinical trials with standardized criteria may have a positive impact on defining optimal cardiovascular care for MINOCA patients.
Core tip: Myocardial infarction with non-obstructive coronary arteries (MINOCA) differs from type 1 myocardial infarction regarding patient characteristics, presentation, physiopathology, management, treatment, and prognosis. In the absence of relevant coronary artery disease, myocardial ischaemia might be triggered by an acute event in epicardial coronary arteries, coronary microcirculation, or both. Epicardial causes of MINOCA include coronary plaque disruption, coronary dissection, and coronary spasm. Diagnostic strategies include invasive and non-invasive techniques recently embracing intravascular ultrasound and cardiac magnetic resonance. MINOCA is not a benign diagnosis, and its polymorphic forms differ in prognosis.
- Citation: Vidal-Perez R, Abou Jokh Casas C, Agra-Bermejo RM, Alvarez-Alvarez B, Grapsa J, Fontes-Carvalho R, Rigueiro Veloso P, Garcia Acuña JM, Gonzalez-Juanatey JR. Myocardial infarction with non-obstructive coronary arteries: A comprehensive review and future research directions. World J Cardiol 2019; 11(12): 305-315
- URL: https://www.wjgnet.com/1949-8462/full/v11/i12/305.htm
- DOI: https://dx.doi.org/10.4330/wjc.v11.i12.305
Remarkable progress in medicine regarding the pathogenesis of heart disease has produced lifesaving and life-extending therapies impacting ischaemic patients worldwide. The definition of angina pectoris is over two hundred years old, but the controversy about the aetiologic role of coronary arteries has never ceased to hold interest. Acute myocardial infarction (MI) without significant coronary artery disease (CAD) was initially described almost 80 years ago by Gross and Sternberg, whereas the term myocardial infarction with non-obstructive coronary arteries (MINOCA) is recent[1,2].
The diagnosis of an acute coronary syndrome should be established according to the fourth universal definition of MI, which is when there is evidence of acute myocardial injury accompanied by clinical data suggesting acute myocardial ischaemia such as relevant symptoms, new ischaemic electrocardiogram (ECG) changes, loss of viable myocardium present in imaging, or identification of coronary thrombus. Several diverse definitions of MI have been used, leading to unbalanced criteria and confusion. Thus, a general universal definition of MI was agreed upon for the first time over 50 years ago with the collaboration of multiple groups that were initially created for epidemiological reasons. With the discovery of cardiac biomarkers, the diagnosis of MI has been simplified, but because an increase in cardiac biomarkers is an entity by itself, it is not pathognomonic of an acute coronary syndrome in isolation. Elevation of cardiac biomarkers, such as cardiac troponin I and T, represents injury to myocardial cells, but such increases do not reflect the underlying pathophysiology because they can arise in a variety of situations, including normal hearts. This variability highlights the need for a global uniform definition of MI and myocardial injury[3-5].
There are multiple classifications of MI. Classically, for discrimination of immediate or delayed treatment strategies, patients who develop new ST-segment elevation in two contiguous leads or new bundle branch block with ischaemic alterations are designated ST-elevation myocardial infarction (STEMI) patients, whereas the subgroup without ST-segment elevation is diagnosed as non-ST elevation MI (NSTEMI). In addition to those two categories, MI can be classified patho-physiologically[4,5]. Evidence of an imbalance between myocardial oxygen supply and demand unrelated to acute atherothrombosis corresponds to type 2 MI; by definition, acute atherothrombotic plaque rupture excludes type 2 MI. Type 1 MI and MINOCA are two separate entities with different underlying mechanisms, management, and prognosis[5,6].
MINOCA is diagnosed in a patient with features of MI with non-obstructive coronary arteries on angiography, is defined as no coronary artery stenosis ≥ 50% in any potential infarct-related artery and is characterized by the absence of a clinically specific cause of the acute presentation. Clinical criteria and biomarker behaviour of MINOCA remain similar to other acute coronary events[3,6].
MINOCA is not an uncommon presentation of acute coronary syndromes. Large MI registries reflect the universal nature of MINOCA with the prevalence ranging between 5% and 25% in different series, with 11% in a recent prospective observational study[7-9]. Throughout the years, MINOCA has remained prevalent with an increasing incidence, as was observed in a Spanish registry[10]. MINOCA patient characteristics differ from those of other Myocardial Infarction and Coronary Artery Disease (MI-CAD) patients because MINOCA subjects are younger, are more often female, and tend to have fewer traditional cardiovascular risk factors. In the VIRGO study, women had 5-fold higher odds of presenting with MINOCA than men; non-white patients also had increased rates of MINOCA than white patients. Pasupathy et al[11] reported that MINOCA patients were less likely to have hyperlipidaemia, whereas a similar distribution was observed regarding hypertension, diabetes mellitus, smoking, and family history of premature coronary disease. Ele-ctrocardiographic patterns also differ, generally presenting as STEMI or NSTEMI, with two-thirds of patients having the latter. It has also been suggested that hormonal changes, such as the time of menarche and menopause, may also play a role in MINOCA.
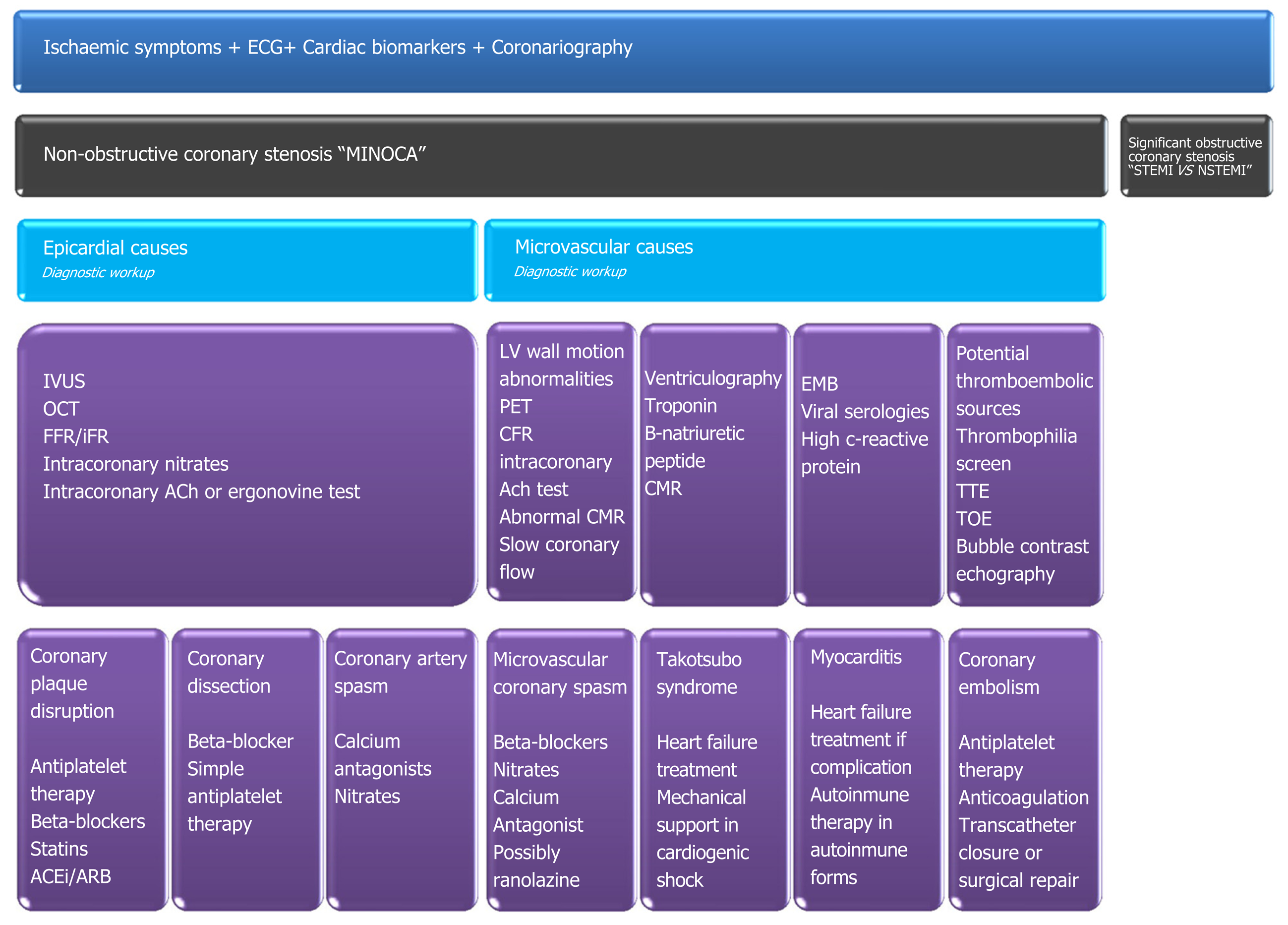
In the absence of relevant CAD, myocardial ischaemia may be triggered by a disorder of epicardial coronary arteries and/or malfunction in the coronary microcirculation (Table 1). Both have multiple presentations[7,8].
| Mechanism | Diagnosis | Prevalence in coronary syndromes | Therapy |
| Epicardial causes | |||
| Coronary artery disease | IVUS/OCT, FFR/iFR | 5%-20% of MI | Antiplatelet therapy, statins, ACEi/ARB, beta-blockers |
| Coronary dissection | IVUS/OCT | 25% of MI in women under 50 yr of age | Beta-blocker and simple antiplatelet therapy |
| Coronary artery spasm | Intracoronary nitrates, intracoronary Ach or ergonovine test by experienced teams | 3%–95% of MI depending on the registry | Calcium antagonists, nitrates |
| Microvascular causes | |||
| Microvascular coronary spasm | Objective evidence of ischaemia (ECG, LV wall motion abnormalities, PET). Impaired microvascular function (CFR, intracoronary Ach test, abnormal CMR, slow coronary flow) | As high as 25% depending on the registry | Beta-blockers and nitrates, calcium antagonist, possibly ranolazine |
| Takotsubo syndrome | Ventriculography, echocardiography, troponin, B-natriuretic peptide, CMR | 1%-3% of general STEMI, 5%-6% women with STEMI, concomitant CAD 10%-29% | Heart failure treatment, mechanical support in cardiogenic shock |
| Myocarditis | CMR, EMB, viral serologies, high c-reactive protein | 33% of MINOCA when determined by CMR | Heart failure treatment if complication, autoimmune therapy in autoimmune forms |
| Coronary embolism | History of potential thromboembolic sources, thrombophilia screen, TTE, TOE, bubble contrast echography | 2.9% MI | Antiplatelet therapy, anticoagulation, transcatheter closure or surgical repair |
Coronary plaque disruption: Many atherosclerotic plaques are positively remodelled, expand outward, and have a lipid-rich body and thin fibrous cap, making them vulnerable to rupture. The transient and partial thrombosis in this type of plaque causes distal thrombus embolization, with possible superimposed vasospasm, and might be responsible for MINOCA; this mechanism resembles type 1 MI. MINOCA represents 5%-20% of all type 1 MI. Since coronary angiography cannot evaluate the vascular lumen, intracoronary imaging modalities such as intravascular ultrasound (IVUS) might play a determinant role in evaluating the lesion. Ouldzein et al[12] performed IVUS in MINOCA patients to evaluate the morphological and quantitative characteristics of the culprit lesion and subsequently classified subjects according to the presence or absence of plaque rupture; the frequency of ruptured plaques in MI patients was estimated to be between 20% and 40%, and patients with plaque rupture had increased plaque burden, plaque volume and positive arterial remodelling.
Coronary dissection: Coronary dissection without visible luminal obstruction and coronary artery intramural haematomas constitute 25% of MI in women younger than 50 years of age. IVUS is a cornerstone in the assessment of coronary dissection. The physiology of this entity is unclear; however, fibromuscular dysplasia is thought to be related. This presentation has a high rate of recurrence[8,13].
Coronary artery spasm: Coronary artery spasm (CAS) represents between 3% and 95% of MINOCA cases depending on the registry. Positive provocative tests with intracoronary, adenosine or ergonovine portend a worse prognosis. The diagnosis does not require confirmation of epicardial coronary spasm, and these tests should only be performed by experienced teams because they have a potential risk of arrhythmic complications. Positive testing has been associated with a higher occurrence of death from any cause and cardiac death during follow up, a higher rate of MI readmission and inferior control of angina symptoms; epicardial spasm also showed worse clinical outcomes than microvascular spasm[14].
Microvascular coronary spasm: Microcoronary microvascular spasm, also referred to as Syndrome X, can occur in up to 25% of MINOCA patients in some registries and is the cause of persistent angina in up to 36% MINOCA subjects. Catecholamines and endothelin exert transient vasoconstrictive effects primarily in the coronary microvasculature, reducing microvascular blood flow in a transient manner. Objective documentation of myocardial ischaemia should be sought. The presence of four clinical criteria for microvascular angina accomplished a definitive diagnosis: Symptoms of myocardial ischaemia, the absence of obstructive CAD [< 50% diameter reduction in fractional flow reserve (FFR) > 0.80], objective evidence of ischaemia (ECG ischaemic changes, wall motion or perfusion abnormalities), and evidence of impaired coronary microvascular function. This last parameter includes having a coronary flow reserve ≤ 2-2.5, coronary microvascular spasm (reproduction of symptoms, ischaemic ECG shifts) without epicardial spasm in acetylcholine testing, abnormal coronary microvascular resistance indices, or coronary slow flow phenomenon. Diagnostic techniques for the evaluation of microvascular disease include invasive and non-invasive measures. Positron emission tomography (PET) is the most accurate non-invasive outlook of coronary vasomotor function; cardiac magnetic resonance (CMR) can also be applied, although post-processing is technically challenging. Invasive techniques include invasive coronary flow reserve, more recent FFR, and instantaneous wave-free ratio with certain limitations[15]. Plaque burden can be present or absent in MINOCA patients, and a broad spectrum of subtypes have been described, but these usually overlap. The guarded prognosis of these patients justifies an invasive approach[16].
Takotsubo syndrome: This stress cardiomyopathy represents 1%-3% of all STEMI, with 5%-6% prevalence in female subgroups, and is characterized by apical ballooning of the left ventricle in the absence of occlusive CAD; although concomitant CAD is described in 10%-29% of Takotsubo syndrome (TTS) cases. The proposed Mayo clinical criteria include transient left ventricle mid-segment wall hypokinesis, akinesis or dyskinesis that extends beyond one vascular territory, absence of significant CAD, new electrocardiographic changes or modest elevation in cardiac biomarkers, and exclusion of pheochromocytoma or myocarditis[17,18]. The more recent international TTS diagnostic criteria (interTAK Diagnostic Criteria) vary from the Mayo criteria by recognizing pheochromocytoma as a secondary cause of TTS by stating that the presence of CAD should not be an exclusion and that cases with wall motion abnormalities restricted to one vascular territory should not be excluded (Table 2 and 3)[19]. The causes and aetiologic mechanisms of TTS are complex and still in debate, but reversible coronary microvascular vasoconstriction is a common mechanism in apical ballooning[20]. Diagnostic tools in TTS diagnosis include ventriculography, transthoracic echocardiogram with adenosine and CMR. In the absence of significant CAD, ballooning ventriculography allows a diagnosis. Contrast echocardiography with adenosine may prove microvascular constriction. CMR provides additional findings suggesting takotsubo; the hyperintense signal on T2 sequences, diffuse or transmural oedema, dysfunctional ventricular contraction matching the TTS typical ballooning, and alterations not restricted to a particular vascular territory in the absence of myocardial necrosis[8,21]. Strain echocardiography and F-18 fluorodeoxyglucose positron emission have shown promising results in the diagnosis of TTS and may play a role in the future[22].
| Diagnostic criteria |
| Left ventricular dysfunction usually extending beyond a single coronary territory. |
| Sometimes triggered by emotional, physical or combined stress. |
| Acute neurologic disorders, including pheochromocytoma, may become triggers. |
| New ECG abnormalities. Rare cases can present with without ECG shifts. |
| Moderate troponin elevation. Usually, significantly high brain natriuretic peptide. |
| Can have concomitant CAD. |
| No evidence of infectious myocarditis usually excluded by CMR. |
| Mostly present in postmenopausal women. |
| Criteria | Points | Diagnosis probability |
| Female sex | 25 points | ≤ 70 points |
| Emotional stress | 24 points | |
| Low/intermediate | ||
| Physical stress | 13 points | |
| TTS probability | ||
| No ST-segment depression | 12 points | |
| Psychiatric disorders | 11 points | > 70 points |
| Neurologic disorders | 9 points | |
| High TTS probability | ||
| QTc prolongation | 6 points |
Myocarditis: This polymorphic inflammatory disease can mimic many conditions and can have a prevalence of approximately 33% among MINOCA patients when determined by CMR imaging[24]. Young patients and high C-reactive protein findings were associated with myocarditis, while male sex, previously treated hyperlipidaemia and high troponin ratio were correlated with type 1 MI. Myocarditis also accounts for 5%-12% of young athlete sudden cardiovascular death[25]. The most common pathogens identified in patients are human herpesvirus 6 and parvovirus B19. Diagnosis of myocarditis is challenging; thus, given the poor yield of endomyocardial biopsies (EMB) and viral serologies, standard criteria such as the European Society of Cardiology 2013 Myocarditis Task Force criteria were established (Table 4)[7]. Certain diagnoses and aetiologies of myocarditis require EMB (histology, immunohistology, infectious agents by polymerase chain reaction). CMR imaging should be included in the workup of myocarditis; it provides tissue characterization but does not identify the underlying cause. Late gadolinium enhancement is observed in the majority of patients and can have several phenotypes with different prognostic implications[26].
| Presence of ≥ 1 clinical presentation and ≥ 1 diagnostic criteria: |
| Clinical presentation: |
| Acute coronary-like syndrome |
| New onset or worsening unexplained heart failure |
| Chronic unexpected heart failure over 3 mo duration |
| Life-threatening unexplained conditions (including arrhythmias, aborted sudden death, cardiogenic shock) |
| Diagnostic criteria: |
| ECG/Holter/stress test shifts: Any degree atrioventricular block or bundle branch block, ST/T or Q wave changes, sinus arrest, cardiac arrest rhythms, low voltage, frequent premature beat or supraventricular tachycardia |
| Elevated cardiac troponins |
| Functional and structural abnormalities on cardiac imaging |
| Oedema and/or late gadolinium enhancement of myocarditis pattern in CMR |
Coronary embolism: Coronary embolism (CE) can affect coronary microcirculation and/or angiographically visible epicardial vessels. Coronary emboli can arise from coronary or systemic arterial thrombi, and coronary thrombosis may be related to thrombotic disorders. The prevalence of de novo CE MINOCA can be 2.9%. Atrial fibrillation is the most common cause of CE. Case definition can be held according to the National Cerebral and Cardiovascular Center criteria for the diagnosis of CE; the 3 major criteria include angiographic evidence of coronary artery embolism and thrombosis without atherosclerotic components, concomitant multivessel CE and concomitant systemic embolization. Minor criteria include CAD with stenosis < 25%, evidence of embolic source detected by imaging, and coexistence of potential thromboembolic disease. Paradoxical embolism due to right-left shunts is a rare cause of MINOCA, and treatment includes trans-catheter device closure or surgical repair. Transthoracic, transoesophageal, and contrast-enhanced echocardiography are the cornerstone methods for uncovering cardiac sources of embolism[8,27].
CMR imaging is a cornerstone in determining underlying myocardial tissue pathology. However, 8%-67% of MINOCA patients have no late gadolinium enhancement, myocardial oedema, or wall motion alterations. Vasospastic angina, coronary plaque disease or CE can have normal CMR findings; in these cases, intracoronary imaging may help shed light on the underlying ischaemic trigger. IVUS and CMR provide complementary mechanistic insights into MINOCA patients and may be useful in identifying potential causes and therapies[28].
Coronary plaque disruption: Dual antiplatelet treatment for 12 mo is recommended if allowed by haemorrhage risk, followed by chronic single antiplatelet therapy and statins[8]. Angiotensin-converting enzyme inhibitors/angiotensin receptor blockers and beta-blocker treatment have the same indication as STEMI and NSTEMI guidelines[4,5].
Coronary dissection: There is no effective treatment to reduce long-term risk. A medical strategy is recommended for coronary interventions, and stenting may cause propagation of the dissection. A conservative strategy along with beta-blockers and single antiplatelet treatment is recommended[8,13].
CAS: Chronic medical treatment includes calcium channel blockers and nitrates. Calcium antagonist dose reduction or discontinuation was associated with worse prognosis regarding mortality, supporting the role of epicardial spasm in the occurrence of adverse events[8].
Microvascular coronary spasm: There are no specific therapies for microvascular dysfunction, and management of underlying cardiovascular risk factors is recommended. Traditional anti-ischaemic drugs, such as beta-blockers and nitrates, should be first-line therapy; calcium antagonists can be added to treat refractory angina and are recommended when vasomotor tone is increased. The data on ranolazine for angina relief are controversial in this subset[9,15].
TTS: There are no randomized trials to guide evidence-based treatment. Empiric strategies include cardio-selective beta-blockers, avoidance of inotropes, angiotensin-converting enzyme inhibitors for persistent myocardial dysfunction, mechanical support devices in cardiogenic shock, and antiplatelet treatment with statins if associated with CAD[21].
Myocarditis: Myocarditis treatment differs from that of coronary disease because it does not require anti-ischaemic therapies. Myocarditis generally has a favourable prognosis resolving in 2 to 4 wk, while a minor subgroup develops cardiovascular complications such as heart failure and should be treated correspondingly. Autoimmune forms have negative infection findings on biopsy, and specific autoimmune therapy is required in these cases[7].
CE: Standard treatment of thromboembolic events remains individualized. Multiple factors play a role in this entity, such as the time of presentation and the presence or absence of multiple embolic sites. Patients with paroxysmal embolism in the presence of atrial septal defects require percutaneous or surgical closure. These patients have a high rate of recurrence and major adverse cardiovascular events (MACEs) in the follow-up[8,27].
MINOCA patients are a conundrum for clinicians; therefore, a systematic global approach should be pursued, and an attempt must be made to determine the specific aetiologic mechanism as prognosis and management vary. The diagnostic arsenal includes invasive and non-invasive techniques. Medical history can suggest a diagnosis of vasospasm angina if the patient has a chronic pattern of recurrent episodic angina. Regional LV motion alterations corresponding to a finite vascular territory suggest vasospasm or thrombosis. Apical ballooning suggests TTS. A history of atrial fibrillation, dilated cardiomyopathy, prosthetic heart valves, infective endocarditis, atrial myxoma, and patent foramen oval suggest CE. IVUS or optical coherence tomography are encouraged in non-severe coronary angiography findings with less than 50% luminal reduction; if intracoronary imaging reveals normal findings, provocative functional testing is recommended. Transthoracic or transoesophageal echocardiography, LV ventriculography, and CMR are other well-documented techniques. The test flow-chart does not have a specific order and should be performed according to clinical suspicion[4,7,8]. In Figure 1, we summarize our diagnostic and therapeutic workup for MINOCA management.
The prognosis of MINOCA patients is heterogeneous and not benign. Patients should be carefully informed about their condition and must not be inaccurately reassured about a favourable course. Because of the aetiological heterogeneity, the extent of MI damage and different inclusion criteria, registries reporting MINOCA prognosis show diverse data regarding major cardiac adverse events during hospitalization and follow-up[10]. In the VIRGO study, similar proportions of cardiac arrest, reduced ejection fraction, and heart failure were observed in patients with MINOCA and MI-CAD, whereas the mortality rates during follow-up were not significantly different. According to a meta-analysis of eight studies that reported all-cause mortality in patients with MINOCA, both in-hospital and 12-month mortality were comparable to MI-CAD[9].
Moreover, different secondary prevention strategies at discharge have been published with discrepancies regarding medical treatment with proven prognostic value, thus possibly interfering with prognosis. In addition, it may be speculated that within the vast spectrum of MINOCA patients, the multiple categories can have dissimilar prognoses and may be under- or overestimated by grouping them together.
Nordenskjöld et al[29] conducted an observational study with 9092 MINOCA subjects and found that 24% of the patients presented a new MACE and 14% died during follow-up. Multiple predictors for MACEs and death among MINOCA patients are similar to those previously shown to predict new events in MI-CAD patients, some of which are older age, diabetes, hypertension, current smoking, previous MI, previous stroke, and reduced LVEF. In this study, a cholesterol paradox was observed, where low levels of total cholesterol were significantly associated with the composite endpoint of MACEs and long-term mortality; this phenomenon was primarily observed in the statin-naive group who received statin treatment after MINOCA.
Nordenskjöld et al[30] also studied the possible mechanisms and prognosis for reinfarction in MINOCA patients, describing an average time to readmission of 17 mo. With a median follow-up of 38 mo, mortality was similar whether the reinfarction event was MINOCA or MI-CAD. A progression of coronary stenosis is described in approximately half of the patients, and thus, the performance of another angiography in the MI event following MINOCA was relevant; all-cause mortality and cardiovascular mortality were higher among patients who did not undergo a new coronary angiography than among those who did. Repeated episodes of MINOCA are not harmless.
In a recent study of the Chinese population, MACEs in MINOCA patients at the 1-year follow-up were lower than those in MI-CAD patients. Multi-factorial survival analysis showed that older age (≥ 60 years old), female sex, atrial fibrillation, and reduced LVEF are independent risk factors for MACEs in MINOCA patients within one year[31]. The atherosclerotic burden in MINOCA patients may also have an additional role in their prognosis and represents a promising research target in the following years[23].
TTS is a special subset of MINOCA patients with regard to triggers that can be identified in two-thirds of cases and should be exposed because they can influence prognosis. Generally, long-term outcomes of TTS are comparable to those of age- and sex-matched MI patients. TTS related to emotional stress have a favourable short- and long-term prognosis, whereas those secondary to physical stress or medical conditions such as neurological events are associated with higher mortality in follow up; patients with neurological triggers tend to have higher mortality[23].
The present study shows a knowledge gap and heterogeneous management of MINOCA patients that need attention. MINOCA is a polymorphic aggregate with much more to be uncovered, with special emphasis on the pathophysiology. Standard MI protocols do not apply systematically to all MINOCA patients. Variations in revascularization strategies and the use of proven medical therapies exist[9]. The era of eyeball angiographic quantification is evolving, and measuring only the degree of stenosis is insufficient. The plaque burden is multi-faceted, and different plaque content, volume, and distribution along with luminal stenosis can have a divergent clinical impact and prognosis[29]. There is a demand for the use of standard criteria in MINOCA research for effective worldwide communication, as such criteria may help understand and compare international registries. Standardized criteria may provide an investigative structure for mechanistic, diagnostic, prognostic and clinical trial studies aimed at developing MINOCA evidence-based guidelines.
MINOCA should be considered a working diagnosis, and challenges must be overcome to identify its underlying cause because its polymorphic nature has various implications. MINOCA is a prevalent, not benign pathology, and misconceptions regarding this condition must be reviewed. Variable practice patterns and disparities in MINOCA care exist. Future multicentric clinical trials will have a strong impact and refine the optimal cardiovascular care of MINOCA patients.
Manuscript source: Invited Manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li JJ, Lin GM, Petix NR S-Editor: Tang JZ L-Editor: A E-Editor: Zhang YL
| 1. | Gross H, Sternberg WH. Myocardial infarction without significant lesions of coronary arteries. Arch Intern Med (Chic). 1939;64:249–267. [RCA] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined--a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36:959-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3050] [Cited by in RCA: 2994] [Article Influence: 119.8] [Reference Citation Analysis (0)] |
| 3. | Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol. 2018;72:2231-2264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1366] [Cited by in RCA: 2496] [Article Influence: 356.6] [Reference Citation Analysis (1)] |
| 4. | Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7073] [Cited by in RCA: 6654] [Article Influence: 950.6] [Reference Citation Analysis (0)] |
| 5. | Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S; ESC Scientific Document Group. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4045] [Cited by in RCA: 4400] [Article Influence: 440.0] [Reference Citation Analysis (0)] |
| 6. | Thygesen K, Mair J, Katus H, Plebani M, Venge P, Collinson P, Lindahl B, Giannitsis E, Hasin Y, Galvani M, Tubaro M, Alpert JS, Biasucci LM, Koenig W, Mueller C, Huber K, Hamm C, Jaffe AS; Study Group on Biomarkers in Cardiology of the ESC Working Group on Acute Cardiac Care. Recommendations for the use of cardiac troponin measurement in acute cardiac care. Eur Heart J. 2010;31:2197-2204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 447] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 7. | Agewall S, Beltrame JF, Reynolds HR, Niessner A, Rosano G, Caforio AL, De Caterina R, Zimarino M, Roffi M, Kjeldsen K, Atar D, Kaski JC, Sechtem U, Tornvall P; WG on Cardiovascular Pharmacotherapy. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J. 2017;38:143-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 265] [Article Influence: 44.2] [Reference Citation Analysis (1)] |
| 8. | Scalone G, Niccoli G, Crea F. Editor's Choice- Pathophysiology, diagnosis and management of MINOCA: an update. Eur Heart J Acute Cardiovasc Care. 2019;8:54-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 122] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 9. | Safdar B, Spatz ES, Dreyer RP, Beltrame JF, Lichtman JH, Spertus JA, Reynolds HR, Geda M, Bueno H, Dziura JD, Krumholz HM, D'Onofrio G. Presentation, Clinical Profile, and Prognosis of Young Patients With Myocardial Infarction With Nonobstructive Coronary Arteries (MINOCA): Results From the VIRGO Study. J Am Heart Assoc. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 295] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 10. | Redondo-Diéguez A, Gonzalez-Ferreiro R, Abu-Assi E, Raposeiras-Roubin S, Aidhodjayeva O, López-López A, Castiñeira-Busto M, Peña-Gil C, García-Acuña JM, González-Juanatey JR. Long-term Prognosis of Patients With Non-ST-segment Elevation Acute Myocardial Infarction and Coronary Arteries Without Significant Stenosis. Rev Esp Cardiol (Engl Ed). 2015;68:777-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. 2015;131:861-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 654] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 12. | Ouldzein H, Elbaz M, Roncalli J, Cagnac R, Carrié D, Puel J, Alibelli-Chemarin MJ. Plaque rupture and morphological characteristics of the culprit lesion in acute coronary syndromes without significant angiographic lesion: analysis by intravascular ultrasound. Ann Cardiol Angeiol (Paris). 2012;61:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Hayes SN, Kim ESH, Saw J, Adlam D, Arslanian-Engoren C, Economy KE, Ganesh SK, Gulati R, Lindsay ME, Mieres JH, Naderi S, Shah S, Thaler DE, Tweet MS, Wood MJ; American Heart Association Council on Peripheral Vascular Disease; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Genomic and Precision Medicine; and Stroke Council. Spontaneous Coronary Artery Dissection: Current State of the Science: A Scientific Statement From the American Heart Association. Circulation. 2018;137:e523-e557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 821] [Article Influence: 117.3] [Reference Citation Analysis (0)] |
| 14. | Montone RA, Niccoli G, Fracassi F, Russo M, Gurgoglione F, Cammà G, Lanza GA, Crea F. Patients with acute myocardial infarction and non-obstructive coronary arteries: safety and prognostic relevance of invasive coronary provocative tests. Eur Heart J. 2018;39:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 15. | Taqueti VR, Di Carli MF. Coronary Microvascular Disease Pathogenic Mechanisms and Therapeutic Options: JACC State-of-the-Art Review. J Am Coll Cardiol. 2018;72:2625-2641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 453] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 16. | Ong P, Camici PG, Beltrame JF, Crea F, Shimokawa H, Sechtem U, Kaski JC, Bairey Merz CN; Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. 2018;250:16-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 542] [Article Influence: 77.4] [Reference Citation Analysis (0)] |
| 17. | Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155:408-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1193] [Cited by in RCA: 1297] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 18. | Sachdev E, Bairey Merz CN, Mehta PK. Takotsubo Cardiomyopathy. Eur Cardiol. 2015;10:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, Cammann VL, Crea F, Galiuto L, Desmet W, Yoshida T, Manfredini R, Eitel I, Kosuge M, Nef HM, Deshmukh A, Lerman A, Bossone E, Citro R, Ueyama T, Corrado D, Kurisu S, Ruschitzka F, Winchester D, Lyon AR, Omerovic E, Bax JJ, Meimoun P, Tarantini G, Rihal C, Y-Hassan S, Migliore F, Horowitz JD, Shimokawa H, Lüscher TF, Templin C. International Expert Consensus Document on Takotsubo Syndrome (Part I): Clinical Characteristics, Diagnostic Criteria, and Pathophysiology. Eur Heart J. 2018;39:2032-2046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 641] [Cited by in RCA: 1020] [Article Influence: 170.0] [Reference Citation Analysis (0)] |
| 20. | Galiuto L, De Caterina AR, Porfidia A, Paraggio L, Barchetta S, Locorotondo G, Rebuzzi AG, Crea F. Reversible coronary microvascular dysfunction: a common pathogenetic mechanism in Apical Ballooning or Tako-Tsubo Syndrome. Eur Heart J. 2010;31:1319-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 215] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 21. | Plácido R, Cunha Lopes B, Almeida AG, Rochitte CE. The role of cardiovascular magnetic resonance in takotsubo syndrome. J Cardiovasc Magn Reson. 2016;18:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, Cammann VL, Crea F, Galiuto L, Desmet W, Yoshida T, Manfredini R, Eitel I, Kosuge M, Nef HM, Deshmukh A, Lerman A, Bossone E, Citro R, Ueyama T, Corrado D, Kurisu S, Ruschitzka F, Winchester D, Lyon AR, Omerovic E, Bax JJ, Meimoun P, Tarantini G, Rihal C, Y-Hassan S, Migliore F, Horowitz JD, Shimokawa H, Lüscher TF, Templin C. International Expert Consensus Document on Takotsubo Syndrome (Part II): Diagnostic Workup, Outcome, and Management. Eur Heart J. 2018;39:2047-2062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 506] [Cited by in RCA: 553] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 23. | Ghadri JR, Kato K, Cammann VL, Gili S, Jurisic S, Di Vece D, Candreva A, Ding KJ, Micek J, Szawan KA, Bacchi B, Bianchi R, Levinson RA, Wischnewsky M, Seifert B, Schlossbauer SA, Citro R, Bossone E, Münzel T, Knorr M, Heiner S, D'Ascenzo F, Franke J, Sarcon A, Napp LC, Jaguszewski M, Noutsias M, Katus HA, Burgdorf C, Schunkert H, Thiele H, Bauersachs J, Tschöpe C, Pieske BM, Rajan L, Michels G, Pfister R, Cuneo A, Jacobshagen C, Hasenfuß G, Karakas M, Koenig W, Rottbauer W, Said SM, Braun-Dullaeus RC, Banning A, Cuculi F, Kobza R, Fischer TA, Vasankari T, Airaksinen KEJ, Opolski G, Dworakowski R, MacCarthy P, Kaiser C, Osswald S, Galiuto L, Crea F, Dichtl W, Empen K, Felix SB, Delmas C, Lairez O, El-Battrawy I, Akin I, Borggrefe M, Horowitz J, Kozel M, Tousek P, Widimský P, Gilyarova E, Shilova A, Gilyarov M, Winchester DE, Ukena C, Bax JJ, Prasad A, Böhm M, Lüscher TF, Ruschitzka F, Templin C. Long-Term Prognosis of Patients With Takotsubo Syndrome. J Am Coll Cardiol. 2018;72:874-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 245] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 24. | Tornvall P, Gerbaud E, Behaghel A, Chopard R, Collste O, Laraudogoitia E, Leurent G, Meneveau N, Montaudon M, Perez-David E, Sörensson P, Agewall S. Myocarditis or "true" infarction by cardiac magnetic resonance in patients with a clinical diagnosis of myocardial infarction without obstructive coronary disease: A meta-analysis of individual patient data. Atherosclerosis. 2015;241:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 25. | Maron BJ, Udelson JE, Bonow RO, Nishimura RA, Ackerman MJ, Estes NA, Cooper LT, Link MS, Maron MS; American Heart Association Electrocardiography and Arrhythmias Committee of Council on Clinical Cardiology, Council on Cardiovascular Disease in Young, Council on Cardiovascular and Stroke Nursing, Council on Functional Genomics and Translational Biology, and American College of Cardiology. Eligibility and Disqualification Recommendations for Competitive Athletes With Cardiovascular Abnormalities: Task Force 3: Hypertrophic Cardiomyopathy, Arrhythmogenic Right Ventricular Cardiomyopathy and Other Cardiomyopathies, and Myocarditis: A Scientific Statement From the American Heart Association and American College of Cardiology. Circulation. 2015;132:e273-e280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 157] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 26. | Aquaro GD, Perfetti M, Camastra G, Monti L, Dellegrottaglie S, Moro C, Pepe A, Todiere G, Lanzillo C, Scatteia A, Di Roma M, Pontone G, Perazzolo Marra M, Barison A, Di Bella G; Cardiac Magnetic Resonance Working Group of the Italian Society of Cardiology. Cardiac MR With Late Gadolinium Enhancement in Acute Myocarditis With Preserved Systolic Function: ITAMY Study. J Am Coll Cardiol. 2017;70:1977-1987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 325] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 27. | Shibata T, Kawakami S, Noguchi T, Tanaka T, Asaumi Y, Kanaya T, Nagai T, Nakao K, Fujino M, Nagatsuka K, Ishibashi-Ueda H, Nishimura K, Miyamoto Y, Kusano K, Anzai T, Goto Y, Ogawa H, Yasuda S. Prevalence, Clinical Features, and Prognosis of Acute Myocardial Infarction Attributable to Coronary Artery Embolism. Circulation. 2015;132:241-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 234] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 28. | Reynolds HR, Srichai MB, Iqbal SN, Slater JN, Mancini GB, Feit F, Pena-Sing I, Axel L, Attubato MJ, Yatskar L, Kalhorn RT, Wood DA, Lobach IV, Hochman JS. Mechanisms of myocardial infarction in women without angiographically obstructive coronary artery disease. Circulation. 2011;124:1414-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 326] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 29. | Nordenskjöld AM, Baron T, Eggers KM, Jernberg T, Lindahl B. Predictors of adverse outcome in patients with myocardial infarction with non-obstructive coronary artery (MINOCA) disease. Int J Cardiol. 2018;261:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 30. | Nordenskjöld AM, Lagerqvist B, Baron T, Jernberg T, Hadziosmanovic N, Reynolds HR, Tornvall P, Lindahl B. Reinfarction in Patients with Myocardial Infarction with Nonobstructive Coronary Arteries (MINOCA): Coronary Findings and Prognosis. Am J Med. 2019;132:335-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 31. | Abdu FA, Liu L, Mohammed AQ, Luo Y, Xu S, Auckle R, Xu Y, Che W. Myocardial infarction with non-obstructive coronary arteries (MINOCA) in Chinese patients: Clinical features, treatment and 1 year follow-up. Int J Cardiol. 2019;287:27-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 32. | Ghanem AM, Hamimi AH, Matta JR, Carass A, Elgarf RM, Gharib AM, Abd-Elmoniem KZ. Automatic Coronary Wall and Atherosclerotic Plaque Segmentation from 3D Coronary CT Angiography. Sci Rep. 2019;9:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |