Published online Dec 26, 2019. doi: 10.4330/wjc.v11.i12.292
Peer-review started: March 15, 2019
First decision: August 2, 2019
Revised: August 21, 2019
Accepted: October 18, 2019
Article in press: October 18, 2019
Published online: December 26, 2019
Processing time: 280 Days and 21 Hours
The incorporation of biomarkers in the actually used risk scores seem to be helpful for early identifying atrial fibrillation (AF) patients at higher risk. The aim of this critical review of the scientific literature is to investigate the potential clinical significance of red blood cell distribution width (RDW) in AF. A systematic electronic search was carried out to identify all articles describing an epidemiological association between RDW and AF in adult human populations. Data abstraction was conducted on a final number of 35 articles (13 cross-sectional, 12 prospective and 10 retrospective studies). The results of these epidemiological investigations were all virtually concordant to emphasize that an enhanced RDW value is not only a predictive factor and a marker of AF but its measurement may also be helpful for predicting the risk of developing many adverse complications in patients with AF, such as recurrence and duration of AF, hospitalization for heart failure, bleeding, left atrial thrombosis and stasis, thromboembolic events and mortality. AF patients with RDW values exceeding the local reference range may be more aggressively investigated and managed, in order to identify and attenuate the impact of possible underlying disorders causing both anisocytosis and AF.
Core tip: This critical review of the scientific literature aims to investigate the potential clinical significance of red blood cell distribution width (RDW) in atrial fibrillation (AF). We concluded that an enhanced RDW value is not only a predictive factor and a marker of AF but its measurement may also be helpful for predicting the risk of developing many adverse complications in patients with AF, such as recurrence and duration of AF, hospitalization for heart failure, bleeding, left atrial thrombosis and stasis, thromboembolic events and mortality.
- Citation: Lippi G, Cervellin G, Sanchis-Gomar F. Red blood cell distribution width: A marker of anisocytosis potentially associated with atrial fibrillation. World J Cardiol 2019; 11(12): 292-304
- URL: https://www.wjgnet.com/1949-8462/full/v11/i12/292.htm
- DOI: https://dx.doi.org/10.4330/wjc.v11.i12.292
Atrial fibrillation (AF) is the most common heart arrhythmia worldwide[1]. Worryingly, AF is related to higher rates of stroke and mortality[2]. Many risk scores and biological markers have been identified and developed to predict future AF events. Among the most frequently used and validated risk scores based on clinical parameters are CHADS2 [congestive heart failure, hypertension, age ≥ 75 years, diabetes, and stroke or transient ischemic attack (2 points)] and CHA2DS2-VASc [cardiac failure or dysfunction, hypertension, age 65-74 (1 point) or ≥ 75 years (2 points), diabetes mellitus, and stroke, TIA or thromboembolism (2 points) –vascular disease, and sex category (female)][3,4]. In addition, biomarkers may significantly contribute to obtain additional information regarding the risk that could influence the management of AF. Therefore, there is also an increasing interest in determining whether biomarkers themselves or in combination with clinical risk scores enhances prognostic accuracy for thromboembolism and mortality in AF patients[5,6]. A wide range of biomarkers have been evaluated as predictors and/or prognostics, such as cardiac troponin I and T, natriuretic peptides, D-dimer, CRP, galectin-3, growth differentiation factor-15, among others[1,5,7,8].
The incorporation of biomarkers in the actually used risk scores seem to be helpful for early identifying AF patients at higher risk (i.e., enhanced risk for stroke, systemic embolic event or death), determining also their eligibility for anticoagulation and/or individualizing the most appropriate treatment strategy. Biomarkers are dynamic, and for that reason, they are also highly recommended to be included into management of patients with AF. Therefore, knowledge of new biomarkers related to AF may provide clinicians with more potential tools to quickly identify patients at higher risk of AF, attenuate its occurrence, improve its management, and decrease the risk of adverse events in patients with AF.
The search for hematological predictors of AF commenced in 1987 with the publication of a seminal study by Imataka et al[9], who demonstrated that plasma volume and erythrocyte biology may be significantly perturbed in patients with AF. Ten years later, Takahashi et al[10] first showed that erythrocyte size was altered both before and after the onset of chronic AF, thus leading to way to subsequent research aimed to define whether high heterogeneity of erythrocytes volumes, conventionally known as anisocytosis, may have clinical significance in AF.
Anisocytosis, defined as the presence of red blood cells (RBCs) with a broad heterogeneity of size and volume in peripheral blood, can be reliably estimated by the vast majority of modern hematological analyzers using different techniques, which provide a similar final index called RBC distribution width (RDW)[11]. The RDW, which is not directly measured by the analyzers, but can be calculated as standard deviation (SD) of the mean corpuscular volume (MCV), and is usually expressed in absolute value (i.e., RDW-SD) or as the coefficient of variation [i.e., RDW-CV: (RDW-SD)/(MCV) × 100]. Albeit largely instrument-dependent, the reference range of RDW-CV is usually comprised between 11.5%-14.5%[12]. Increased RDW values, thus reflecting anisocytosis, may be due to many pathological conditions including congenital erythrocyte disorders (i.e., β-thalassemia, sickle cell disease, hereditary spherocytosis), anemia (e.g., due to iron, folate or vitamin B deficiencies), blood transfusions, some forms of hemolytic anemias, oxidative stress, inflammation and impaired renal function[13-15]. Since the measurement of RDW has now become a useful part in diagnostic and prognostic assessment of many cardiovascular disorders such as acute coronary syndrome (ACS), heart failure and venous thromboembolism[16,17], the aim of this critical review of the scientific literature is to investigate the potential clinical significance of measuring RDW in patients with, or at risk of, AF.
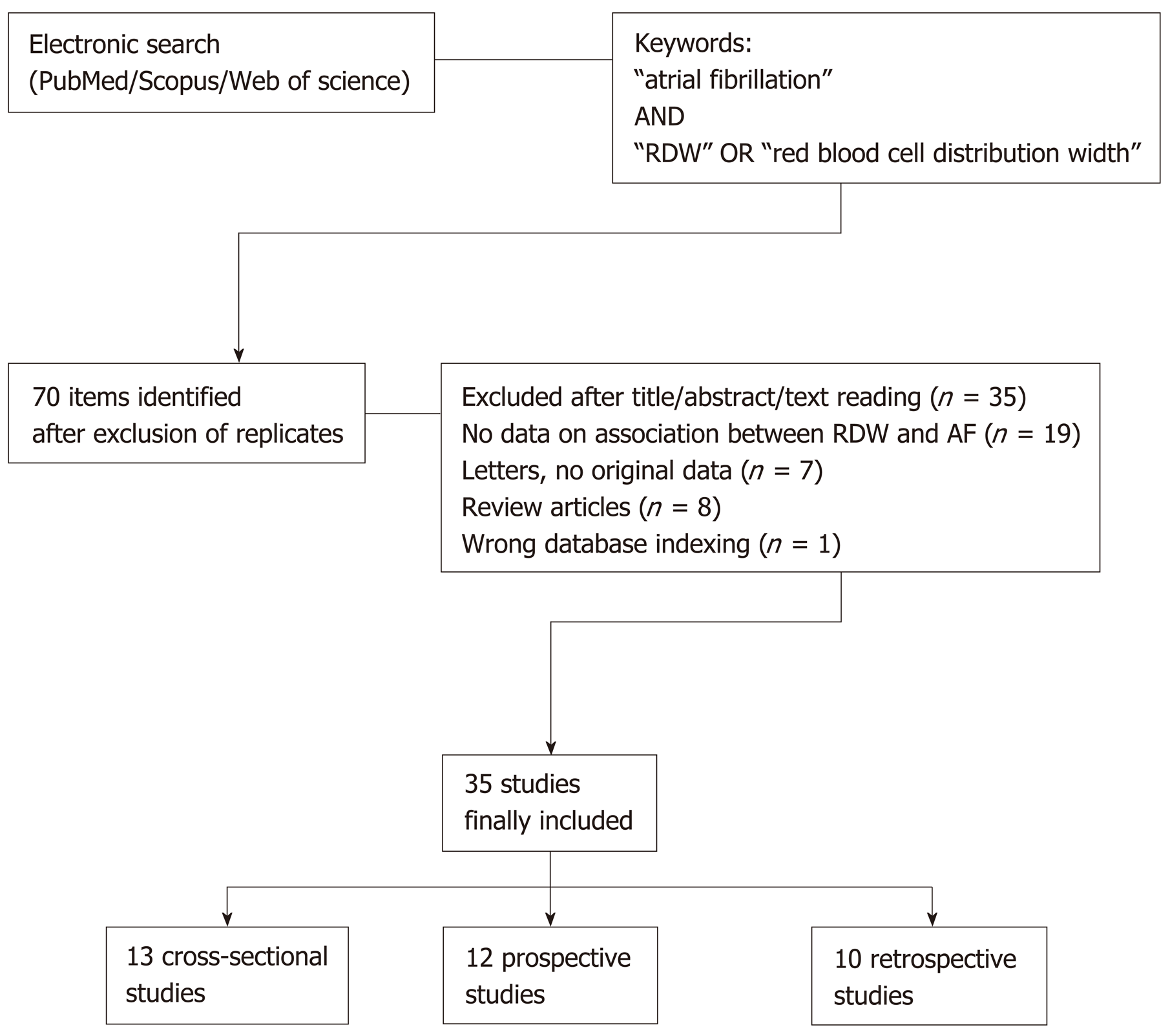
A systematic electronic search was carried out using the three well-recognized and widely accessed scientific databases (i.e., Medline interface PubMed, Web of Science and Scopus/EMBASE)[18], with no date or language limits, to identify all articles which described the association between RDW and AF in epidemiological investigations involving human adult populations (cross-sectional, retrospective and prospective studies). The following keywords were used: “atrial fibrillation” AND “red blood cell distribution width” OR “RDW”. The bibliographic references of selected items were also carefully checked for identifying additionally relevant documents. The title, abstract and full text of the articles were accurately reviewed by two authors (Lippi G and Cervellin G), and potential disagreement for inclusion was eventually resolved by the opinion of the third author. Although no meta-analysis was specified before the electronic search, since it was already clear that the studies could not be combined due to large heterogeneity in sample size, setting, and endpoints, it was our aim to explore whether this approach would still be possible after analyzing the data of the included studies.
The search strategy retrieved a total number of 70 documents after elimination of replicates among the three scientific search platforms. Thirty five studies ought to be excluded since they did not match our search criteria (Figure 1). Data abstraction was hence conducted on a final number of 35 articles describing an epidemiological association between RDW and atrial fibrillation in adult populations, published between the years 2010 and 2019 (13 cross-sectional, 12 prospective and 10 retrospective studies) (Figure 1). It was finally decided that, as predictable, a meta-analysis was unfeasible due to large heterogeneity of the different studies (difference in nature, clinical settings, and endpoints, sample size from 49 to over 69000, no clear description of comorbidities in all studies, use of rather different RDW thresholds) (Table 1).
| Authors | Study design | Study population | Endpoints | Outcome |
| Horne et al[19], 2010 | Prospective | 3927 patients undergoing coronary angiography, endpoints collected at 30-d and 1-yr | Risk of developing cardiovascular diseases and complications | RDW positively correlated with the frequency of incident AF |
| Providência et al[20], 2013 | Cross-sectional | 247 patients presenting with symptomatic AF | Association with outcomes of transesophageal echocardiography | High RDW associated with left atrial appendage thrombosis |
| Liu et al[21], 2014 | Cross-sectional | 133 patients with paroxysmal AF and 101 healthy controls | Difference between groups | High RDW independently associated with AF |
| Ertaş et al[22], 2013 | Retrospective | 132 patients undergoing nonemergency CABG | Risk of new-onset AF until hospital discharge | RDW independently predicted the risk of developing AF |
| Ertaş et al[23], 2013 | Cross-sectional | 126 patients with AF (39 with stroke and 87 without) and 126 healthy controls | Difference among groups | RDW significantly higher in patients with AF than in controls, but non different between AF patients with or without stroke |
| Kurt et al[24], 2014 | Cross-sectional | 320 patients with AF | Relationship with CHA2DS2-VASc score | High RDW independently associated with higher CHA2DS2-VASc score |
| Güngör et al[25], 2014 | Cross-sectional | 117 patients with AF and 60 health control subjects | Difference among groups | RDW significantly higher in AF patients than in controls |
| Adamsson Eryd et al[26], 2014 | Prospective | 27124 subjects free from AF at enrollment, followed-up for 13.6 yr | Risk of developing AF | RDW independently predicted the risk of developing AF |
| Sarikaya et al[27], 2014 | Cross-sectional | 126 hypertensive patients (63 with AF and 63 without) | Difference among groups | High RDW significantly associated with AF |
| Gurses et al[28], 2015 | Prospective | 299 AF patients undergoing cryoballoon-based ablation, followed-up for 24 mo | Outcome of cryoballoon-based ablation | RDW independently predicted the risk of recurrence and duration of AF |
| Korantzopoulos et al[29], 2015 | Prospective | 109 patients undergoing elective cardiac surgery, followed-up throughout hospitalization | Risk of AF lasting > 5 min during hospitalization | RDW independently predicted the risk of postoperative AF |
| Wan et al[30], 2015 | Prospective | 300 patients with AF followed-up for a median up period of 3.2 yr | Risk of adverse clinical outcomes | RDW independently predicted the risk of major adverse events and death |
| Lee et al[31], 2015 | Prospective | 567 patients with newly diagnosed paroxysmal AF | Risk of adverse clinical outcomes | RDW independently predicted the risk of new-onset stroke, composite outcome and bleeding |
| Zhao et al[32], 2015 | Cross-sectional | 90 AF patients, 24 with evidence of left atrial thrombus (n = 11) or left atrial spontaneous echo contrast (n = 13) | Evidence of left atrial thrombus or left atrial spontaneous echo contrast | RDW associated with presence of left atrial thrombus or left atrial spontaneous echo contrast |
| Aksu et al[33], 2015 | Prospective | 49 patients with AF followed-up for 10 mo | Risk of AF recurrence | RDW predicted the risk of AF recurrence |
| Korantzopoulos et al[34], 2016 | Cross-sectional | 101 patients with sick sinus syndrome (32 with AF) | Difference between groups | High RDW independently associated with AF |
| Karataş et al[35], 2016 | Retrospective | 621 patients with myocardial infarction undergoing primary percutaneous coronary intervention | Risk of new-onset AF throughout hospitalization | RDW independently predicted the risk of new-onset AF |
| Yanagisawa et al[36], 2016 | Prospective | 757 AF patients undergoing radiofrequency catheter ablation followed-up for 22 mo | Risk of adverse clinical outcomes | RDW independently predicted the risk of recurrent AF and major adverse events |
| Vizzardi et al[37], 2016 | Retrospective | 232 patients with stable heart failure 1 yr after enrolment | Risk of adverse events 1 yr after enrolment | RDW independently predicted the risk of cardiovascular death and/or hospitalization for heart failure |
| Geçmen et al[38], 2016 | Prospective | 94 patients undergoing isolated on-pump CABG surgery followed-up until discharge from cardiovascular intensive care unit | Risk of postoperative AF | RDW independently predicted the risk postoperative AF |
| Zhang et al[39], 2017 | Prospective | 172 patients with nonvalvular AF undergoing catheter ablation, followed-up for 3 mo | Risk of bleeding | RDW predicted the risk of bleeding events |
| Al-Kindi et al[40], 2017 | Retrospective | 46720 patients with a diagnosis of HIV infection followed-up for development of cardiovascular complications | Risk of cardiovascular complications | RDW independently predicted the risk of AF |
| Liu et al[41], 2017 | Cross-sectional | 99 patients with AF, categorized according to their CHADS2 and CHA2DS2-VASc scores | Association with risk of stroke | High RDW independently associated with higher CHADS2 and CHA2DS2-VASc scores |
| Saliba et al[42], 2017 | Retrospective | 69412 patients with AF | Risk of death 2 yr after study entry | RDW independently predicted the risk of death; persistently increased RDW values at two time points stronger predictors of death than a single increased RDW value |
| Kaya et al[43], 2017 | Cross-sectional | 619 patients with AF (325 with left atrial stasis and 294 without) | Association with left atrial stasis | High RDW independently associated with left atrial stasis |
| Cha et al[44], 2017 | Retrospective | 5082 patients with AF | Risk of thromboembolic events during 5.2 yr | High peak RDW value during follow-up independently associated with the risk of thromboembolic events |
| Nam et al[45], 2017 | Cross-sectional | 103 healthy control subjects and 117 patients with AF patients, 65 of whom with paroxysmal and 52 with persistent AF | Difference among groups | RDW values non significantly different between controls and all AF cases; RDW values significantly higher in patients with persistent than in those with paroxysmal AF |
| Wasilewski et al[46], 2017 | Retrospective | 1734 patients with LVEF ≤ 35% and without ACS | Risk of AF after 660 d | High RDW independently predicted the risk of AF |
| Kilicgedik et al[48], 2018 | Retrospective | 358 patients after who underwent CABG surgery (57 with PSAF and 301 patients with non-PSAF) | Risk of AF after CABG surgery | High RDW was predictive of PSAF |
| Cerşit et al[47], 2018 | Retrospective | 50 patients with AF and 62 age- and sex- matched controls, who had presented with ACS | Association and predictive value of RDW with AF in patients with ACS. | High RDW was associated with AF and had long-term predictive value |
| Ozsin et al[49], 2018 | Retrospective | 93 patients who underwent off-pump CABG (24 patients with PSAF and 69 without PSAF) | Association and predictive value of RDW for development PSAF | Elevated RDW levels may be predictive of PSAF |
| Pilling et al[50], 2018 | Prospective | 240477 healthy UK Biobank study volunteers aged 40 ± 70 yr at baseline (follow-up ≤ 9 yr) | Association of RDW with AF in healthy subjects. | High RDW was associated with AF and had long-term predictive value |
| Han et al[51], 2019 | Cross-sectional | 303 patients with nonvalvular AF living at low altitude (3.5 m above the sea level) and high altitude (2260 m above the sea level). | Association of RDW with AF in subjects living at low and high altitude. | Elevated RDW levels were an independent risk marker for AF and is affected by type of AF and altitude |
| Jurin et al[52], 2019 | Prospective | 579 patients with AF (non-permanent and permanent AF ), with a median follow-up time of 21 mo | Association of RDW values with progression to permanent AF | RDW was independently associated with AF progression |
| Li et al[53], 2019 | Cross-sectional | 106998 Chinese individuals | Relationship between RDW and AF | Elevated RDW is significantly related to higher prevalence of AF in a general Chinese population |
The first epidemiological investigation which could be identified in this critical literature review was published in 2010 by Horne and collaborators[19]. In this prospective investigation, based on the Intermountain Heart Collaborative Study, a total number of 3927 patients undergoing coronary angiography were evaluated after 1 year and 30 d, with the aim of defining the frequency of incident cardiovascular disorders and complications (including AF). When patients were classified according to quintiles of RDW, the frequency of incident AF steadily increased from the lowest up to the highest (i.e., from 2% to 14%) RDW quintiles. A highly significant trend towards increasing frequency of AF was consistently observed across RDW quintiles (P < 0.001).
Providência et al[20] carried out a cross-sectional study including 247 patients presenting with symptomatic AF to the emergency department, who were then subjected to transesophageal echocardiography for ruling out left atrial appendage thrombus. Overall, eft atrial appendage thrombus was evidenced in 21/247 (8.5%) of all AF patients, and its presence was found to be significantly more frequent in patients with RDW ≥ 15.0% than in those with lower RDW values (14.8% vs 5.4%; P = 0.013).
Liu et al[21] carried out another cross-sectional study including 133 patients with paroxysmal AF and 101 healthy controls. In multivariate logistic regression analysis, a RDW value > 12.55% was associated with a 63% enhanced risk of AF (odds ratio, 1.63; 95%CI: 1.01-2.61).
Ertaş et al[22] retrospectively studied 132 patients undergoing nonemergency coronary artery bypass graft (CABG) surgery. A RDW > 13.45% was associated with a nearly 1.5-fold increased risk of new-onset AF (hazard ratio 1.48; 95%CI: 1.07-2.06). The same team of authors published another cross-control study, in which RDW was measured in 126 patients with non-valvular AF (39 with stroke and 87 without) and in 126 healthy controls with no AF[23]. The value of RDW was found to be significantly higher in AF patients with (14.1% ± 1.7%) or without stroke (14.3% ± 1.8%) compared to the control population (13.2% ± 0.9%), but its value did not differ among AF patients with or without stroke (P > 0.05).
Kurt et al[24] measured RDW in 320 patients with AF and found that those with a higher CHA2DS2-VASc score had also significantly higher RDW values than those with a lower CHA2DS2-VASc score (14.9% ± 2.7% vs 13.6% ± 1.7%; P < 0.001). A highly significant correlation could be observed between RDW and CHA2DS2-VASc score (r = 0.383; P < 0.001). In multivariate analysis, a RDW value > 14.05% was associated with a 25% higher risk (odds ratio, 1.25; 95%CI: 1.11-1.42) of having high CHA2DS2-VASc score (i.e., ≥ 2).
In an ensuing investigation, Güngör et al[25] studied 117 patients with AF and 60 healthy control subjects, concluding that RDW values were significantly higher in AF cases than in controls (13.4% vs 12.6%; P = 0.01). In multivariate regression analysis, a RDW > 12.9% was associated with a nearly 4-fold higher risk (odds ratio, 4.18; 95%CI: 2.15-8.15) of AF.
Adamsson Eryd et al[26] carried out a large prospective study including 27124 subjects free from AF at enrollment, who were followed-up for a mean period of 13.6 years. Subjects in the highest quartile of RDW had a 33% enhanced risk (hazard ratio, 1.33; 95%CI: 1.16-1.53) of developing AF on follow-up compared to those in the lowest quartile. Moreover, each 1 SD increase of RDW value was associated with a 8% higher risk (hazard ratio, 1.08; 95%CI: 1.04-1.12) of incident AF.
Sarikaya et al[27] studied 126 patients with hypertension (63 with AF and 63 without) and reported that RDW values were significantly higher in patients with AF than in those without (15.1% ± 1.6% vs 14.0% ± 1.1%; P = 0.001). In multivariate logistic regression analysis, a RDW value > 14.2% was found to be independently associated with 1.8-fold higher risk (odds ratio, 1.85; 95%CI: 1.22-2.79) of AF.
Gurses et al[28] measured RDW in 299 patients with paroxysmal or persistent AF undergoing cryoballoon-based ablation, and who were then followed-up for a mean period of 24 mo. A RDW value > 13.75% was independently associated with both early (hazard ratio, 6.39; 95%CI: 3.41-11.97) and late (hazard ratio, 1.88; 95%CI: 1.41-2.50) recurrence of AF, enhanced left atrial diameter (hazard ratio, 3.09; 95%CI: 1.81-5.27), as well as with duration of AF (hazard ratio, 1.04; 95%CI: 1.01-1.07).
Korantzopoulos et al[29] studied 109 patients undergoing elective cardiac surgery, who were then prospectively followed-up throughout hospitalization. In multivariate logistic regression analysis, a RDW > 13.35% was independently associated with a 46% higher risk (odds ratio, 1.46; 95%CI: 1.08-1.99) of developing postoperative AF during hospital stay.
Wan et al[30] carried out a prospective study including 300 patients with AF who were followed-up at a median period of 3.2 years. Patients in the fourth quartile of RDW values had a 2.7-fold higher risk (hazard ratio, 2.70; 95%CI: 1.35-5.83) of major adverse events (all-cause mortality, ACS, stroke and major hemorrhage) and a 3.8-fold higher risk (hazard ratio, 3.83; 95%CI: 1.53-9.58) of death during follow-up.
Lee et al[31] measured RDW values in 567 patients with newly diagnosed paroxysmal AF, who were followed-up for a median period of 4.8 years. In multivariate analysis, an increased RDW value (no indications provided on the cut-off used) was independently associated with 47% higher risk (hazard ratio, 1.47; 95%CI: 1.05-2.05) of new-onset stroke, 26% higher risk (hazard ratio, 1.26; 95%CI: 1.02-1.54) of composite outcome (mortality, new-onset stroke and hospitalization for heart failure), and 74% enhanced risk of bleeding (hazard ratio, 1.74; 95%CI: 1.28-2.36) throughout follow-up.
Zhao et al[32] retrospectively analyzed a local echocardiology database for identifying all AF patients who underwent transesophageal echocardiography before catheter ablation or electrical cardioversion. The final study population consisted of 90 AF patients, 24 of whom had evidence of left atrial thrombus (n = 11) or left atrial spontaneous echo contrast (n = 13). The mean RDW value was found to be significantly higher in patients with these two complications than in those without (13.0% ± 0.9% vs 12.6% ± 0.8%; P = 0.039).
Aksu et al[33] studied 49 patients with symptomatic paroxysmal AF who underwent cryoballoon ablation and were then followed-up for a mean period of 10 mo. Patients with AF recurrence on follow-up had significantly higher RDW values than those without (16.1% ± 1.4% vs 14.9% ± 0.5%; P = 0.033). Interestingly, the post-ablation RDW value remained almost unchanged in patents without recurrence of AF, but in those with AF recurrence the RDW significantly increased from 16.1% ± 1.4% to 16.3% ± 2.4% (P < 0.05).
In another study, Korantzopoulos et al[34] measured RDW in 101 patients with sick sinus syndrome (32 with AF), and found that a RDW value > 14.0% was independently associated with AF (odds ratio, 1.58; 95%CI: 1.06-2.85).
Karataş et al[35] studied 621 patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention, and who were followed-up throughout hospitalization. Patients with RDW > 13.4% had a 55% higher risk (odds ratio, 1.55; 95%CI: 1.20-2.01) of developing new-onset AF until hospital discharge.
Yanagisawa et al[36] measured RDW in 757 patients undergoing radiofrequency catheter ablation for AF, who were then followed-up for a mean period of 22 mo. In multivariate linear regression analysis, a RDW value > 13.9% was associated with 20% higher risk (hazard ratio, 1.20; 95%CI: 1.01-1.40) of recurrent AF in patients with heart failure, whilst no significant association was found in those without heart failure. In patients with heart failure, a RDW value > 14.8% was also associated with 83% higher risk (hazard ratio, 1.83; 95%CI: 1.13-2.72) of developing major adverse events (all-cause mortality, hospitalization for heart failure and cerebral ischemia) during follow-up.
Vizzardi et al[37] carried out a retrospective study including 232 patients with stable heart failure, whose clinical outcome was assessed 1 year after enrolment. In multivariate logistic regression analysis, a RDW value > 14.45% was independently associated with 3.9-fold enhanced risk (odds ratio, 3.89; 95%CI: 1.04-14.55) of cardiovascular death and/or hospitalization for heart failure in the first year after enrolment.
Geçmen et al[38] carried out a prospective study including 94 patients undergoing isolated on-pump CABG surgery, who were followed-up until discharge from the cardiovascular intensive care unit. In univariate analysis, higher RDW values were associated with a 41% higher risk (odds ratio, 1.41; 95%CI: 1.01-1.96) of postoperative AF during cardiovascular intensive care unit stay. The cut-off value of RDW was unavailable in the publication and the association between RDW and postoperative AF was not tested in multivariate analysis.
Zhang et al[39] measured RDW in 172 patients diagnosed with nonvalvular AF, who were followed up for 3 mo after catheter ablation. The overall number of bleeding events was found to be higher in patients with RDW values > 12.8% than in those with lower RDW values (11.8% vs 3.4%). Interestingly, the diagnostic efficiency (i.e., area under the receiver operating characteristics curve; AUC) for predicting bleeding occurrence was higher for RDW than for activated partial thromboplastin time (0.737 vs 0.558; P < 0.01).
Al-Kindi et al[40] used a large commercial database including electronic health records of many participating hospitals, with the aim of identifying patients aged 18 years or older with a diagnosis of HIV and who had at least one available RDW measurement. The search allowed the extraction of a total number of 46720 records (mean or median follow-up period for development of cardiovascular complications is unavailable in the article). In these HIV patients, a RDW value > 14.5% was independently associated with a 96% higher risk (odds ratio, 1.96; 95%CI: 1.64-2.33) of incident AF.
Liu et al[41] studied 99 patients with AF, divided into two groups according to their CHADS2 and CHA2DS2-VASc scores. In multivariate logistic regression analysis, a RDW value > 12.55% was found to be significantly associated with higher (≥ 2) CHADS2 score (odds ratio, 2.18; 95CI%: 1.14-3.22), whilst a RDW value > 12.75% was found to be significantly associated with higher (≥ 2) CHA2DS2-VASc score (odds ratio, 5.75; 95%CI: 3.70-7.79).
Saliba et al[42] searched the electronic database for a large national health maintenance for identifying all patients diagnosed with AF in whom at least two RDW measurements were performed 1 year before study entry. Mortality data were retrospectively reviewed for up to 2 years after patients inclusion in the database. The electronic search identified a total of 69412 records. A RDW value > 14.5% was independently associated with a 49% increased risk (hazard ratio, 1.49; 95%CI, 1.43-1.55) of all-cause mortality during the follow-up period. More importantly, persistently increased RDW values at the two-time points were independently associated with an even higher risk of death during the same follow-up period (HR, 1.70; 95%CI: 1.61-1.79).
Kaya et al[43] analyzed the data of 619 AF patients undergoing transesophageal echocardiography examination before cardioversion or AF ablation. In multivariate regression analysis, a RDW value > 13.7% was associated with a 67% increased risk of left atrial stasis (odds ratio, 1.67; 95%CI: 1.44-1.94).
Cha et al[44] carried out a retrospective study including 5082 patients with non-valvular AF, who were followed-up for a mean period of 5.2 years. The RDW was measured several times during follow-up, allowing to identify nadir (i.e., the lowest), peak (i.e., the highest) and mean RDW values. Among the various RDW measures, a peak value ≥ 13.9% was independently associated with a 66% enhanced risk (odds ratio, 1.66; 95%CI: 1.41-1.96) of thromboembolic events, including ischemic stroke and systemic embolism.
Nam et al[45] carried out a cross-sectional study including 103 healthy control subjects and 117 patients with AF, 65 of whom with paroxysmal AF and 52 with persistent AF. Overall, no significant difference was found in mean RDW values between controls and AF cases (13.4% ± 1.6% vs 13.5% ± 0.8%; P = 0.343), whilst patients with persistent AF exhibited significantly higher mean RDW values than those with paroxysmal AF (13.9% ± 0.9% vs 13.3% ± 0.6%; P < 0.05).
Wasilewski et al[46] performed a sub-analysis of the COMMIT-HF (COnteMporary Modalities In Treatment of Heart Failure) registry, including 1734 patients with left ventricular ejection fraction ≤ 35% and without ACS at baseline, who were retrospectively investigated for a median period of 660 d. Patients in the highest RDW tertile had a more than double risk of developing AF on follow-up compared to those in the lowest tertile (44.1% vs 20.2%; P < 0.01).
Cerşit et al [47] investigated the association between RDW and AF in 50 patients with and without AF after an ACS. RDW was significantly higher in patients with AF than the control group (14.5% ± 2% vs 12.6% ± 1%, P < 0.001). A RDW of > 11.7% also predicted AF (sensitivity 56% and specificity of 64%; AUC = 0.637, P < 0.001).
Kılıcgedik et al[48] evaluated the RDW values in 358 patients who underwent CABG surgery [57 with post-surgery AF (PSAF) and 301 patients with non-PSAF]. Interestingly, RDW values were significantly higher in PSAF group. In multivariate analysis, RDW [OR:1.16 (95%CI: 1.0-1.36), P = 0.05] was found to be predictive for PSAF (68.4% sensitivity and 51.2% specificity; P = 0.001). Likewise, Ozsin et al[49] analyzed the RDW levels in 93 patients who underwent off-pump CABG surgery. 24 patients developed PSAF while 69 did not. RDW was significantly correlated with PSAF and was also found to be predictive for PSAF (79.2% sensitivity and 65.2% specificity; P = 0.001).
Pilling et al[50] analyzed the RDW levels in 240477 healthy volunteers (40 ± 70 at baseline) during a follow-up period of ≤ 9 years. Higher RDW levels (≥ 15% variation, n = 6050) was associated with AF (sHR 1.37: 1.21 to 1.55). RDW was also predictive of new-onset AF.
Han et al[51] investigated the effects of low altitude (3.5 m above the sea level) and high altitude (2260 m above the sea level) on RDW levels of 303 patients with nonvalvular AF. RDW levels were higher in AF than control individuals (P < 0.05) and higher in persistent AF than paroxysmal AF (P < 0.05) in both low and high altitudes. Moreover, RDW, was independently associated with AF in low altitude (RDW, OR: 1.687, 95%CI: 1.021–2.789; P < 0.05), whereas it was an independent predictor for AF (RDW, OR: 1.755, 95%CI: 1.179–2.613; P < 0.05) in high altitude.
Jurin et al[52] recruited 579 patients with AF, 412 with non-permanent AF and 167 with permanent AF, and followed-up the patients with non-permanent AF during a median time of 21 mo. The main endpoint was progression of non-permanent AF to permanent AF. 109 patients (26.6%) progressed to permanent AF. Moreover, increased RDW levels showed a significant independent association with the progression to permanent AF (HR 1.19, 95%CI: 1.03–1.39, P = 0.022).
Finally, Li et al[53] recently examined the relationship between RDW and AF in a general Chinese population (106998 subjects). The authors concluded that RDW was significantly related to a higher prevalence of AF; the OR (95%CI) of AF for increasing tertiles of RDW were 1.00 (reference), 1.08 (0.69, 1.67), and 2.65 (1.75, 4.07) (P for trend < 0.0001), respectively.
Taken together, the results of these epidemiological studies, as well as results from two systematic reviews and meta-analysis recently published[54,55], are all virtually concordant to emphasize that an enhanced RDW value not only is a predictive factor and a marker of AF but its measurement may also be helpful for predicting the risk of developing many adverse complications in patients with AF, such as recurrence and duration of AF, hospitalization for heart failure, bleeding, left atrial thrombosis and stasis, thromboembolic events (including new-onset stroke) and mortality.
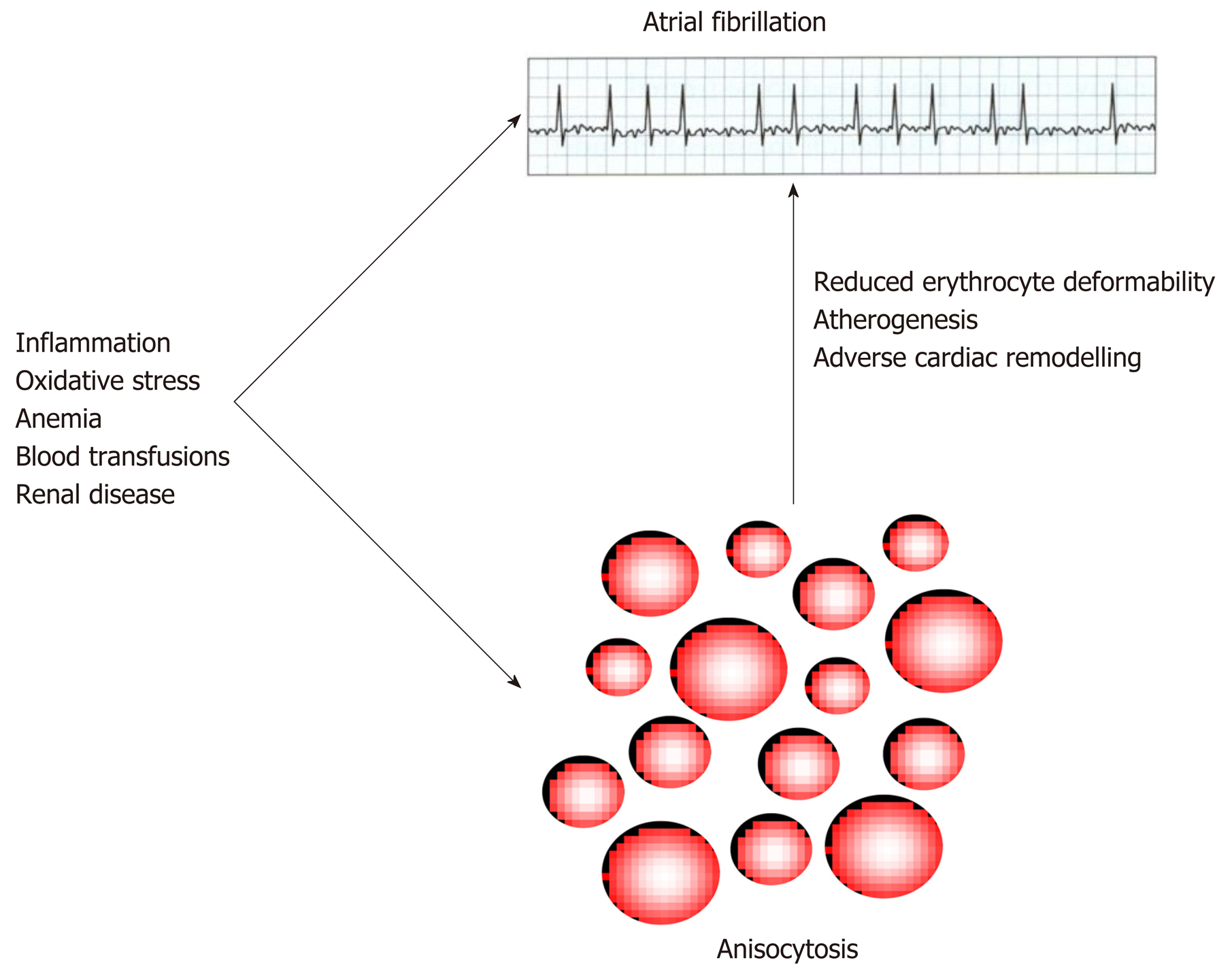
There are at least two biological explanations which can be brought for justifying the strong epidemiological association observed between anisocytosis and AF, either of which is plausible (Figure 2).
The first and rather predictable scenario is that the same causative factors for AF may also impair erythropoiesis, and thereby the observation of an increased RDW value may only be a coincident epiphenomenon in AF[56]. For example, a high RDW value is commonplace in patients with recent blood transfusions or severe anemia[13], and both RBC transfusion[57] and anemia[58] are associated with an excess incidence of AF, as consequence of onset of heart failure and impairment of renal function. Inflammation is probably the most frequent cause of anisocytosis[59], but its contribution to the pathogenesis of AF is now almost unquestionable, since many inflammatory cytokines are known to impair atrial electrophysiology and structure[60]. Oxidative stress is another important inducer of anisocytosis[61], whilst the oxidation of myofibrillar protein and cardiomyocyte membrane lipids is also a well-recognized mechanism leading to AF[62]. Finally, it is now clearly acknowledged that renal diseases may generate a kaleidoscope of inflammatory, neurohumoral, metabolic and hemodynamic stresses to the heart[63], whilst impaired erythropoiesis and anisocytosis are also commonplace in patients with impaired renal function, mainly due to impaired erythropoietin production[13] (Figure 2).
On the other hand, a support to the thesis that anisocytosis not only may be an innocent bystander in AF, but may also trigger, or contribute to worsening, AF has emerged from a discrete number of studies. Hirayama et al showed that the onset of arrhythmias is strongly associated with reduced erythrocyte deformability[64], which is a conventional hallmark of anisocytic erythrocytes[65]. A large variation of erythrocytes volume is also associated with a greater cholesterol content in the RBC membrane, which can then be directly transferred to atherosclerotic plaques enriched in erythrocytes[66,67], thus finally promoting atherogenesis and ultimately predisposing to cardiac arrhythmias, since AF atherosclerosis and AF are now considered two strictly intertwined disorders[68]. Finally, the presence of anisocytic erythrocytes has also been involved in the mechanisms underlying adverse cardiac remodeling[69], thus leading to atrial fibrosis and predisposing the patients to a higher risk of developing AF[70].
The value of the RDW can be automatically generated, along with the other parameters of the complete blood cell count, by the majority of modern hematological analyzers. It can therefore be considered an easier, faster and less expensive test compared to other potentially useful biomarkers in AF[1]. Regardless of the fact that anisocytosis may be a simple bystander or an active player in the pathogenesis of AF and of its life-threatening complications, the current epidemiological evidence convincingly suggests that routine measurement of RDW may provide valuable clinical information for diagnosis and management of AF, alone or combined with traditional risk scores such as CHADS2 and CHA2DS2-VASc[71]. In particular, the strong and often independent association observed between high RDW values and unfavorable outcomes (e.g., recurrence of AF, heart failure, bleeding, thromboembolic events and death) (Table 1), would lead us to conclude that AF patients with RDW values exceeding the local reference range may be more aggressively investigated and managed, in order to identify and reduce the impact of possible underlying disorders causing both anisocytosis and AF (Figure 2), and also for preventing the possible risk of adverse events potentially attributable to anisocytosis. Additional studies are then advised to define whether the inclusion of RDW within conventional risks scores may be effective in providing more accurate risk stratification in AF.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Barik R, Pastromas S, Ueda H, S-Editor: Zhang L L-Editor: A E-Editor: Zhang YL
| 1. | O'Neal WT, Venkatesh S, Broughton ST, Griffin WF, Soliman EZ. Biomarkers and the prediction of atrial fibrillation: state of the art. Vasc Health Risk Manag. 2016;12:297-303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2373] [Cited by in RCA: 2928] [Article Influence: 266.2] [Reference Citation Analysis (0)] |
| 3. | Melgaard L, Gorst-Rasmussen A, Lane DA, Rasmussen LH, Larsen TB, Lip GY. Assessment of the CHA2DS2-VASc Score in Predicting Ischemic Stroke, Thromboembolism, and Death in Patients With Heart Failure With and Without Atrial Fibrillation. JAMA. 2015;314:1030-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 254] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 4. | Jacobs V, May HT, Bair TL, Crandall BG, Cutler M, Day JD, Weiss JP, Osborn JS, Muhlestein JB, Anderson JL, Mallender C, Bunch TJ. The impact of risk score (CHADS2 versus CHA2DS2-VASc) on long-term outcomes after atrial fibrillation ablation. Heart Rhythm. 2015;12:681-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 5. | Ruff CT, Giugliano RP, Braunwald E, Murphy SA, Brown K, Jarolim P, Mercuri M, Antman EM, Morrow DA. Cardiovascular Biomarker Score and Clinical Outcomes in Patients With Atrial Fibrillation: A Subanalysis of the ENGAGE AF-TIMI 48 Randomized Clinical Trial. JAMA Cardiol. 2016;1:999-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Fernández-Ruiz I. Atrial fibrillation: New score for stroke risk. Nat Rev Cardiol. 2016;13:634-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Hijazi Z, Oldgren J, Lindbäck J, Alexander JH, Connolly SJ, Eikelboom JW, Ezekowitz MD, Held C, Hylek EM, Lopes RD, Yusuf S, Granger CB, Siegbahn A, Wallentin L; ARISTOTLE and RE-LY Investigators. A biomarker-based risk score to predict death in patients with atrial fibrillation: the ABC (age, biomarkers, clinical history) death risk score. Eur Heart J. 2018;39:477-485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 8. | Hijazi Z, Lindahl B, Oldgren J, Andersson U, Lindbäck J, Granger CB, Alexander JH, Gersh BJ, Hanna M, Harjola VP, Hylek EM, Lopes RD, Siegbahn A, Wallentin L. Repeated Measurements of Cardiac Biomarkers in Atrial Fibrillation and Validation of the ABC Stroke Score Over Time. J Am Heart Assoc. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Imataka K, Nakaoka H, Kitahara Y, Fujii J, Ishibashi M, Yamaji T. Blood hematocrit changes during paroxysmal atrial fibrillation. Am J Cardiol. 1987;59:172-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Takahashi N, Ashida T, Kiraku J, Fujii J. Increase in erythrocyte volume in patients with chronic atrial fibrillation. Jpn Heart J. 1997;38:387-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Lippi G, Pavesi F, Bardi M, Pipitone S. Lack of harmonization of red blood cell distribution width (RDW). Evaluation of four hematological analyzers. Clin Biochem. 2014;47:1100-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 12. | Fischbach FT, Dunning MB. A manual of laboratory and diagnostic tests. 9th ed. Philadelphia: Wolters Kluwer Health; 2015; . |
| 13. | Sarma PR. Red Cell Indices. Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. Boston; 1990; 720-723. |
| 14. | Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. 2015;52:86-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 714] [Article Influence: 64.9] [Reference Citation Analysis (1)] |
| 15. | Lippi G, Cervellin G, Sanchis-Gomar F. Red blood cell distribution width and cardiovascular disorders. Does it really matter which comes first, the chicken or the egg? Int J Cardiol. 2016;206:129-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Lippi G, Cervellin G. Risk assessment of post-infarction heart failure. Systematic review on the role of emerging biomarkers. Crit Rev Clin Lab Sci. 2014;51:13-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Lippi G, Buonocore R, Cervellin G. Value of Red Blood Cell Distribution Width on Emergency Department Admission in Patients With Venous Thrombosis. Am J Cardiol. 2016;117:670-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Lippi G, Favalor EJ, Simundic AM. Biomedical research platforms and their influence on article submissions and journal rankings: an update. Biochem Med (Zagreb). 2012;22:7-14. [PubMed] |
| 19. | Horne BD, May HT, Kfoury AG, Renlund DG, Muhlestein JB, Lappé DL, Rasmusson KD, Bunch TJ, Carlquist JF, Bair TL, Jensen KR, Ronnow BS, Anderson JL. The Intermountain Risk Score (including the red cell distribution width) predicts heart failure and other morbidity endpoints. Eur J Heart Fail. 2010;12:1203-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Providência R, Ferreira MJ, Gonçalves L, Faustino A, Paiva L, Fernandes A, Barra S, Pimenta J, Leitão-Marques AM. Mean corpuscular volume and red cell distribution width as predictors of left atrial stasis in patients with non-valvular atrial fibrillation. Am J Cardiovasc Dis. 2013;3:91-102. [PubMed] |
| 21. | Liu T, Shao Q, Miao S, Liu E, Xu G, Yuan R, Li G. Red cell distribution width as a novel, inexpensive marker for paroxysmal atrial fibrillation. Int J Cardiol. 2014;171:e52-e53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Ertaş G, Aydin C, Sönmez O, Erdoğan E, Turfan M, Tasal A, Bacaksiz A, Vatankulu MA, Uyarel H, Ergelen M, Zeybek R, Göktekin Ö. Red cell distribution width predicts new-onset atrial fibrillation after coronary artery bypass grafting. Scand Cardiovasc J. 2013;47:132-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Ertaş G, Sönmez O, Turfan M, Kul S, Erdoğan E, Tasal A, Bacaksiz A, Vatankulu MA, Altıntaş O, Uyarel H, Göktekin O. Neutrophil/lymphocyte ratio is associated with thromboembolic stroke in patients with non-valvular atrial fibrillation. J Neurol Sci. 2013;324:49-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 24. | Kurt M, Tanboga IH, Buyukkaya E, Karakas MF, Akçay AB, Sen N. Relation of red cell distribution width with CHA2DS2-VASc score in patients with nonvalvular atrial fibrillation. Clin Appl Thromb Hemost. 2014;20:687-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Güngör B, Özcan KS, Erdinler İ, Ekmekçi A, Alper AT, Osmonov D, Çalık N, Akyuz S, Toprak E, Yılmaz H, Yıldırım A, Bolca O. Elevated levels of RDW is associated with non-valvular atrial fibrillation. J Thromb Thrombolysis. 2014;37:404-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Adamsson Eryd S, Borné Y, Melander O, Persson M, Smith JG, Hedblad B, Engström G. Red blood cell distribution width is associated with incidence of atrial fibrillation. J Intern Med. 2014;275:84-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 27. | Sarikaya S, Şahin Ş, Akyol L, Börekçi E, Yilmaz YK, Altunkaş F, Karaman K. Is there any relationship between RDW levels and atrial fibrillation in hypertensive patient? Afr Health Sci. 2014;14:267-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Gurses KM, Yalcin MU, Kocyigit D, Evranos B, Ates AH, Yorgun H, Sahiner ML, Kaya EB, Ozer N, Oto MA, Aytemir K. Red blood cell distribution width predicts outcome of cryoballoon-based atrial fibrillation ablation. J Interv Card Electrophysiol. 2015;42:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Korantzopoulos P, Sontis N, Liu T, Chlapoutakis S, Sismanidis S, Siminelakis S, Apostolakis E. Association between red blood cell distribution width and postoperative atrial fibrillation after cardiac surgery: A pilot observational study. Int J Cardiol. 2015;185:19-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Wan H, Yang Y, Zhu J, Huang B, Wang J, Wu S, Shao X, Zhang H. The relationship between elevated red cell distribution width and long-term outcomes among patients with atrial fibrillation. Clin Biochem. 2015;48:762-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Lee KH, Park HW, Cho JG, Yoon NS, Kim SS, Kim MR, Kim MC, Cho KH, Kim HK, Kim CH, Kim KH, Jun SJ, Kim WJ, Lee KJ, Jeong HC, Cho JY, Park KH, Sim Ds, Yoon HJ, Kim KH, Hong YJ, Kim JH, Ahn Y, Jeong MH, Park JC. Red cell distribution width as a novel predictor for clinical outcomes in patients with paroxysmal atrial fibrillation. Europace. 2015;17 Suppl 2:ii83-ii88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Zhao J, Liu T, Korantzopoulos P, Fu H, Shao Q, Suo Y, Zheng C, Xu G, Liu E, Xu Y, Zhou C, Li G. Red blood cell distribution width and left atrial thrombus or spontaneous echo contrast in patients with non-valvular atrial fibrillation. Int J Cardiol. 2015;180:63-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Aksu T, Baysal E, Guler TE, Golcuk SE, Erden İ, Ozcan KS. Predictors of atrial fibrillation recurrence after cryoballoon ablation. J Blood Med. 2015;6:211-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Korantzopoulos P, Kyrlas K, Liu T, Li G, Goudevenos JA. Red blood cell distribution width and atrial fibrillation in patients with sick sinus syndrome. J Cardiol. 2016;67:551-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Karataş MB, Çanga Y, İpek G, Özcan KS, Güngör B, Durmuş G, Onuk T, Öz A, Şimşek B, Bolca O. Association of admission serum laboratory parameters with new-onset atrial fibrillation after a primary percutaneous coronary intervention. Coron Artery Dis. 2016;27:128-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Yanagisawa S, Inden Y, Kato H, Miyoshi A, Mizutani Y, Ito T, Kamikubo Y, Kanzaki Y, Hirai M, Murohara T. Elevated Red Blood Cell Distribution Width Predicts Recurrence After Catheter Ablation for Atrial Fibrillation in Patients With Heart Failure - Comparison With Non-Heart Failure Patients. Circ J. 2016;80:627-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Vizzardi E, Sciatti E, Bonadei I, Pezzali NL, Lombardi CM, Metra M. Red cell distribution width and chronic heart failure: prognostic role beyond echocardiographic parameters. Monaldi Arch Chest Dis. 2016;84:59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 38. | Geçmen Ç, Babür Güler G, Erdoğan E, Hatipoğlu S, Güler E, Yılmaz F, Unkun T, Cap M, Bengi Bakal R, Bayram T, Deniz Acar R, Candan Ö, Özdemir N. SYNTAX score predicts postoperative atrial fibrillation in patients undergoing on-pump isolated coronary artery bypass grafting surgery. Anatol J Cardiol. 2016;16:655-661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Zhang Q, Qin Y, Zhao DS, Zhao HP, Zhou L. Prognostic Value of Red Blood Cell Distribution Width on Bleeding Events in Nonvalvular Atrial Fibrillation Patients Taking Dabigatran (110 mg b.i.d.) after Catheter Ablation. Cardiology. 2017;136:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Al-Kindi SG, Kim CH, Morris SR, Freeman ML, Funderburg NT, Rodriguez B, McComsey GA, Dalton JE, Simon DI, Lederman MM, Longenecker CT, Zidar DA. Brief Report: Elevated Red Cell Distribution Width Identifies Elevated Cardiovascular Disease Risk in Patients With HIV Infection. J Acquir Immune Defic Syndr. 2017;74:298-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Liu T, Shao Q, Korantzopoulos P, Miao S, Zhang Z, Xu G, Yuan R, Li G. Relation of red blood cell distribution width with CHADS2 and CHA2DS2-VASc score in Chinese patients with non-valvular atrial fibrillation. Int J Cardiol. 2017;228:861-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Saliba W, Barnett-Griness O, Rennert G. Red cell distribution width and all-cause mortality in patients with atrial fibrillation: A cohort study. J Arrhythm. 2017;33:56-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Kaya A, Tukkan C, Alper AT, Gungor B, Ozcan KS, Tatlisu MA, Tekkesin AI, Karadeniz FO, Gok G, Kayapinar O. Increased levels of red cell distribution width is correlated with presence of left atrial stasis in patients with non-valvular atrial fibrillation. North Clin Istanb. 2017;4:66-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Cha MJ, Lee HS, Kim HM, Jung JH, Choi EK, Oh S. Association between red cell distribution width and thromboembolic events in patients with atrial fibrillation. Eur J Intern Med. 2017;46:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 45. | Nam JH, Park KH, Lee JH, Lee CH, Son JW, Kim U, Park JS, Shin DG. Discordant Relationships between Systemic Inflammatory Markers and Burden of Oxidative Stress in Patients with Atrial Fibrillation. Korean Circ J. 2017;47:752-761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 46. | Wasilewski J, Pyka Ł, Hawranek M, Tajstra M, Skrzypek M, Wasiak M, Suliga K, Bujak K, Gąsior M. Prognostic value of red blood cell distribution width in patients with left ventricular systolic dysfunction: Insights from the COMMIT-HF registry. Cardiol J. 2018;25:377-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 47. | Cerşit S, Kalçik M, Öcal L, Bayam E. Association between atrial fibrillation and red cell distribution width in patients with acute coronary syndrome. Medeniyet Medical Journal. 2018;33:89-93. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 48. | Kılıcgedik A, Naser A, Gurbuz AS, Kulahcioglu S, Bakal RB, Unkun T, Yilmaz F, Kahveci G, Kirma C. Red Cell Distribution Width with CHADS2 and CHA2DS2-VASc score is associated with Post-operative Atrial Fibrillation after Coronary Artery Bypass Grafting. Heart Surg Forum. 2018;21:E170-E174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 49. | Ozsin KK, Sanri US, Toktas F, Yavuz S. Relationship between red cell distribution width and mean platelet volume with new onset atrial fibrillation afteroff-pump coronary artery bypass grafting. Bratisl Lek Listy. 2018;119:335-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 50. | Pilling LC, Atkins JL, Kuchel GA, Ferrucci L, Melzer D. Red cell distribution width and common disease onsets in 240,477 healthy volunteers followed for up to 9 years. PLoS One. 2018;13:e0203504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 51. | Han K, Su X, Liu J, Yao F, Lu F. Red Cell Distribution Width as a Novel Marker for Different Types of Atrial Fibrillation in Low and High Altitude. Cardiol Res Pract. 2019;2019:6291964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 52. | Jurin I, Hadžibegović I, Durlen I, Jakšić Jurinjak S, Mišković D, Ajduk M, Jerkić H, Letilović T. Left atrium size and red cell distribution width predict atrial fibrillation progression from paroxysmal or persistent to permanent. Acta Clin Belg. 2019;1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 53. | Li H, Gu Y, Liu M, Wang X, Chi VTQ, Zhang Q, Liu L, Meng G, Yao Z, Wu H, Bao X, Zhang S, Kumari S, Sun S, Zhou M, Jia Q, Song K, Wu Y, Liu T, Niu K. The relationship between red blood cell distribution width and atrial fibrillation in Asian population: A cross-sectional study. Pacing Clin Electrophysiol. 2019;42:1197-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 54. | Shao Q, Korantzopoulos P, Letsas KP, Tse G, Hong J, Li G, Liu T. Red blood cell distribution width as a predictor of atrial fibrillation. J Clin Lab Anal. 2018;32:e22378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 55. | Weymann A, Ali-Hasan-Al-Saegh S, Popov AF, Sabashnikov A, Mirhosseini SJ, Liu T, Tse G, Lotfaliani M, Ghanei A, Testa L, D'Ascenzo F, Benedetto U, Dehghan H, Roever L, Sá MPBO, Baker WL, Yavuz S, Zeriouh M, Mashhour A, Nombela-Franco L, Jang JS, Meng L, Gong M, Deshmukh AJ, Palmerini T, Linde C, Filipiak KJ, Biondi-Zoccai G, Calkins H, Stone GW. Haematological indices as predictors of atrial fibrillation following isolated coronary artery bypass grafting, valvular surgery, or combined procedures: a systematic review with meta-analysis. Kardiol Pol. 2018;76:107-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 56. | Jagannathan-Bogdan M, Zon LI. Hematopoiesis. Development. 2013;140:2463-2467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 256] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 57. | Alameddine AK, Visintainer P, Alimov VK, Rousou JA. Blood transfusion and the risk of atrial fibrillation after cardiac surgery. J Card Surg. 2014;29:593-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 58. | Ali AN, Athavale NV, Abdelhafiz AH. Anemia: An Independent Predictor Of Adverse Outcomes In Older Patients With Atrial Fibrillation. J Atr Fibrillation. 2016;8:1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 59. | Lippi G, Targher G, Montagnana M, Salvagno GL, Zoppini G, Guidi GC. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med. 2009;133:628-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 60. | Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. 2015;12:230-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 747] [Article Influence: 74.7] [Reference Citation Analysis (0)] |
| 61. | Ghaffari S. Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxid Redox Signal. 2008;10:1923-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 279] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 62. | Yang KC, Dudley SC. Oxidative stress and atrial fibrillation: finding a missing piece to the puzzle. Circulation. 2013;128:1724-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 63. | McManus DD, Saczynski JS, Ward JA, Jaggi K, Bourrell P, Darling C, Goldberg RJ. The Relationship Between Atrial Fibrillation and Chronic Kidney Disease : Epidemiologic and Pathophysiologic Considerations for a Dual Epidemic. J Atr Fibrillation. 2012;5:442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 64. | Hirayama T, Roberts D, Allers M, Belboul A, al-Khaja N, William-Olsson G. Association between arrhythmias and reduced red cell deformability following cardiopulmonary bypass. Scand J Thorac Cardiovasc Surg. 1988;22:179-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 65. | Patel KV, Mohanty JG, Kanapuru B, Hesdorffer C, Ershler WB, Rifkind JM. Association of the red cell distribution width with red blood cell deformability. Adv Exp Med Biol. 2013;765:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 153] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 66. | Tziakas D, Chalikias G, Grapsa A, Gioka T, Tentes I, Konstantinides S. Red blood cell distribution width: a strong prognostic marker in cardiovascular disease: is associated with cholesterol content of erythrocyte membrane. Clin Hemorheol Microcirc. 2012;51:243-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 67. | Tziakas DN, Chalikias GK, Stakos D, Tentes IK, Papazoglou D, Thomaidi A, Grapsa A, Gioka G, Kaski JC, Boudoulas H. Independent and additive predictive value of total cholesterol content of erythrocyte membranes with regard to coronary artery disease clinical presentation. Int J Cardiol. 2011;150:22-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 68. | Willeit K, Kiechl S. Atherosclerosis and atrial fibrillation--two closely intertwined diseases. Atherosclerosis. 2014;233:679-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 69. | Fujii T, Nishigaki R, Kawahara K, Yamada N, Onda M, Yokoyama M, Naito Z, Asano G, Shimizu-Suganuma M, Shichinohe K. Ultrastructural changes and immunohistochemical localization of advanced glycation end products in the heart of streptozotocin-treated Mongolian gerbils. Med Electron Microsc. 1999;32:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 70. | Shimano M, Tsuji Y, Inden Y, Kitamura K, Uchikawa T, Harata S, Nattel S, Murohara T. Pioglitazone, a peroxisome proliferator-activated receptor-gamma activator, attenuates atrial fibrosis and atrial fibrillation promotion in rabbits with congestive heart failure. Heart Rhythm. 2008;5:451-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 71. | Chen JY, Zhang AD, Lu HY, Guo J, Wang FF, Li ZC. CHADS2 versus CHA2DS2-VASc score in assessing the stroke and thromboembolism risk stratification in patients with atrial fibrillation: a systematic review and meta-analysis. J Geriatr Cardiol. 2013;10:258-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |