Published online Sep 26, 2018. doi: 10.4330/wjc.v10.i9.110
Peer-review started: March 29, 2018
First decision: April 23, 2018
Revised: June 28, 2018
Accepted: August 5, 2018
Article in press: August 5, 2018
Published online: September 26, 2018
Processing time: 185 Days and 8.2 Hours
To compare myocardial viability assessment accuracy of cardiac magnetic resonance imaging (CMR) compared to [18F]-fluorodeoxyglucose (FDG)- positron emission tomography (PET) depending on left ventricular (LV) function.
One-hundred-five patients with known obstructive coronary artery disease (CAD) and anticipated coronary revascularization were included in the study and examined by CMR on a 1.5T scanner. The CMR protocol consisted of cine-sequences for function analysis and late gadolinium enhancement (LGE) imaging for viability assessment in 8 mm long and contiguous short axis slices. All patients underwent PET using [18F]-FDG. Myocardial scars were rated in both CMR and PET on a segmental basis by a 4-point-scale: Score 1 = no LGE, normal FDG-uptake; score 2 = LGE enhancement < 50% of wall thickness, reduced FDG-uptake ( ≥ 50% of maximum); score 3 = LGE ≥ 50%, reduced FDG-uptake (< 50% of maximum); score 4 = transmural LGE, no FDG-uptake. Segments with score 1 and 2 were categorized “viable”, scores 3 and 4 were categorized as “non-viable”. Patients were divided into three groups based on LV function as determined by CMR: Ejection fraction (EF), < 30%: n = 45; EF: 30%-50%: n = 44; EF > 50%: n = 16). On a segmental basis, the accuracy of CMR in detecting myocardial scar was compared to PET in the total collective and in the three different patient groups.
CMR and PET data of all 105 patients were sufficient for evaluation and 5508 segments were compared in total. In all patients, CMR detected significantly more scars (score 2-4) than PET: 45% vs 40% of all segments (P < 0.0001). In the different LV function groups, CMR found more scar segments than PET in subjects with EF< 30% (55% vs 46%; P < 0.0001) and EF 30%-50% (44% vs 40%; P < 0.005). However, CMR revealed less scars than PET in patients with EF > 50% (15% vs 23%; P < 0.0001). In terms of functional improvement estimation, i.e., expected improvement after revascularization, CMR identified “viable” segments (score 1 and 2) in 72% of segments across all groups, PET in 80% (P < 0.0001). Also in all LV function subgroups, CMR judged less segments viable than PET: EF < 30%, 66% vs 75%; EF = 30%-50%, 72% vs 80%; EF > 50%, 91% vs 94%.
CMR and PET reveal different diagnostic accuracy in myocardial viability assessment depending on LV function state. CMR, in general, is less optimistic in functional recovery prediction.
Core tip: Both cardiac magnetic resonance imaging (CMR) and [18F]-fluorodeoxyglucose-positron emission tomography (PET) are considered standard methods and reliable in myocardial viability imaging in coronary artery disease. However, CMR in general detects more scar and is, therefore, less optimistic in functional recovery prediction. Moreover, CMR and PET reveal different diagnostic accuracy depending on left ventricular (LV) function state: Particularly in severe and moderate LV function impairment, where revascularization is performed to improve function, CMR detects more scar and less viable myocardium - most probably due to higher spatial resolution. This aspect has not been reported, yet. Irrespective of LV function, PET might overestimate the improvement of regional and global function after revascularization.
- Citation: Hunold P, Jakob H, Erbel R, Barkhausen J, Heilmaier C. Accuracy of myocardial viability imaging by cardiac MRI and PET depending on left ventricular function. World J Cardiol 2018; 10(9): 110-118
- URL: https://www.wjgnet.com/1949-8462/full/v10/i9/110.htm
- DOI: https://dx.doi.org/10.4330/wjc.v10.i9.110
Left ventricular (LV) dysfunction due to myocardial ischemia is one of the most common manifestations in chronic coronary artery disease (CAD), but does not necessarily represent non-viable, irreversibly injured tissue[1-4]. Although large multicenter studies such as the positron-emission-tomography (PET) and recovery following revascularization PARR-2 trial[5,6] or the surgical treatment for ischemic heart failure STICH-trial have been performed, even today there is no general consensus as to when assigning patients to either optimized medical treatment alone or to a combination of medical treatment plus revascularization procedures [(percutaneous coronary intervention or coronary artery bypass graft (CABG) surgery)][7-12]. Arriving at the optimal management strategy for these patients is a complex multifactorial process that includes not only viability but also processes such as ischemia and remodeling[4,7,8,13-15].
Currently, several imaging modalities are used for the evaluation of myocardial viability, each of them assessing different myocardial features: e.g., low-dose dobutamine stress echocardiography (DSE), nuclear techniques such as PET or single-photon-emission-computed-tomography (SPECT) as well as cardiac magnetic resonance imaging (CMR). Traditionally, nuclear techniques were regarded as gold standard for viability testing owing to their high sensitivity and negative predictive value (NPV) [e.g., fluorodeoxyglucose (FDG)-PET 92% and 87%, respectively][7,12,16,17]. For this purpose mainly three tracers are used evaluating cell membrane integrity, perfusion and intact mitochondria ([201Tl] thallium- or [99mTc] technetium-SPECT)[18] or maintained metabolism ([18F] FDG-PET)[2].
With recent advances of the hard and software, especially the introduction of non-breath-hold sequences and arrhythmia rejection protocols[19,20], CMR has become a versatile alternative to nuclear imaging, coming along with an excellent spatial resolution[17]. While late gadolinium enhancement (LGE) allows visualization of the transmural extent of the scar by achieving signal intensity differences of nearly 500% between irreversibly injured and viable myocardium[14,18,21], dobutamine stress CMR analyzes contractile reserve of dysfunctional myocardium similar to DSE[2,7]. Although the low specificity of LGE-CMR is well known, which is mainly attributable to the variable functional recovery in segments with LGE covering 25%-75% of the wall[1,14,22], its general high diagnostic accuracy for detecting myocardial scars has been proven in different studies[2,7,14,22-25]. However, so far, the value of CMR and PET has not been defined considering different LV function states. The aim of this study therefore was to evaluate the diagnostic accuracy of LGE-CMR viability assessment and PET and to compare them in patient groups with different LV functions.
The local institutional review board (Ethics Committee of the Medical Faculty, University Essen) approved the study protocol and informed written consent has been given by all participants.
Within 30 mo, 105 patients (87 men, 18 women; mean age, 61 ± 11 years) with known obstructive CAD as proven by catheter coronary angiography and indication for CABG surgery were enrolled in the study. All of them underwent nuclear myocardial viability testing for clinical indication. After completion of the nuclear imaging contrast-enhanced CMR was performed; all imaging examinations took place within 6 ± 4 d before scheduled CABG surgery.
CMR scans were performed on a 1.5T scanner (Magnetom Sonata, Siemens Medical Solutions, Erlangen, Germany). Patients were examined in supine position. The spine array coil (two elements) and a body flex phased array coil (two elements) were combined for signal reception.
The CMR protocol consisted of a LV functional study by an electrocardiography (ECG)-triggered breath-hold segmented steady-state free precession (SSFP) cine sequence [repetition time (TR)/echo time (TE), 3.0/1.5 ms; flip angle, 60°; bandwidth, 975 Hertz per pixel]. Slice thickness was 8 mm. At first, three standard long axis views were acquired (four-chamber view, two-chamber view, LV three-chamber view); thereafter, the entire LV was covered by contiguous short axis slices without interslice gap. LGE images were acquired after administration of 0.2 mmol/kg bw Gadolinium-DTPA (Magnevist™, Bayer AG, Leverkusen, Germany) at a flow rate of 2 mL/s. Again, three long and all short axis slices were scanned utilizing an established ECG-triggered segmented 2D inversion-recovery gradient-recalled echo sequence (TR/TE, 8/4 ms; flip angle, 25°) during breath-hold[21]. LGE images were acquired 8 to 15 min after contrast media injection. To null the signal of normal myocardium the inversion time (TI, non-selective inversion pulse) had to be manually adjusted between 200 and 260 ms. The rectangular field-of-view (FOV) provided an in-plane resolution of 1.6 × 1.3 mm2 for all sequences.
Two experienced radiologists, who were blinded to nuclear study results, analyzed all CMR data in a consensus reading. SSFP images were reviewed as cine-loops on an interactive workstation. LV volumetry was done using the ARGUS™ software (Siemens Medical Systems, Erlangen, Germany) by manual drawing of the endocardial contours on all short axes in end-diastolic and end-systolic phase including the papillary muscles to the LV lumen[26]. End-diastolic volumes (EDV) and end-systolic volumes (ESV) were measured by slice summation; ejection fraction (EF) was calculated using the equation: EF = (EDV - ESV)/EDV.
For quantification of myocardial viability, all short axis images were segmented using a 6-segment model. The LGE extent was assessed and quantified in each short axis segment by the 4-point scoring system given in Table 1. As recommended in the guidelines, a cutoff value of 50%-transmurality was set to discriminate myocardium with a chance to functionally recover after revascularization (“viable”, score 1 and 2) from myocardium without beneficial functional prognosis (“non-viable”, score 3 and 4)[15,27] .
| Score | CMR | PET |
| 1 | No enhancement | Normal FDG uptake |
| 2 | Enhancement < 50% of wall thickness | Reduced FDG uptake, ≥ 50% of maximum |
| 3 | Enhancement ≥ 50% of wall thickness | Reduced FDG uptake, < 50% of maximum |
| 4 | Transmural enhancement | No FDG uptake |
The PET study was done under fasting conditions (at least 4 h) and after oral administration of two doses of acipimox 500 mg (Olbemox™, Pharmacia, Erlangen, Germany) and 75 g of glucose. Imaging was performed using a high-resolution PET camera (Siemens ECAT HR+, Erlangen, Germany). Forty minutes after intravenous application of [18F]-FDG (370 MBq) PET data was acquired in a 2-dimensional fashion: (1) transmission scan (duration, 10 min) 60 min after injection; (2) emission scan (duration, 30 min). After attenuation and scatter correction the emission data was reconstructed in an iterative fashion (OSEM algorithm, 2 iterations, 32 subsets, Gauss filter FWHM 6 mm). Furthermore, the 2-dimensional data stack was reformatted into a 3-dimensional volume to create 8 mm long and short axis slices corresponding to the acquired MR data.
As with CMR data, myocardial FDG-uptake was evaluated using the same 4-point scale (Table 1) for each myocardial segment. Preserved or increased glucose utilization and subsequent FDG-uptake indicated cell survival, while reduced FDG-uptake defined myocardial scar.
For statistical analyses SPSS Statistics software (version 22, IBM Corp., Amonk, NY, United States) was used. Statistical significance was assumed with P < 0.05. Moreover, Bonferroni correction was performed, yielding an adapted level of significance of P = 0.008.
At first, 3 different patient groups were composed based on the global LV function as assessed by CMR: (1) severely impaired LV function (EF < 30%); (2) moderately decreased LV function (EF 30%-50%); and (3) non-compromised LV function (EF > 50%). After that, CMR viability scores were compared segment-based to PET scores in two ways: first, normal segments (score 1) and segments with any kind of scar (score 2-4) were analyzed. Secondly, according to data published by Kim et al[22], evaluation of segments with no or little scar (scores 1 and 2, “viable”), which are expected to improve after revascularization, were compared to segments with score 3 and 4 (“non-viable”). In each case, sensitivity, specificity, positive predictive value (PPV), NPV, and accuracy were evaluated in contingency tables for CMR as test variable compared to PET. Diagnostic accuracy of CMR and PET were compared for the three LV function groups separately. Parametric data is expressed as mean ± SD. Two-tailed Fisher’s exact test was applied to compare frequencies of scar detection (scores 2-4) and functional recovery estimation (scores 1 and 2) between CMR and PET. Cohen’s Kappa was calculated for the agreement of CMR and PET in detecting scar and functional recovery estimation.
All patients successfully finished the study protocol, therefore complete data sets of 105 subjects underwent analysis. Depending on the long axis diameter, the LV was covered in CMR by 8 to 14 short axis slices. The 3D PET data set was then separated accordingly into the same number of slices yielding a total of 5508 segments. Mean CMR volumetric measures were: EDV, 198 ± 69 mL (range, 63-386 mL) and ESV, 137 ± 66 mL (range, 24-316 mL), resulting in a mean EF of 34% ± 14% (range, 9%-78%). Forty-five patients had an EF < 30%, 44 patients 30%-50%, and 16 patients > 50%.
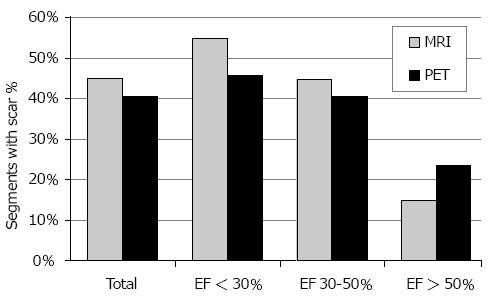
As demonstrated by Figure 1, CMR detected myocardial scars (score 2-4) in 45% of all segments, while PET depicted scars in 40% of all segments (P < 0.0001). Inter-observer agreement (Cohen’s Kappa) between CMR and PET in scar detection was 0.39 (fair to moderate). Analysis of the different patient groups revealed that CMR found more scars than PET in subjects with EF < 30% (55% vs 46%; P < 0.0001) and EF 30%-50% (44% vs 40%; P < 0.005). However, CMR revealed less scars than PET in patients with EF > 50% (15% vs 23%; P < 0.0001). Statistical values (sensitivity, specificity, PPV, NPV, and accuracy) within the 3 different patient groups are summarized in Table 2.
| Sensitivity | Specificity | PPV | NPV | Accuracy | |
| All patients | 69% | 71% | 62% | 77% | 70% |
| EF< 30% | 79% | 66% | 66% | 79% | 72% |
| EF 30%-50% | 64% | 69% | 58% | 74% | 67% |
| EF > 50% | 25% | 89% | 41% | 79% | 74% |
Viable segments which can be expected to improve after revascularization (score 1 and 2) were detected by CMR in 72% (3949/5508) compared to non-viable segments (score 3 and 4) in 28% (1559/5508). For PET, viability of segments was declared in 80% (4396/5508) and non-viability in 20% (1112/5508). CMR and PET significantly differed in depicting viable and non-viable segments (P < 0.0001). Inter-observer agreement (Cohen’s Kappa) between CMR and PET in functional recovery estimation was 0.48 (moderate). Analysis of the different patient groups Analysis of the different subgroups revealed that CMR judged segments as viable in 66% in patients with severely compromised LV function, in 72% in subjects with moderately reduced LV function and in 91% in patients with non-compromised LV function. For PET, these values were 75%, 80%, and 94% respectively. Comparison of CMR and PET showed that CMR declared significantly less segments as viable than PET in patients with severely or moderately reduced LV function (for all, P < 0.0001). In patients with uncompromised LV function no statistical significance was evident between both modalities after Bonferroni correction (P = 0.03).
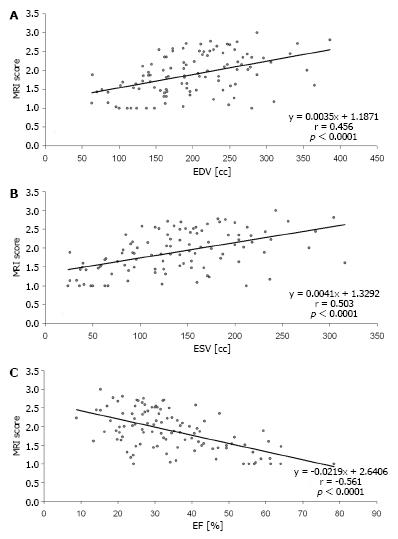
Table 3 provides statistical values for CMR in the estimation of functional recovery (MRI score 1 and 2) compared to PET. Comparison of Tables 2 and 3 reveals that overall performance of CMR was better in Table 3, when small scars (< 50% transmurality) were excluded. Figure 2 gives an example of a transmural scar detected both by CMR and PET as well as of small subendocardial scars that were found by CMR but overseen in PET. Figure 3 shows diagrams of the volumetric measures in relation to the total extent of scar (mean scar score).
| Sensitivity | Specificity | PPV | NPV | Accuracy | |
| All patients | 72% | 83% | 51% | 92% | 81% |
| EF < 30% | 74% | 79% | 55% | 90% | 78% |
| EF 30%-50% | 73% | 82% | 50% | 93% | 81% |
| EF > 50% | 30% | 92% | 19% | 95% | 89% |
Results from myocardial viability testing play an important role in clinical decision making especially in patients with impaired myocardial function, who might require invasive treatment. Albeit several studies reported on patients who benefited from restoration of blood flow despite pre-interventional proof of non-viable tissue and without post-operative functional recovery consequently[11,28,29], general consensus exists that information on the transmural extent of myocardial scar is of importance because it holds a close relationship with recovery of segmental function at follow-up[3]. We, therefore, sought to evaluate the diagnostic accuracy of LGE-CMR viability assessment and PET in patient groups with different LV functions. The main findings were: (1) in subjects with severely or moderately reduced LV function, CMR detects considerably more myocardial scars than PET; (2) scars, which are only seen in PET in patients with non-compromised LV function are probably false-positive results or artifacts; and (3) in patients with impaired LV function (EF < 50%), CMR demonstrates more non-viable myocardium compared to PET and is generally less optimistic concerning functional recovery after revascularization procedures.
Within the last decade CMR has more and more replaced nuclear imaging techniques in myocardial viability assessment. In particular, CMR seems to be useful in identifying patients, who most likely will not benefit from coronary revascularization[11]. Kühl et al[11] demonstrated that none of the segments which were classified as viable by PET/SPECT and nonviable by CMR showed functional recovery 6 mo after revascularization procedures, while 42% of segments described as viable by CMR and non-viable by PET/SPECT still improved[7].
To our knowledge, the present study is the first to compare CMR and PET differentiated in groups depending on LVEF. In patients with severely or moderately reduced LVEF, our study revealed a high sensitivity, specificity and NPV, indicating that CMR is good in detecting myocardial scars. Especially, the high NPV is of great importance in decision-making: if CMR indicates non-viable tissue, low likelihood for functional improvement after revascularization would be expected, which might prevent patients from unnecessary invasive procedures and potential peri-interventional risks. Moreover, CMR demonstrated considerably more nonviable myocardial tissue than PET with better sensitivity, PPV, NPV and accuracy (excluding small scars with < 50% transmural extent, score 2; Tables 2 and 3). One reason for that most probably is the significantly higher spatial resolution of CMR compared to PET, which is of benefit in finding subendocardial scars and in analyzing the thinned myocardial walls in subjects with severely reduced LVEF. Another explanation might be related to the fact that FDG-uptake represents viability, so that small amounts of viable cells lead to visible FDG-uptake indicating viability[11,29,30], although structural changes may already be present (e.g., expansion of extracellular spaces), leading to altered gadolinium kinetics. In addition to that, PET evaluates myocardial viability semiquantitatively: FDG-uptake in a given segment is expressed relative to the segment with maximum FDG-uptake. As a consequence, in thinned myocardium already small rims of reduced FDG-uptake may decrease relative percentage of FDG-uptake to below the threshold value set for viability, albeit viable tissue exists[11], resulting in false-negative evaluation of myocardial viability.
PET detected more scars in subjects with noncompromised LVEF (> 50%), similar to what Klein et al[30] described in their study. These surplus segments seen in PET only are most likely false-positive results or artifacts: As LVEF is only marginally or not impaired, only small amounts of non-viable subendocardial tissue are expected. Larger scars would have had more impact on myocardial function. However, a considerable number of non-viable cells is needed for detectable reduction of the relative FDG-uptake below the threshold-value considered for viability, which seems less probable in small scars[31-33]. Owing to the lower spatial resolution of PET it appears unlikely that these small scars were depicted by PET with higher sensitivity than by CMR[3,17]. This is further underlined by studies describing that more than half of the small subendocardial scars depicted by CMR cannot be delineated in PET[30]. Moreover, a minimum of 2 g irreversibly injured myocardium can be detected by LGE-CMR compared to a minimum tissue of 10 g required in PET[33]. Because of that recent studies have denominated LGE-CMR as method of choice for small subendocardial unrecognized myocardial infarction scars[34,35]. Other shortcomings of PET are its radiation exposure, the long examination time and the allocation of appropriate tracers[11]. CMR has evolved as a valuable alternative to PET for evaluation of viable and infarcted myocardium by different techniques (morphology, edema, function, perfusion and scar) in a single examination[2]. Estimated examination time for a complete CMR work-up is 30-60 min. Whether the recently emerging combination of PET and CMR (PET/CMR-hybrid) might be an alternative to CMR alone in assessment of myocardial viability will be the task of future studies[36,37].
We are aware of the following limitations of our study: First, the possibility of anatomical misalignment between different imaging modalities cannot be excluded. Within the last years, hybrid PET/CT-systems had increasingly replaced single PET-scanners. However, as published in a review by Anagnostopoulos et al[38] the impact of PET/CT-imaging on clinical outcomes in patients suffering from CAD is still unclear. They therefore recommended that until further studies are performed anatomical or functional imaging should be done sequentially by cardiac CT or PET depending on the pre-test probability of CAD[38]. In patients with higher probability PET should be the first line modality as its ability to guide patient management decisions regarding revascularization or medical treatment has been shown[39]. Therefore, we think that our results can be regarded representative, even though we are aware of the limitations of comparing semi-quantitative assessment of radiotracer uptake in PET as comparator for evaluating LGE-CMR. Furthermore, we defined myocardial scar as area with decreased FDG-uptake, even though reduced FDG-uptake might also be caused by stunned myocardium. The most appropriate approach would have been to perform both a functional and perfusion scan, in which stunned myocardium would have been detected as area with reduced FDP-uptake but normal perfusion (perfusion-metabolism reverse mismatch)[33]. However, for the given clinical indication in our patients the functional scan was sufficient and therefore no perfusion data was available. Another technical limitation might be that we did not use a glucose-insulin clamp to standardize the glucose metabolism within the whole myocardium. Like others[36,37], in our institution PET imaging is done under fasting condition and oral administration of two doses of acipimox and 75 g glucose prior to FDG application. Therefore, we believe the number of false-negative segments to be negligible. However, the lack of clinical approval of acipimox in the United States hampers representativeness of our data abroad. Moreover, no correction technique was applied for PET. And finally, a detailed segment-to-segment attenuation comparison was only partly done in our study, but would have been of interest to get deeper information on exact differences between PET and CMR regarding scar detection, e.g., considering the location of scar keeping in mind that the exact delineation of the inferior LV segments is often impaired in PET[30,40]. This has to be investigated by further studies.
In conclusion, our study demonstrates differences in diagnostic accuracy of CMR and PET differentiating between patient groups according to LV ejection fraction. Advantages of CMR compared to PET were found in detecting scars and non-viable tissue in subjects with severely or moderately reduced LVEF. CMR is generally less optimistic concerning functional recovery after revascularization procedures, which is of great importance in clinical decision-making.
Cardiac magnetic resonance imaging (CMR) and positron emission tomography (PET) have been established for myocardial viability imaging in coronary artery disease (CAD). However, differences in accuracy have been reported. It has been shown that CMR provides higher sensitivity in detecting small scars due to the significantly higher spatial resolution. So far, no data are available on differences in diagnostic accuracy depending on left ventricular (LV) function although it might be suggested that LV volumes and wall thickness, for example, might have an impact on the sensitivity.
The above mentioned missing data have been collected in our large study and have now been made available. This study might help to better understand the advantages and disadvantages of the two different methods.
The primary objective of this research was to compare contrast-enhanced CMR and fluorodeoxyglucose-PET for the evaluation of myocardial viability in known CAD under different LV function conditions.
One-hundred-five CAD patients were examined by CMR and PET. Myocardial scars were rated in both CMR and PET on a segmental basis in each 8 mm thick short axis slice concerning presence and extent of myocardial scar after myocardial infarction. For each of the evaluated 5518 segments, direct comparison was performed and three patient groups with different LV function were analyzed. In particular two aspects, diagnostic accuracy has been evaluated: (1) scar detection; and (2) functional improvement estimation by the two methods.
As expected, CMR has a higher sensitivity for scar detection and, therefore, is less optimistic than PET in the prediction of functional recovery after revascularization. In the different LV function groups, CMR found more scar segments than PET in subjects with EF < 30% and EF 30%-50% (44% vs 40 %; P < 0.005), whereas CMR revealed less scars than PET in patients with EF > 50%.
There are differences in the diagnostic accuracy between both modalities that have not been described, yet. This new knowledge helps to understand the strengths and weaknesses of the two modalities. One should keep in mind that particularly in severely impaired LV function - where viability really matters - CMR is able to detect more scars. In those cases, using CMR instead of PET could prevent unnecessary revascularizations and accompanying complications.
This study could initiate more research on particular myocardial viability imaging aspects to better sort outpatient conditions that influence the accuracy of available techniques.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Nacak M, Tomizawa N S- Editor: Ji FF L- Editor: A E- Editor: Wu YXJ
| 1. | Tomlinson DR, Becher H, Selvanayagam JB. Assessment of myocardial viability: comparison of echocardiography versus cardiac magnetic resonance imaging in the current era. Heart Lung Circ. 2008;17:173-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Grover S, Srinivasan G, Selvanayagam JB. Evaluation of myocardial viability with cardiac magnetic resonance imaging. Prog Cardiovasc Dis. 2011;54:204-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Wu YW, Tadamura E, Kanao S, Yamamuro M, Marui A, Komeda M, Toma M, Kimura T, Togashi K. Myocardial viability by contrast-enhanced cardiovascular magnetic resonance in patients with coronary artery disease: comparison with gated single-photon emission tomography and FDG position emission tomography. Int J Cardiovasc Imaging. 2007;23:757-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Shabana A, El-Menyar A. Myocardial viability: what we knew and what is new. Cardiol Res Pract. 2012;2012:607486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Abraham A, Nichol G, Williams KA, Guo A, deKemp RA, Garrard L, Davies RA, Duchesne L, Haddad H, Chow B. 18F-FDG PET imaging of myocardial viability in an experienced center with access to 18F-FDG and integration with clinical management teams: the Ottawa-FIVE substudy of the PARR 2 trial. J Nucl Med. 2010;51:567-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 6. | Beanlands RS, Nichol G, Huszti E, Humen D, Racine N, Freeman M, Gulenchyn KY, Garrard L, deKemp R, Guo A. F-18-fluorodeoxyglucose positron emission tomography imaging-assisted management of patients with severe left ventricular dysfunction and suspected coronary disease: a randomized, controlled trial (PARR-2). J Am Coll Cardiol. 2007;50:2002-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 334] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 7. | Schinkel AF, Bax JJ, Poldermans D, Elhendy A, Ferrari R, Rahimtoola SH. Hibernating myocardium: diagnosis and patient outcomes. Curr Probl Cardiol. 2007;32:375-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 256] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 8. | Ling LF, Marwick TH, Flores DR, Jaber WA, Brunken RC, Cerqueira MD, Hachamovitch R. Identification of therapeutic benefit from revascularization in patients with left ventricular systolic dysfunction: inducible ischemia versus hibernating myocardium. Circ Cardiovasc Imaging. 2013;6:363-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 9. | Tarakji KG, Brunken R, McCarthy PM, Al-Chekakie MO, Abdel-Latif A, Pothier CE, Blackstone EH, Lauer MS. Myocardial viability testing and the effect of early intervention in patients with advanced left ventricular systolic dysfunction. Circulation. 2006;113:230-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 113] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 10. | Slart RH, Bax JJ, van Veldhuisen DJ, van der Wall EE, Dierckx RA, de Boer J, Jager PL. Prediction of functional recovery after revascularization in patients with coronary artery disease and left ventricular dysfunction by gated FDG-PET. J Nucl Cardiol. 2006;13:210-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Kühl HP, Lipke CS, Krombach GA, Katoh M, Battenberg TF, Nowak B, Heussen N, Buecker A, Schaefer WM. Assessment of reversible myocardial dysfunction in chronic ischaemic heart disease: comparison of contrast-enhanced cardiovascular magnetic resonance and a combined positron emission tomography-single photon emission computed tomography imaging protocol. Eur Heart J. 2006;27:846-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Uebleis C, Hellweger S, Laubender RP, Becker A, Sohn HY, Lehner S, Haug A, Bartenstein P, Cumming P, Van Kriekinge SD. The amount of dysfunctional but viable myocardium predicts long-term survival in patients with ischemic cardiomyopathy and left ventricular dysfunction. Int J Cardiovasc Imaging. 2013;29:1645-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Schinkel AF, Valkema R, Geleijnse ML, Sijbrands EJ, Poldermans D. Single-photon emission computed tomography for assessment of myocardial viability. EuroIntervention. 2010;6 Suppl G:G115-G122. [PubMed] |
| 14. | Selvanayagam JB, Kardos A, Francis JM, Wiesmann F, Petersen SE, Taggart DP, Neubauer S. Value of delayed-enhancement cardiovascular magnetic resonance imaging in predicting myocardial viability after surgical revascularization. Circulation. 2004;110:1535-1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 240] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 15. | Nagel E, Schuster A. Myocardial viability: dead or alive is not the question! JACC Cardiovasc Imaging. 2012;5:509-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Bax JJ, Poldermans D, Elhendy A, Boersma E, Rahimtoola SH. Sensitivity, specificity, and predictive accuracies of various noninvasive techniques for detecting hibernating myocardium. Curr Probl Cardiol. 2001;26:147-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 137] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 17. | Nekolla SG, Martinez-Moeller A, Saraste A. PET and MRI in cardiac imaging: from validation studies to integrated applications. Eur J Nucl Med Mol Imaging. 2009;36 Suppl 1:S121-S130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Weinsaft JW, Klem I, Judd RM. MRI for the assessment of myocardial viability. Magn Reson Imaging Clin N Am. 2007;15:505-525, v-vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Uecker M, Zhang S, Voit D, Karaus A, Merboldt KD, Frahm J. Real-time MRI at a resolution of 20 ms. NMR Biomed. 2010;23:986-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 263] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 20. | Zhang S, Uecker M, Voit D, Merboldt KD, Frahm J. Real-time cardiovascular magnetic resonance at high temporal resolution: radial FLASH with nonlinear inverse reconstruction. J Cardiovasc Magn Reson. 2010;12:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | Simonetti OP, Kim RJ, Fieno DS, Hillenbrand HB, Wu E, Bundy JM, Finn JP, Judd RM. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218:215-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1038] [Cited by in RCA: 986] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 22. | Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2376] [Cited by in RCA: 2211] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 23. | Mahrholdt H, Klem I, Sechtem U. Cardiovascular MRI for detection of myocardial viability and ischaemia. Heart. 2007;93:122-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Romero J, Xue X, Gonzalez W, Garcia MJ. CMR imaging assessing viability in patients with chronic ventricular dysfunction due to coronary artery disease: a meta-analysis of prospective trials. JACC Cardiovasc Imaging. 2012;5:494-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 25. | Hunold P, Brandt-Mainz K, Freudenberg L, Vogt FM, Neumann T, Knipp S, Barkhausen J. [Evaluation of myocardial viability with contrast-enhanced magnetic resonance imaging--comparison of the late enhancement technique with positronemission tomography]. Rofo. 2002;174:867-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Miller S, Simonetti OP, Carr J, Kramer U, Finn JP. MR Imaging of the heart with cine true fast imaging with steady-state precession: influence of spatial and temporal resolutions on left ventricular functional parameters. Radiology. 2002;223:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 124] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E; Society for Cardiovascular Magnetic Resonance Board of Trustees Task Force on Standardized Protocols. Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update. J Cardiovasc Magn Reson. 2013;15:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 543] [Cited by in RCA: 552] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 28. | Hombach V, Merkle N, Bernhard P, Rasche V, Rottbauer W. Prognostic significance of cardiac magnetic resonance imaging: Update 2010. Cardiol J. 2010;17:549-557. [PubMed] |
| 29. | Samady H, Elefteriades JA, Abbott BG, Mattera JA, McPherson CA, Wackers FJ. Failure to improve left ventricular function after coronary revascularization for ischemic cardiomyopathy is not associated with worse outcome. Circulation. 1999;100:1298-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 144] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Klein C, Nekolla SG, Bengel FM, Momose M, Sammer A, Haas F, Schnackenburg B, Delius W, Mudra H, Wolfram D. Assessment of myocardial viability with contrast-enhanced magnetic resonance imaging: comparison with positron emission tomography. Circulation. 2002;105:162-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 382] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 31. | Wang L, Yan C, Zhao S, Fang W. Comparison of (99m)Tc-MIBI SPECT/18F-FDG PET imaging and cardiac magnetic resonance imaging in patients with idiopathic dilated cardiomyopathy: assessment of cardiac function and myocardial injury. Clin Nucl Med. 2012;37:1163-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Wagner A, Mahrholdt H, Holly TA, Elliott MD, Regenfus M, Parker M, Klocke FJ, Bonow RO, Kim RJ, Judd RM. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet. 2003;361:374-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 970] [Cited by in RCA: 891] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 33. | Rischpler C, Langwieser N, Souvatzoglou M, Batrice A, van Marwick S, Snajberk J, Ibrahim T, Laugwitz KL, Nekolla SG, Schwaiger M. PET/MRI early after myocardial infarction: evaluation of viability with late gadolinium enhancement transmurality vs. 18F-FDG uptake. Eur Heart J Cardiovasc Imaging. 2015;16:661-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 34. | Achenbach S, Barkhausen J, Beer M, Beerbaum P, Dill T, Eichhorn J, Fratz S, Gutberlet M, Hoffmann M, Huber A. Consensus recommendations of the German Radiology Society (DRG), the German Cardiac Society (DGK) and the German Society for Pediatric Cardiology (DGPK) on the use of cardiac imaging with computed tomography and magnetic resonance imaging. Rofo. 2012;184:345-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Krumm P, Zitzelsberger T, Weinmann M, Mangold S, Rath D, Nikolaou K, Gawaz M, Kramer U, Klumpp BD. Cardiac MRI left ventricular global function index and quantitative late gadolinium enhancement in unrecognized myocardial infarction. Eur J Radiol. 2017;92:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Nensa F, Poeppel T, Tezgah E, Heusch P, Nassenstein K, Mahabadi AA, Forsting M, Bockisch A, Erbel R, Heusch G. Integrated FDG PET/MR Imaging for the Assessment of Myocardial Salvage in Reperfused Acute Myocardial Infarction. Radiology. 2015;276:400-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 37. | Nensa F, Schlosser T. Cardiovascular hybrid imaging using PET/MRI. Rofo. 2014;186:1094-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Anagnostopoulos C, Georgakopoulos A, Pianou N, Nekolla SG. Assessment of myocardial perfusion and viability by positron emission tomography. Int J Cardiol. 2013;167:1737-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | Rohatgi R, Epstein S, Henriquez J, Ababneh AA, Hickey KT, Pinsky D, Akinboboye O, Bergmann SR. Utility of positron emission tomography in predicting cardiac events and survival in patients with coronary artery disease and severe left ventricular dysfunction. Am J Cardiol. 2001;87:1096-1099, A6. [PubMed] |
| 40. | Slart RH, Bax JJ, van Veldhuisen DJ, van der Wall EE, Dierckx RA, Jager PL. Imaging techniques in nuclear cardiology for the assessment of myocardial viability. Int J Cardiovasc Imaging. 2006;22:63-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |