Peer-review started: November 14, 2018
First decision: November 29, 2018
Revised: December 11, 2018
Accepted: January 5, 2019
Article in press: January 6, 2019
Published online: January 26, 2019
Processing time: 75 Days and 9 Hours
Friedreich’s ataxia (FRDA), which occurs in 1/50000 live births, is the most prevalent inherited neuromuscular disorder. Nearly all FRDA patients develop cardiomyopathy at some point in their lives. The clinical manifestations of FRDA include ataxia of the limbs and trunk, dysarthria, diabetes mellitus, and cardiac diseases. However, the broad clinical spectrum makes FRDA difficult to identify. The diagnosis of FRDA is based on the presence of suspicious clinical factors, the use of the Harding criteria and, more recently, the use of genetic testing for identifying the expansion of a triplet nucleotide sequence. FRDA is linked to a defect in the mitochondrial protein frataxin; an epigenetic alteration interferes with the folding of this protein, causing a relative deficiency of frataxin in affected patients. Frataxins are small essential proteins whose deficiency causes a range of metabolic disturbances, including oxidative stress, iron-sulfur cluster deficits, and defects in heme synthesis, sulfur amino acid metabolism, energy metabolism, stress responses, and mitochondrial function. The cardiac involvement seen in FRDA is a consequence of mitochondrial proliferation as well as the loss of contractile proteins and the subsequent development of myocardial fibrosis. The walls of the left ventricle become thickened, and different phenotypic manifestations are seen, including concentric or asymmetric hypertrophy and (less commonly) dilated cardiomyopathy. Dilated cardiomyopathy and arrhythmia are associated with mortality in patients with FRDA, whereas hypertrophic cardiomyopathy is not. Systolic function tends to be low-normal in FRDA patients, with an acute decline at the end of life. However, the literature includes only a few long-term prospective studies of cardiac progression in FRDA, and the cause of death is often attributed to heart failure and arrhythmia postmortem. Cardiomyopathy tends to be correlated with the clinical neurologic age of onset and the nucleotide triplet repeat length (i.e., markers of phenotypic disease severity) rather than the duration of disease or the severity of neurologic symptoms. As most patients are wheelchair-bound within 15 years of diagnosis, the clinical determination of cardiac involvement is often complicated by comorbidities. Researchers are currently testing targeted therapies for FRDA, and a centralized database, patient registry, and natural history study have been launched to support these clinical trials. The present review discusses the pathogenesis, clinical manifestations, and spectrum of cardiac disease in FRDA patients and then introduces gene-targeted and pathology-specific therapies as well as screening guidelines that should be used to monitor cardiac disease in this mitochondrial disorder.
Core tip: The present review discusses the pathogenesis, clinical manifestations, and spectrum of cardiac disease in Friedreich’s Ataxia, and introduces gene-targeted and pathology-specific therapies, in addition to the screening guidelines that should be used to monitor cardiac disease in this mitochondrial disorder.
- Citation: Hanson E, Sheldon M, Pacheco B, Alkubeysi M, Raizada V. Heart disease in Friedreich’s ataxia. World J Cardiol 2019; 11(1): 1-12
- URL: https://www.wjgnet.com/1949-8462/full/v11/i1/1.htm
- DOI: https://dx.doi.org/10.4330/wjc.v11.i1.1
Friedreich’s ataxia (FRDA) is the most common autosomal recessive spinocerebellar ataxia. FRDA was first reported in the 1860s but remained difficult to distinguish from other spinocerebellar ataxias until the causative gene was determined in 1996. Since then, evidence has accumulated regarding the pathogenesis, specialized treatment and prognosis of this disease. We now know that FRDA is a mitochondrial disorder that primarily affects neural pathways and cardiac muscle. While FRDA is most clinically renowned for its progressive, unremitting ataxia and neuromuscular decline, significant mortality occurs as a result of cardiomyopathy.
In the 1860s, Nikolaus Friedreich, a professor of medicine at the University of Heidelberg, first identified the disease when he described two symptomatic siblings with asymptomatic parents[1]. Diagnostic criteria based on clinical features were first published in 1976. The modified criteria published by Harding[2] in 1981 were the diagnostic tool of choice until 1996, when Campuzano et al[3] identified the causative alteration of the frataxin gene. This outcome paved the way for genetic testing. Even with the availability of genetic testing, the diagnosis of FRDA often relies on the exclusion of acute neurologic abnormalities and the presence of a slowly progressive clinical course. Thus, diagnosis is often delayed.
FRDA exhibits variable phenotypes, and distinct early-onset and late-onset groups may be classified based on the symptom onset before or after age 25, respectively[4]. Late-onset FRDA is characterized by less severe cardiomyopathy and neurologic symptoms, while early-onset typically exhibits more rapid progression with higher morbidity and mortality. For early-onset FRDA, the typical age of onset is approximately 10 years; most patients are wheelchair-bound between 19 and 26 years of age, with mortality by a mean age of 39 years[5]. In comparison, late-onset FRDA is much less decapacitating with nearly average mortality.
FRDA occurs in 1/50000 live births, is most prevalent in Caucasians and is nearly absent in Sub-Saharan Africans, Asians and Native Americans. This factor reflects a single founder event that is responsible for 90% of the large normal alleles that constitute the primary reservoir of this triplet repeat disease. Heterozygous carriers constitute approximately 1% of the population, and homozygous point mutations in one or the other allele account for 5% of FRDA manifestations[6,7].
The characteristic clinical picture of FRDA reflects malfunction of the central sensory pathways found in the posterior columns of the spinal cord, the spinocerebellar tracts, the cerebellar efferent pathway, and the distal portion of the corticospinal motor tracts. Other abnormalities include atrophy of cerebellar regions, including the dentate nuclei. Peripheral nerves show a loss of large myelinated sensory fibers, resulting in the loss of large primary sensory neurons in the dorsal root ganglia. These neurologic abnormalities cause the progressive and unremitting mixed cerebellar and sensory ataxia that characterizes the disease.
The clinical manifestations of FRDA include ataxia of the limbs and trunk, dysarthria, diabetes mellitus, and cardiac diseases. The diagnosis is first made on the presence of suspicious clinical factors. Harding et al[2] initially described the primary criteria of progressive gait and limb ataxia, absent patellar and ankle reflexes, dysarthria, muscle weakness, loss of vibration or position and onset before the age of 25 years; the secondary criteria were a positive Babinski reflex, pes cavus, scoliosis, and cardiomyopathy. If secondary criteria were not present, then the patient must have an affected sibling. Most often, FRDA patients first present at a young age with increasing clumsiness but normal findings on an magnetic resonance imaging (MRI). The onset is typically before the age of 18 years, and electromyogram studies can help confirm the diagnosis. Genetic testing is now available but is only given when the clinical suspicion is high. Thus, diagnosis is often delayed. As nearly all patients develop cardiomyopathy at some point in their lives, and this aspect of the disease can be the most severe in the youngest cohort, FRDA patients must be referred to a cardiologist upon diagnosis. Indeed, 5% of FRDA patients may present with severe cardiomyopathy in the absence of neurologic symptoms. Cerebellar atrophy on an MRI and the absence of cardiomyopathy are both negative predictors of an FRDA diagnosis[8].
FRDA is the most frequent hereditary ataxia, with an estimated prevalence of 3-4 cases per 50000 individuals. This autosomal-recessive neurodegenerative disease is characterized by progressive gait and limb ataxia, dysarthria, lower-limb areflexia, decreased vibration sense, muscular weakness in the legs, and a positive extensor plantar response. Nonneurological signs include hypertrophic cardiomyopathy and diabetes mellitus. The symptom onset typically occurs around puberty, and the life expectancy of FRDA patients is 40-50 years. The disease is usually caused by a large GAA triplet repeat expansion within the first intron of the frataxin gene. Frataxin mutations cause deficiencies of the iron-sulfur cluster-containing subunits of mitochondrial electron transport complexes I, II, and III and of the iron-sulfur protein, aconitase. The mitochondrial dysfunction in FRDA patients has been addressed in several open-label, nonplacebo-controlled trials, whose results indicate that treatment with idebenone might ameliorate hypertrophic cardiomyopathy. Indeed, a well-designed phase II clinical trial suggested that idebenone may yield concentration-dependent functional improvements in nonwheelchair-bound children and adolescents. Other current experimental approaches seek to address the iron-mediated toxicity or to increase the frataxin protein level.
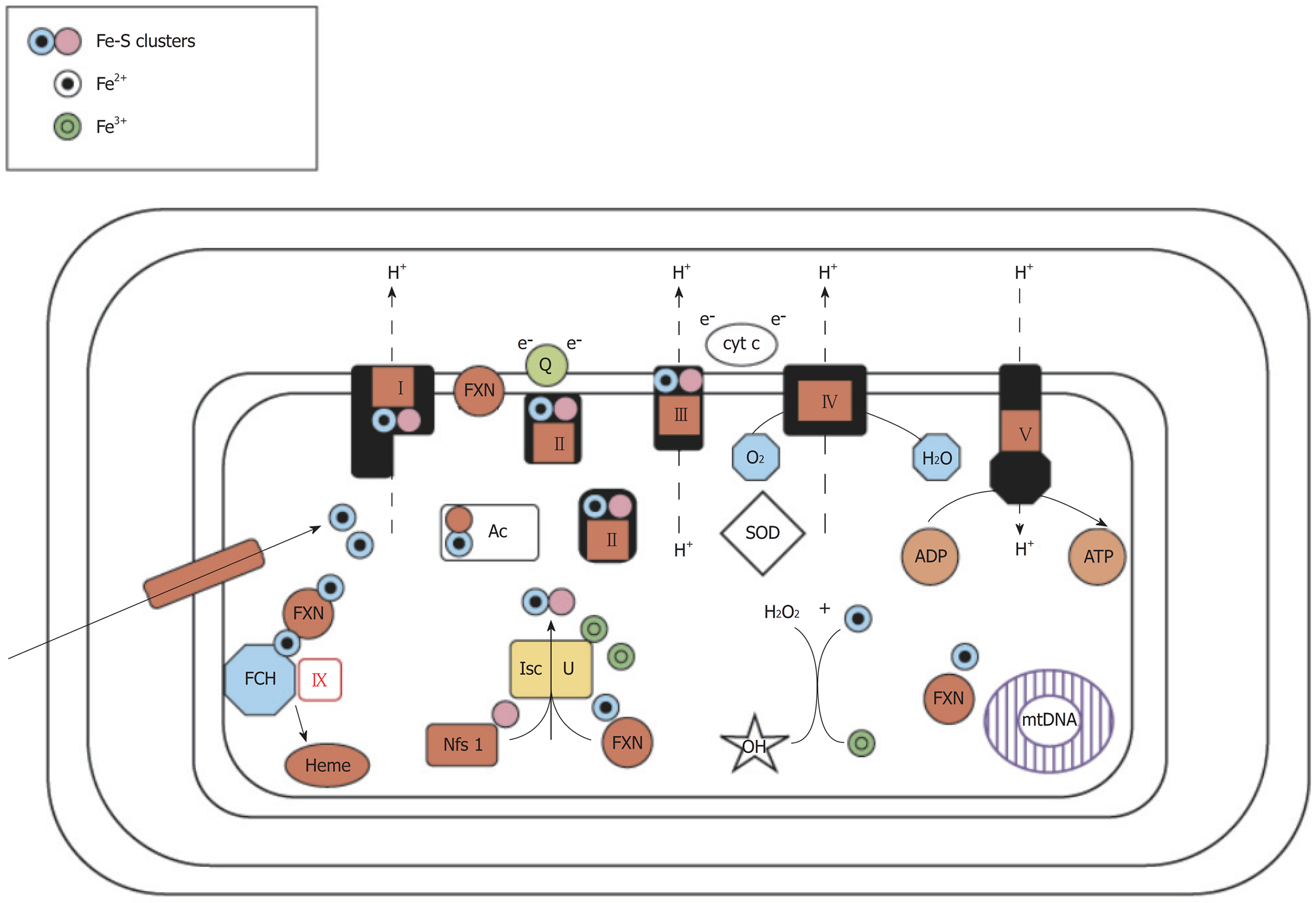
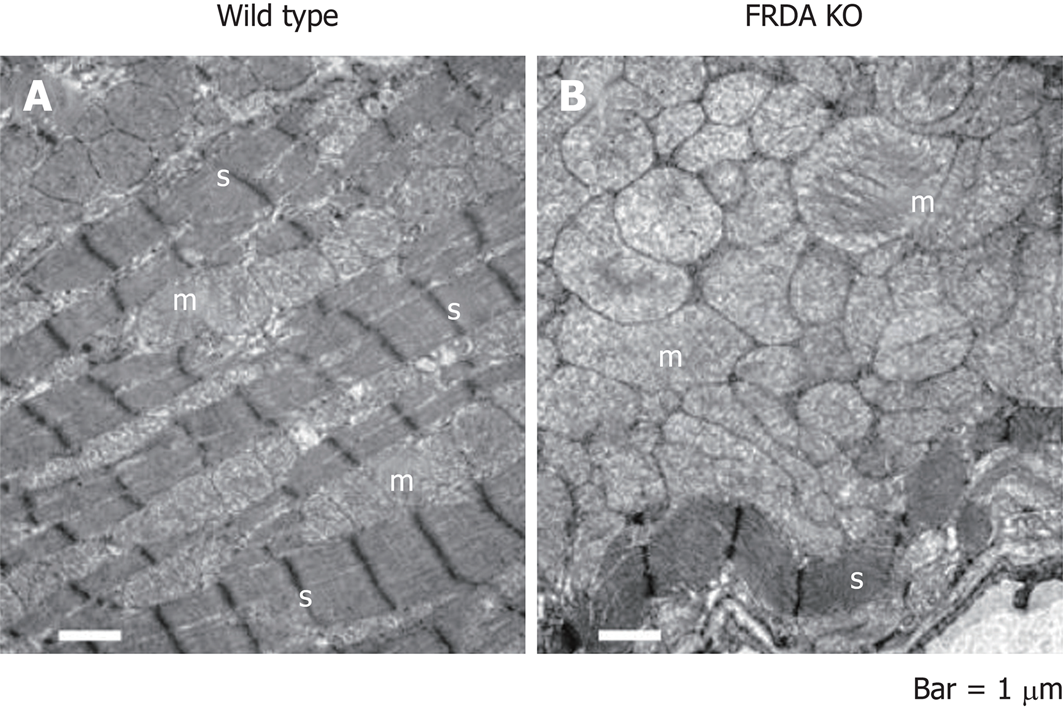
FRDA is linked to a defect in the mitochondrial protein, frataxin, through epigenetic alterations that interfere with protein folding to cause a relative deficiency of frataxin in affected patients (Figure 1). The frataxin gene, which is located at chromosome 9q21.11, harbors an intronic trinucleotide repeat sequence (guanine-adenine-adenine; GAA). Most FRDA patients are homozygous for an expansion of this GAA repeat, whereas a typical gene contains 6-36 trinucleotide repeats; those associated with FRDA typically have between 66 and 1700 trinucleotide repeats[9]. This repeat expansion and other mutations in the frataxin gene impact the ability of the encoded protein to participate in mitochondrial oxidative phosphorylation, and the cell suffers in terms of energy production. More specifically, FRDA-associated mutations impair mitochondrial function, increase reactive oxygen species, and trigger redistribution of iron in the mitochondria and the cytosol. Mitochondria proliferate but remain dysfunctional. Physiologically, these changes reduce the myocardial reserve as evidenced by the enhancement of the late gadolinium signal on cardiac MRI (cMRI)[10] (Figure 2).
Frataxin also participates in iron metabolism; its deficiency interferes with iron hemostasis, leading to the deposition of iron in cells. Such deposition in cardiomyocytes often accompanies myocardial hypertrophy in FRDA patients, suggesting that iron toxicity-mediated oxidative tissue damage may play a role in this disease[10]. Indeed, autopsies of FRDA patients suggested that iron-induced myocarditis may be involved in the pathology of this unique cardiomyopathy[11]. Iron deposition, myocardial hypertrophy and oxidative tissue damage are also associated with an impaired lipid metabolism and a lower threshold for oxidative stress, which may contribute to cardiac disease progression. FRDA is known to most strongly affect tissues that are primarily involved in oxidative phosphorylation and are rich with mitochondria (e.g., dorsal root ganglion, cardiomyocytes and B-islet cells of the pancreas). However, we still do not know why some spinal and brainstem motor neurons are affected, while others remain normal.
Structural studies carried out on different orthologs have shown that eukaryotic frataxin proteins take on a folded conformation (called the frataxin fold) that involves a flexible N-terminal region present only in eukaryotes, whereas all frataxins have a highly conserved C-terminal globular domain. Frataxins bind iron directly but show very unusual properties in this regard, as iron coordination is achieved solely by glutamates and aspartates exposed on the protein surface. It has been suggested that frataxin functions as a ferritin-like protein, as an iron chaperone of the iron-sulfur cluster machinery and heme metabolism and/or as a controller of cellular oxidative stress. If we hope to fully understand the pathology of FRDA and to design novel therapeutic strategies, we must first precisely identify the cellular role of frataxin[9].
Histologically, frataxin deficiency causes failure of iron clearance from myocytes as well as myocardial necrosis, myocardial apoptosis, chronic inflammation and scarring or fibrosis. However, iron deposits are not thought to be the initial direct cause of this disease; in the frataxin knockout model, mice die in utero but do not have manifestations of iron deposition. Although animal models have been used to describe the pathogenesis of FRDA and test targeted treatments, the causes of necrosis or cardiomyocyte apoptosis have not yet been determined in human FRDA patients, and it is not clear whether frataxin is a protective factor or a pathogenic contributor in cardiomyopathy. The protein stores of frataxin in FRDA patients are 20%-25% of those seen in normal individuals, but we do not yet know how much protein is required for a normal phenotype. The embryonic lethality of frataxin knockout mice indicates that a complete lack of frataxin is incompatible with life, whereas the conditional mouse models with a post developmental knockout of frataxin demonstrate mitochondrial pathologies[12].
Centralized database, patient registry, and natural history studies have been launched to support clinical trials in FRDA. The 2011 Neurobiology of Disease in Children symposium, which is held in conjunction with the 40th annual Child Neurology Society meeting, aimed to: (1) describe the clinical features surrounding FRDA, including the cardiomyopathy and the genetics of the disorder; (2) discuss recent advances in our understanding of FRDA pathogenesis and the development of clinical trials; (3) review new investigations of characteristic symptoms; and (4) establish clinical and biochemical overlaps in neurodegenerative diseases and possible directions for future basic, translational and clinical studies.
The cardiac involvement in this mitochondrial disorder is a consequence of mitochondrial proliferation, the loss of contractile proteins, and the subsequent development of myocardial fibrosis. The left ventricular walls become thickened and show a range of phenotypic manifestations, including concentric/asymmetric hypertrophy or dilated cardiomyopathy. Concentric/asymmetric hypertrophy is less common, but dilated cardiomyopathy with arrhythmia is more often associated with mortality compared to hypertrophic cardiomyopathy. The systolic function of FRDA patients tends to be low-normal and show an acute decline at the end of life. However, there is little data from long-term prospective studies of cardiac progression in these patients, and the cause of death is often attributed to heart failure and arrhythmia postmortem. As a marker of phenotypic disease severity, cardiomyopathy tends to be correlated with the clinical neurologic age of onset and the GAA triplet repeat length but not the duration of disease or the severity of neurologic symptoms[13]. However, the clinical determination of cardiac involvement is difficult, as most patients are wheelchair-bound within 15 years of diagnosis due to comorbidities associated with the systemic disease process.
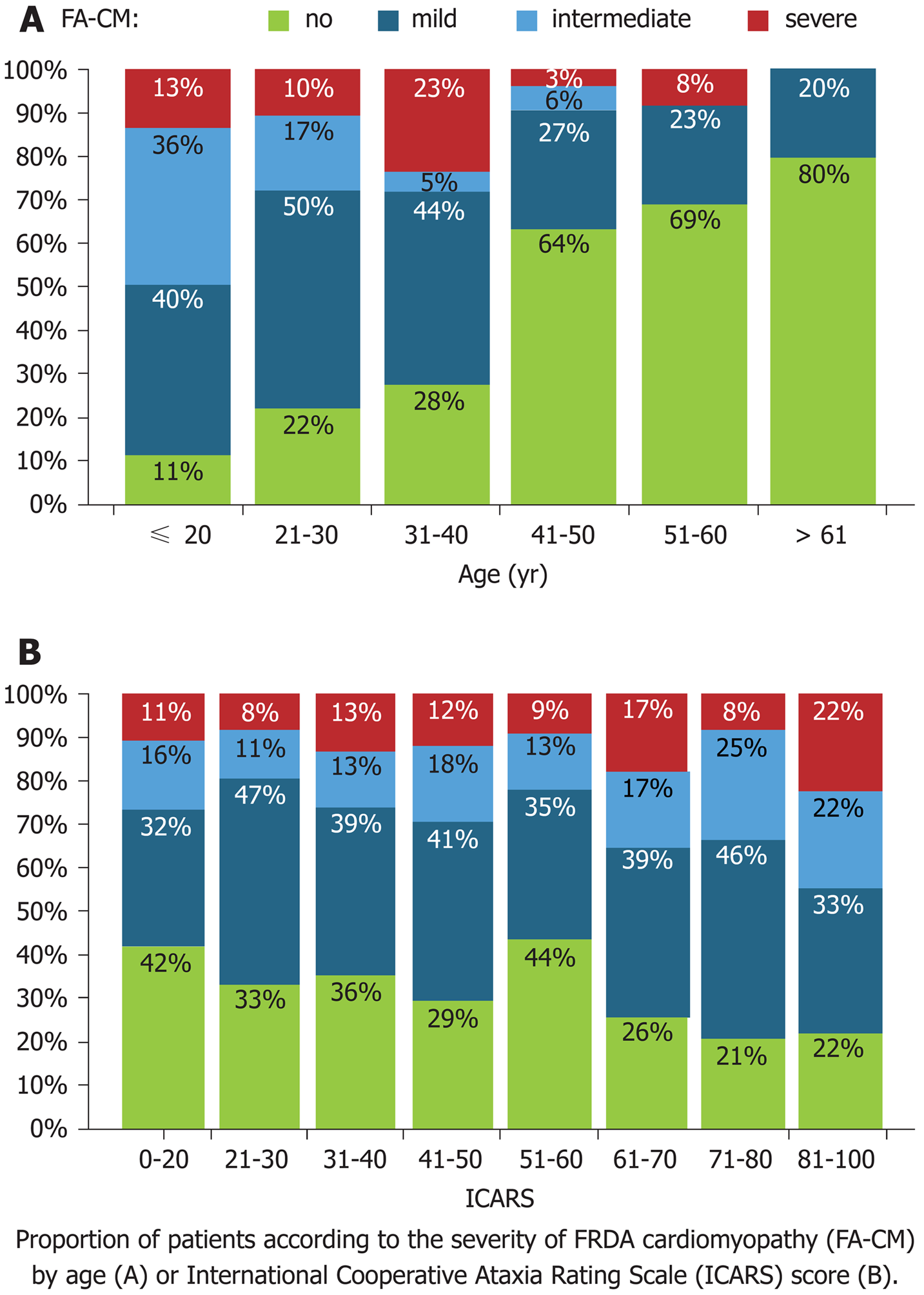
The cardiac diseases of FRDA patients include concentric LV hypertrophy, which leads to the most common causes of death, arrhythmia and heart failure, among these patients. Heart disease can be asymptomatic, and shortness of breath or palpitations are the most common clues. Early age of FRDA onset and GAA repeat length predict cardiac severity and worse LV hypertrophy, LV function, LV mass and eventual mortality, with most cardiac-related deaths occurring prior to age 40[13]. FRDA patients with cardiac-related death usually have a disease duration of 10 years or less, and a disease duration of greater than 20 years significantly reduces the predisposition to cardiac-related death[14] (Figure 3).
Asymmetric septal hypertrophy with an LV outflow gradient is uncommon in FRDA patients, and only a handful of reported cases have undergone septal myomectomy. In fact, the typical beta-blockade-based treatment for hypertrophic cardiomyopathy may be harmful in FRDA patients, given their loss of contractile fibers in the myocardium[15]. Dilated cardiomyopathy is also rare in FRDA; however, when present, it is accompanied by a more severe systolic dysfunction. It has been postulated that ventricular hypertrophy progresses to dilation with fibrotic replacement of myocardium in FRDA, but these observations may also represent different cardiac phenotypes[16,17].
Other cardiac abnormalities of FRDA include echocardiographic findings of a granular speckle-like appearance similar to that seen in amyloidosis, though without pericardial effusion or biatrial enlargement[18]. cMRI studies have detected subclinical LV fibrosis and concentric remodeling even prior to hypertrophy, along with a late gadolinium enhancement indicative of a decreased myocardial perfusion reserve[18]. As systolic dysfunction may indicate certain severe phenotypes late in the course of disease, longitudinal LV strain has been identified as a potential early marker of cardiomyopathy and systolic dysfunction. Although, once again, longitudinal studies of progression from longitudinal LV strain to systolic dysfunction are lacking[19]. These findings of early disease progression may have importance for identifying future therapeutic targets or developing methods to screen for cardiac disease that may otherwise progress undetected in asymptomatic patients who lack exertional symptoms of heart failure because they are non-ambulatory[20] (Figure 4).
The conduction disease seen in FRDA patients is thought to be a result of fibrotic myocardial replacement and scarring, which predispose the patient to atrioventricular conduction blocks and atrial or ventricular tachy- and bradyarrhythmias. Atrial arrhythmias, atrial flutter and atrial fibrillation occur in FRDA patients, but they are not frequent; ventricular arrhythmias are seen even less frequently. A cardiac pacemaker or defibrillator may benefit affected FRDA patients and should be implanted when indicated according to the American Heart Association and American College of Cardiology Guidelines for the general population.
In many cohort studies, T-wave repolarization abnormalities, especially in the inferior and lateral leads, were the most common abnormalities seen on the electrocardiogram (ECG) of FRDA patients (approximately 85%). Bundle branch blocks were found in 15% of FRDA patients. The QT interval tended to be normal, as did the QRS, indicating that there was a relatively low risk for ventricular tachyarrhythmias[10,13,15,18].
It is generally thought that the only increased risk factor for FRDA patients is a predisposition to diabetes mellitus due to beta islet cell disease of the pancreas. However, some histopathologic studies suggest that there is also an increased risk for coronary artery disease. Although one study found no occlusive coronary disease in postmortem patients[21], another study of three postmortem patients identified potential occlusive coronary disease in the microvasculature of the coronary arteries[20]. Fibrotic replacement of the coronary intima has also been identified, and it has been proposed that the coronary arteries of FRDA patients are susceptible to fibromuscular dysplasia. Inducible subendocardial defects detected by late enhancement gadolinium on cMRI indicate a reduced myocardial perfusion reserve as a source of ischemia that may warrant further study as a potential clinical correlate of microvascular coronary disease[20].
There has been debate regarding the progression of cardiac disease and the presence of variable phenotypes in FRDA. A small study of 28 FRDA patients over 5 years examined the TTE of these patients in childhood. Though LV systolic function was diminished in at least one examination, all were normal on subsequent examinations, leading the investigators to assume that the cardiac disease of these patients was stable until at least the age of 22 years[22]. The authors proposed that this cardiac disease progressed from hypertrophic to dilated cardiomyopathy and was not correlated with the GAA repeat length. A prospective open-label trial of 105 patients identified their baseline characteristics as hypertrophic cardiomyopathy with either septal or posterior wall asymmetric hypertrophy of the left ventricle. After treatment with high-dose idebenone, these patients experienced reductions in left ventricular mass with only small declines in their systolic function. Dilated cardiomyopathy was present in only one patient[23].
Notably, cross-sectional and retrospective data support the contention that there are clear differences in the clinical course and presence of systolic cardiomyopathy across FRDA patients. Investigators have proposed that various phenotypes reflect the GAA repeat length, although additional factors likely contribute to the differences seen in cardiac left ventricular hypertrophy, mass and dilatation. One study of 103 patients over a mean of 10 years found that the majority of patients (78%) were distributed in the low-risk group with normal LVEF at baseline and stable (ejection fraction) EF over time. In contrast, the LVEF of patients in the high-risk group (22%) declined an average of 21% over the 10-year period. Such phenotypes have already been observed and described as early- and late-onset FRDA[13].
Late-onset FRDA, which may be diagnosed beyond the criterion age of 25 years, includes a phenotypically variable group of patients who show later disease onset and slower progression compared to patients with early-onset FRDA. This finding emphasizes the need for a dynamic definition of the genetic and clinical criteria used to diagnose FRDA and suggests that the variable phenotypes may represent more than the simple expansion of the specific trinucleotide repeat sequence.
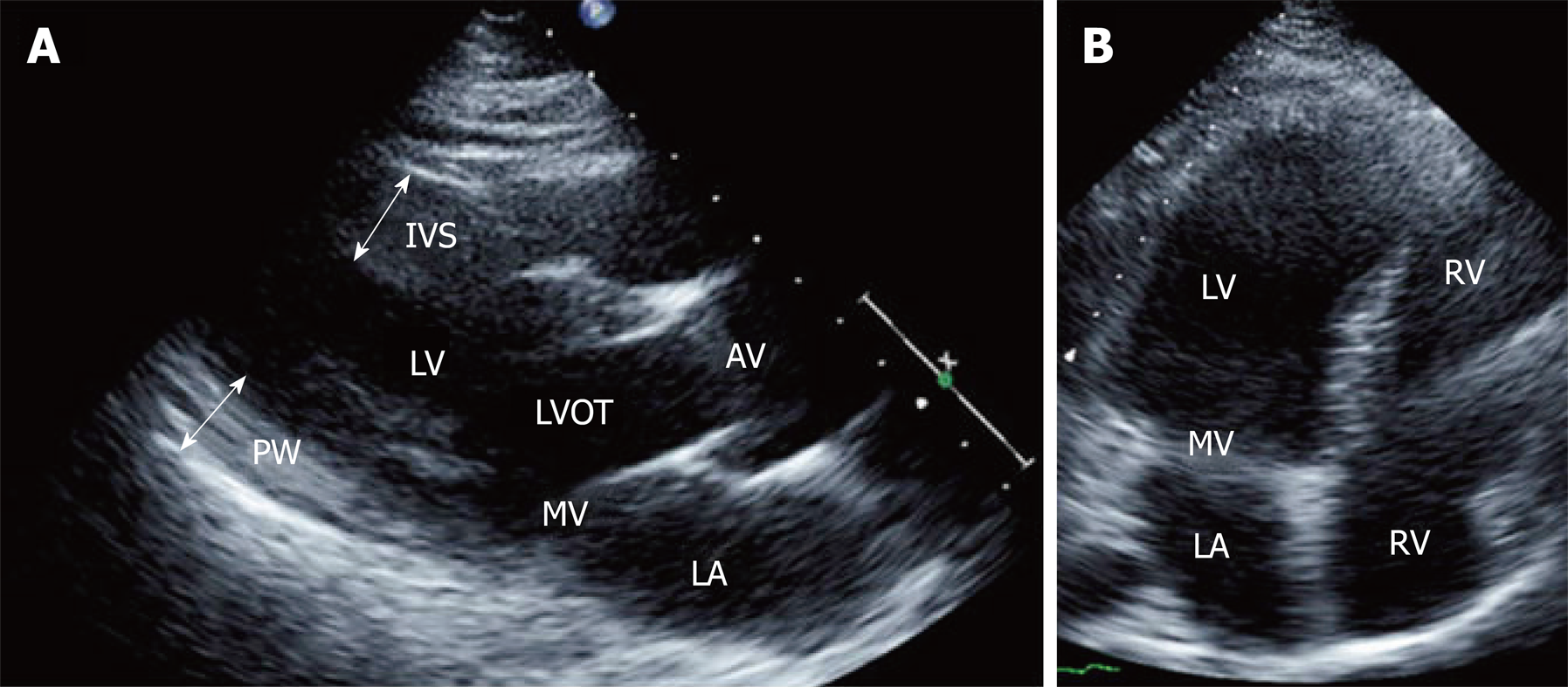
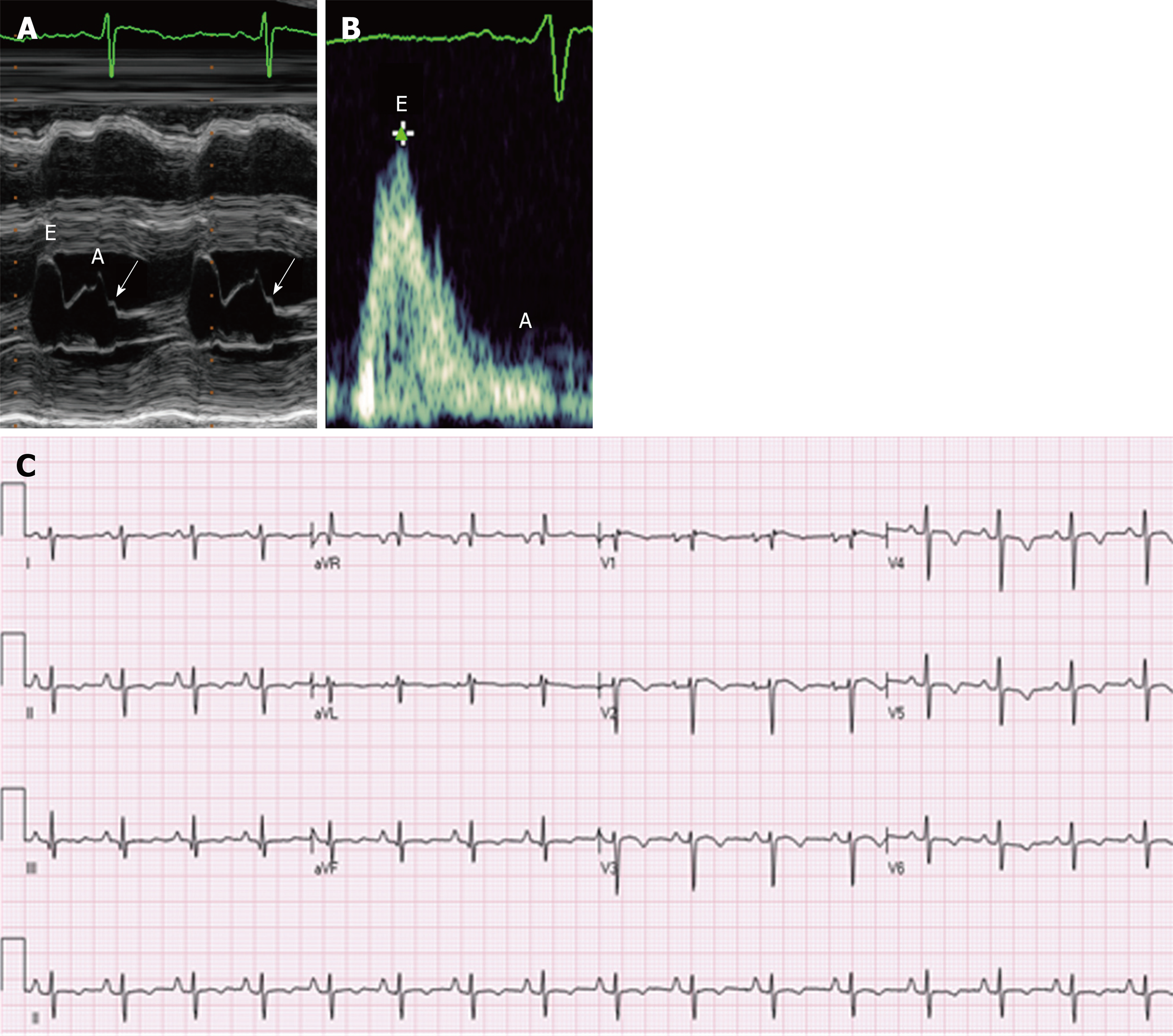
The cardiac abnormalities seen in one of our patients are illustrated in Figure 3. A 25-year-old female recently diagnosed with FRDA presented to the cardiology clinic with 6 months of dyspnea on exertion, chest pressure radiating to her neck and paroxysmal nocturnal dyspnea. An ECG showed T wave inversions in the precordial leads, which in the context of chest pain, were suggestive of coronary artery disease. A TTE showed a normal ejection fraction and left ventricular wall thickness, though diastolic dysfunction was present and showed a restrictive pattern (Figure 5).
Cardiac catheterization revealed normal coronary arteries but showed that the left ventricular end-diastolic pressure was severely elevated to 28 mmHg. The patient was diagnosed with left ventricular diastolic heart failure with a preserved ejection fraction.
Heart failure and sudden cardiac death are the most commonly reported causes of death among FRDA patients (Table 1)[24].
| Causes of death | Frequency | Percentage |
| Cardiac | 36 | 59.0 |
| CHF/cardiac failure | 18 | 28.5 |
| CHF complicated significant arrhythmia | 5 | 8.2 |
| Arrhythmia | 5 | 8.2 |
| Ischemic disease | 3 | 4.9 |
| Stroke (associated with atrial fibrillation or mural thrombus) | 4 | 6.6 |
| Other | 1 | 1.6 |
| Probable cardiac | 2 | 3.3 |
| Severe cardiomyopathy | 1 | 1.6 |
| Arrhythmia | 1 | 1.6 |
| Non-cardiac | 17 | 27.9 |
| Pneumonia | 6 | 9.8 |
| Sepsis | 1 | 1.6 |
| Renal failure | 1 | 1.6 |
| Breast cancer | 1 | 1.6 |
| Accidental drowning | 1 | 1.6 |
| Suicide | 1 | 1.6 |
| Other | 6 | 9.8 |
| Unknown | 6 | 9.8 |
Cardiomyopathy is thus important, especially in early-onset patients, who exhibit more severe cardiac disease. In addition, the progression to heart failure and deterioration of LVEF may be difficult to detect, as decreased systolic function usually occurs shortly before death, and this is difficult to correlate clinically given the lack of ambulation in most patients without correlation of neurologic severity to cardiac severity. Sudden progression of cardiac disease may not be detectable due to these comorbidities. Given the above issues, routine screening of the structural indicators of cardiomyopathy may be more valuable than the symptom review in FRDA patients, and the use of current imaging modalities may be indicated. A consensus statement proposed in 2014 for the multidisciplinary treatment of patients with FRDA recommended that ECG and echocardiography should be performed at the initial presentation and that patients should be referred to a cardiologist only for cardiac symptoms or abnormal cardiac testing[25]. However, we feel that patients should be screened at least annually with an ECG and a TTE. Moreover, cMRI (which can detect remodeling and decreased myocardial perfusion reserve) may prove useful in the future for the early detection of disease and/or monitoring the therapeutic response. The arrhythmias of FRDA patients are normally atrial in origin and may indicate the severity of left ventricular involvement rather than acting as a risk factor for sudden cardiac death due to arrhythmia. Although cMRI is not currently used to detect structural abnormalities in classifying heart failure, it may prove useful in this role, especially in situations (such as FRDA) where structural disease may be the best indicator of cardiac mortality risk.
The cardiomyopathy of FRDA has a unique pathogenesis, and specific targeted pharmaceuticals have yielded mixed results. Idebenone is a coenzyme Q10 analog that has been shown to have antioxidant activity and to facilitate mitochondrial phosphorylation as an electron carrier, which is important in mitochondrial function and energy production. Idebenone has improved cardiomyopathy in a very limited number of patients, as assessed by cMRI, but the clinical impact of this treatment has not yet been fully assessed[26]. Studies of shorter duration (e.g., 6 wk) failed to observe any benefit[26], and a review of the only two studies that were 12 mo or longer showed no change in baseline systolic function on an echocardiogram, although cMRI was not included as a measure in these randomized controlled trials[27]. MICONOS published an interim report in 2010 (the full study has not yet been reported) stating that, in their study, idebenone had not reached its primary endpoint of change with respect to the International Cooperative Ataxia Rating Scale (ICARS) score or cardiologic secondary endpoint. Idebenone has shown the potential to benefit hypertrophy in terms of septal wall thickness, posterior wall thickness and left ventricular mass (LVM) in open-label studies, but randomized controlled trials have not yet shown any clear benefit[26]. A study by Mariotti et al[27] showed that LVM was reduced over 12 mo in patients receiving idebenone compared to placebo, but the cohort was limited to 28 patients. Thus, idebenone has yielded mixed results with respect to cardiac function, and the clinical implication and timing of this pharmaceutical intervention have not yet been determined in detail.
One drawback of antioxidant or iron chelation therapy, in which plasma-bound iron is removed from the body, is the lack of evidence for cardiomyopathy reversal and convincing proof of clinical impact. Alternatively, gene therapy showed potential for reversing the biochemical, cellular and physiologic changes of FRDA cardiomyopathy in a mouse model, offering proof of concept[28]. Mice with a conditional knockout of frataxin in cardiac muscle show progressive and severe cardiomyopathy characterized by systolic dysfunction and increased left ventricular mass. Treatment of these mice with a vector that restored the capacity to transcribe frataxin rapidly normalized systolic cardiac function, halted histologic fibrosis and restored mitochondrial function in terms of iron accumulation, iron hemostatic protein levels and mitochondrial proliferation. When the mice of the asymptomatic mouse model were pretreated with this therapeutic vector, their cardiac function was indistinguishable from that of wild-type mice[28]. These findings suggest that such treatment might prevent cardiomyopathy in early-onset patients and reverse cardiomyopathy in the progressive cardiac disease associated with FRDA of any phenotype.
Cardiac transplantation was offered in approximately five FRDA patients between 2001 and 2011[29]. Some of these patients had both classic hypertrophy and dilated cardiomyopathy. In one case, the transplant occurred in a pediatric patient with cardiomyopathy who later developed FRDA. This is not surprising, as cardiomyopathy may present, even in a severe form, prior to the onset of neurologic disease[29]. The transplanted patients appeared to experience an arrest in the progression of neurologic disease and had some recovery of motor skills and strength. In this population, transplant decisions must be made with considerations of life span and comorbidities.
The cardiac disease of FRDA is variable in its clinical phenotype and severity, complicating its timely and appropriate diagnosis and treatment. The research has progressed since frataxin was first shown to play a role in cardiomyopathy, and new treatments using antioxidants and gene therapy have been trialed, most successfully in animal models. To prevent cardiac mortality in FRDA patients, we may need targeted treatments and management guidelines tailored to this unique mitochondrial pathology. Clinicians are urged to provide genetic testing for patients with highly suggestive clinical pictures, as well as yearly cardiac monitoring and counseling on treatment decisions for this patient population.
FRDA is the most prevalent inherited neuromuscular disorder associated with cardiomyopathy. It is caused by a genetic defect in a mitochondrial protein, frataxin, involving skeletal as well as cardiac muscle leading to physical incapacitation later in life. Interventricular posterior wall thickness was found to be the most sensitive echocardiographic criteria to determine the actual LV mass when compared to Cardiac MRI[30]. Cardiac function tends to be low normal initially in these patients, with an acute decline at the end of life. However neurological status cannot be determined by cardiomyopathy status as correlation was negative[30]. Several investigators have proposed criteria to stage cardiomyopathy based on several markers which were positive in up to two thirds of patients: (1) ECG abnormalities including supraventricular tachycardia and T wave inversion[30]; (2) fibrosis on Cardiac MRI and Hs TNT > 14 ng/mL[31]; and (3) reduction in both S’ and E’ by Tissue Doppler (Mott), which, however, did not demonstrate a consistent correlation with GAA repeats[32]. Furthermore, gene targeted therapies based on these studies have not been successful in reversing the progression of FRDA.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Anan R, Falconi M, Kharlamov AN, Pastromas S S- Editor: Ji FF L- Editor: A E- Editor: Song H
| 1. | Hewer R. The heart in Friedreich's ataxia. Br Heart J. 1969;31:5-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 75] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Harding AE. Friedreich's ataxia: a clinical and genetic study of 90 families with an analysis of early diagnostic criteria and intrafamilial clustering of clinical features. Brain. 1981;104:589-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 612] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 3. | Campuzano V, Montermini L, Lutz Y, Cova L, Hindelang C, Jiralerspong S, Trottier Y, Kish SJ, Faucheux B, Trouillas P, Authier FJ, Dürr A, Mandel JL, Vescovi A, Pandolfo M, Koenig M. Frataxin is reduced in Friedreich ataxia patients and is associated with mitochondrial membranes. Hum Mol Genet. 1997;6:1771-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 536] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 4. | Pandolfo M. Friedreich ataxia. Arch Neurol. 2008;65:1296-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 159] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 5. | Reetz K, Dogan I, Costa AS, Dafotakis M, Fedosov K, Giunti P, Parkinson MH, Sweeney MG, Mariotti C, Panzeri M, Nanetti L, Arpa J, Sanz-Gallego I, Durr A, Charles P, Boesch S, Nachbauer W, Klopstock T, Karin I, Depondt C, vom Hagen JM, Schöls L, Giordano IA, Klockgether T, Bürk K, Pandolfo M, Schulz JB. Biological and clinical characteristics of the European Friedreich's Ataxia Consortium for Translational Studies (EFACTS) cohort: a cross-sectional analysis of baseline data. Lancet Neurol. 2015;14:174-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 154] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 6. | Dürr A, Cossee M, Agid Y, Campuzano V, Mignard C, Penet C, Mandel JL, Brice A, Koenig M. Clinical and genetic abnormalities in patients with Friedreich's ataxia. N Engl J Med. 1996;335:1169-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 763] [Cited by in RCA: 705] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 7. | Labuda M, Labuda D, Miranda C, Poirier J, Soong BW, Barucha NE, Pandolfo M. Unique origin and specific ethnic distribution of the Friedreich ataxia GAA expansion. Neurology. 2000;54:2322-2324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Schulz JB, Boesch S, Bürk K, Dürr A, Giunti P, Mariotti C, Pousset F, Schöls L, Vankan P, Pandolfo M. Diagnosis and treatment of Friedreich ataxia: a European perspective. Nat Rev Neurol. 2009;5:222-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 176] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 9. | Ashley CN, Hoang KD, Lynch DR, Perlman SL, Maria BL. Childhood ataxia: clinical features, pathogenesis, key unanswered questions, and future directions. J Child Neurol. 2012;27:1095-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Payne RM, Wagner GR. Cardiomyopathy in Friedreich ataxia: clinical findings and research. J Child Neurol. 2012;27:1179-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Koeppen AH, Ramirez RL, Becker AB, Bjork ST, Levi S, Santambrogio P, Parsons PJ, Kruger PC, Yang KX, Feustel PJ, Mazurkiewicz JE. The pathogenesis of cardiomyopathy in Friedreich ataxia. PLoS One. 2015;10:e0116396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 12. | Pandolfo M, Pastore A. The pathogenesis of Friedreich ataxia and the structure and function of frataxin. J Neurol. 2009;256 Suppl 1:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 174] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 13. | Pousset F, Legrand L, Monin ML, Ewenczyk C, Charles P, Komajda M, Brice A, Pandolfo M, Isnard R, Tezenas du Montcel S, Durr A. A 22-Year Follow-up Study of Long-term Cardiac Outcome and Predictors of Survival in Friedreich Ataxia. JAMA Neurol. 2015;72:1334-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | Weidemann F, Scholz F, Florescu C, Liu D, Hu K, Herrmann S, Ertl G, Störk S. [Heart involvement in Friedreich's ataxia]. Herz. 2015;40 Suppl 1:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Payne RM, Peverill RE. Cardiomyopathy of Friedreich's ataxia (FRDA). Ir J Med Sci. 2012;181:569-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Casazza F, Morpurgo M. The varying evolution of Friedreich's ataxia cardiomyopathy. Am J Cardiol. 1996;77:895-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Regner SR, Lagedrost SJ, Plappert T, Paulsen EK, Friedman LS, Snyder ML, Perlman SL, Mathews KD, Wilmot GR, Schadt KA, Sutton MS, Lynch DR. Analysis of echocardiograms in a large heterogeneous cohort of patients with friedreich ataxia. Am J Cardiol. 2012;109:401-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Weidemann F, Störk S, Liu D, Hu K, Herrmann S, Ertl G, Niemann M. Cardiomyopathy of Friedreich ataxia. J Neurochem. 2013;126 Suppl 1:88-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 19. | St John Sutton M, Ky B, Regner SR, Schadt K, Plappert T, He J, D'Souza B, Lynch DR. Longitudinal strain in Friedreich Ataxia: a potential marker for early left ventricular dysfunction. Echocardiography. 2014;31:50-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Raman SV, Phatak K, Hoyle JC, Pennell ML, McCarthy B, Tran T, Prior TW, Olesik JW, Lutton A, Rankin C, Kissel JT, Al-Dahhak R. Impaired myocardial perfusion reserve and fibrosis in Friedreich ataxia: a mitochondrial cardiomyopathy with metabolic syndrome. Eur Heart J. 2011;32:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Raman SV, Dickerson JA, Al-Dahhak R. Myocardial ischemia in the absence of epicardial coronary artery disease in Friedreich's ataxia. J Cardiovasc Magn Reson. 2008;10:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Kipps A, Alexander M, Colan SD, Gauvreau K, Smoot L, Crawford L, Darras BT, Blume ED. The longitudinal course of cardiomyopathy in Friedreich's ataxia during childhood. Pediatr Cardiol. 2009;30:306-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Rustin P, Bonnet D, Rötig A, Munnich A, Sidi D. Idebenone treatment in Friedreich patients: one-year-long randomized placebo-controlled trial. Neurology. 2004;62:524-525; author reply 525; discussion 525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Tsou AY, Paulsen EK, Lagedrost SJ, Perlman SL, Mathews KD, Wilmot GR, Ravina B, Koeppen AH, Lynch DR. Mortality in Friedreich ataxia. J Neurol Sci. 2011;307:46-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 227] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 25. | Corben LA, Lynch D, Pandolfo M, Schulz JB, Delatycki MB; Clinical Management Guidelines Writing Group. Consensus clinical management guidelines for Friedreich ataxia. Orphanet J Rare Dis. 2014;9:184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 26. | Meier T, Buyse G. Idebenone: an emerging therapy for Friedreich ataxia. J Neurol. 2009;256 Suppl 1:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Mariotti C, Solari A, Torta D, Marano L, Fiorentini C, Di Donato S. Idebenone treatment in Friedreich patients: one-year-long randomized placebo-controlled trial. Neurology. 2003;60:1676-1679. [PubMed] |
| 28. | Perdomini M, Belbellaa B, Monassier L, Reutenauer L, Messaddeq N, Cartier N, Crystal RG, Aubourg P, Puccio H. Prevention and reversal of severe mitochondrial cardiomyopathy by gene therapy in a mouse model of Friedreich's ataxia. Nat Med. 2014;20:542-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 179] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 29. | Leonard H, Forsyth R. Friedreich's ataxia presenting after cardiac transplantation. Arch Dis Child. 2001;84:167-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Weidemann F, Rummey C, Bijnens B, Störk S, Jasaityte R, Dhooge J, Baltabaeva A, Sutherland G, Schulz JB, Meier T; Mitochondrial Protection with Idebenone in Cardiac or Neurological Outcome (MICONOS) study group. The heart in Friedreich ataxia: definition of cardiomyopathy, disease severity, and correlation with neurological symptoms. Circulation. 2012;125:1626-1634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 31. | Weidemann F, Liu D, Hu K, Florescu C, Niemann M, Herrmann S, Kramer B, Klebe S, Doppler K, Üçeyler N, Ritter CO, Ertl G, Störk S. The cardiomyopathy in Friedreich's ataxia - New biomarker for staging cardiac involvement. Int J Cardiol. 2015;194:50-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 32. | Mottram PM, Delatycki MB, Donelan L, Gelman JS, Corben L, Peverill RE. Early changes in left ventricular long-axis function in Friedreich ataxia: relation with the FXN gene mutation and cardiac structural change. J Am Soc Echocardiogr. 2011;24:782-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |