Published online Mar 26, 2018. doi: 10.4330/wjc.v10.i3.15
Peer-review started: November 24, 2017
First decision: December 27, 2017
Revised: January 12, 2018
Accepted: February 6, 2018
Article in press: February 6, 2018
Published online: March 26, 2018
Processing time: 119 Days and 3 Hours
It is well known that calcium channel blockers (CCBs) are the first line of therapy for vasospastic angina (VSA). Here, we report two cases of VSA with an increase in the frequency of angina attacks after switching from a brand-name to a generic CCB. In both cases, angina recurred upon switching from a brand-name CCB to a generic CCB during follow-up. The patients’ condition improved upon switching back to the original CCB. Both cases involved a high severity of VSA, based on the results of spasm provocation testing. These findings suggest that, in some patients with severe VSA, the frequency of angina attacks increases when switching from a brand-name CCB to a generic CCB. Cardiologists should consider this factor when prescribing drugs for angina.
Core tip: Calcium-channel blockers (CCBs) are the first line of therapy for vasospastic angina (VSA). Here, we report two cases of VSA with an increase in the frequency of angina attacks after switching from a brand-name to a generic CCB. Switching back to the original CCB improved the patients’ condition in both cases. Both cases involved highly severe VSA. It is important for cardiologists to check whether VSA patients who have refractory angina attacks while taking a CCB are taking a brand-name or generic CCB.
- Citation: Goto-Semba R, Fujii Y, Ueda T, Oshita C, Teragawa H. Increased frequency of angina attacks caused by switching a brand-name vasodilator to a generic vasodilator in patients with vasospastic angina: Two case reports. World J Cardiol 2018; 10(3): 15-20
- URL: https://www.wjgnet.com/1949-8462/full/v10/i3/15.htm
- DOI: https://dx.doi.org/10.4330/wjc.v10.i3.15
Vasospastic angina (VSA) is caused by myocardial ischemia due to sudden excessive vasoconstriction of the epicardial coronary arteries[1,2]. It has been demonstrated that coronary spasm causes not only unstable angina but also exertional angina, acute coronary syndrome, and acute sudden death[1,2]. In every patient, it is important to assess whether coronary spasm is associated with the patients’ symptoms or with their pathology. VSA is generally diagnosed on the basis of a combination of characteristic chest symptoms and transient ST-T segment changes on the electrocardiogram (ECG)[3]. However, our personal experience is that in many patients, the diagnosis of VSA cannot be made based on these findings. In such cases, the spasm provocation test (SPT) has been very effective in making a correct diagnosis of VSA[4,5]. The treatment for VSA is based on life-style management and drug therapy. The use of calcium-channel blockers (CCBs) to prevent VSA is recommended as the first line of therapy[3].
Generic drugs are identical to, but substantially less expensive than their brand-name counterparts with respect to the dosage, strength, route of administration, quality, performance characteristics, and intended use[6]. Thus, the use of generic drugs can help reduce overall medical expenses, and their market share is expanding even in Japan. In general, the effects of brand-name and generic drugs are identical[6,7]. However, the effects may not be applicable for all patients and may differ according to the patients’ disease severity and/or subtype.
Here, we present two cases of VSA with increase in the frequency of angina attacks after switching from a brand-name CCB to a generic CCB.
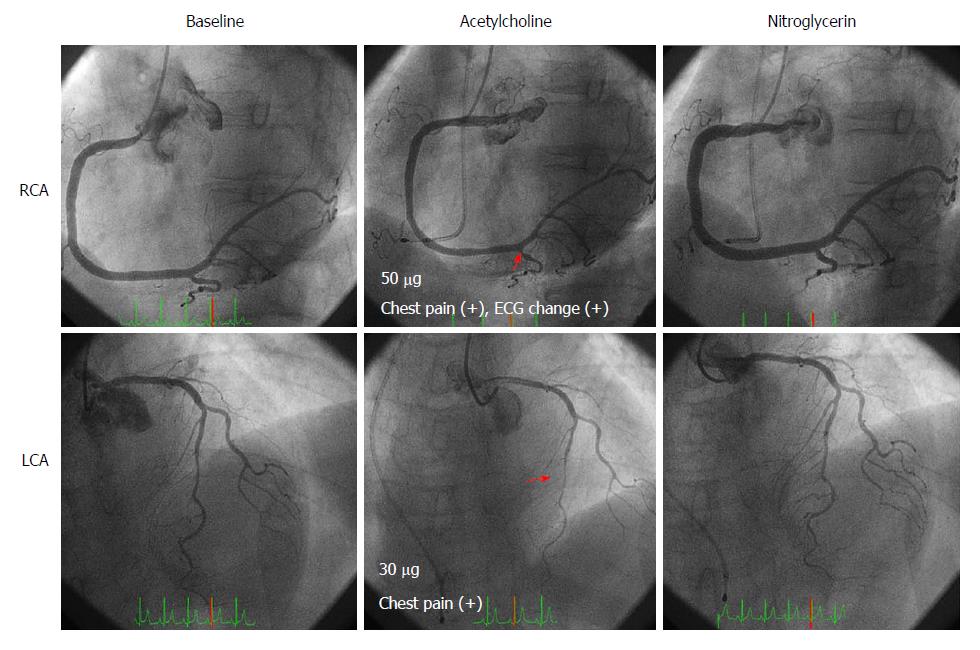
A 70-year-old woman experienced chest pain at rest. She was admitted to our institution to undergo coronary angiography (CAG) because her cardiac CT scan indicated coronary artery stenosis. On admission, her vitals were stable. Blood testing revealed hyperlipidemia. ECG, echocardiography, and chest radiography showed no specific findings. CAG showed no significant coronary stenosis, and subsequent SPT using acetylcholine (ACh) revealed bilateral spasm at the distal segment of the right coronary artery (RCA) at a dose of 50 µg of ACh, and a focal spasm at the mid-segment of the left anterior descending (LAD) coronary artery at a dose of 30 µg of ACh. Intracoronary infusions of nitroglycerin relieved the bilateral coronary spasms (Figure 1). She was diagnosed with VSA and was prescribed diltiazem hydrochloride (100 mg capsule, BID) and pitavastatin calcium hydrate (2 mg tablet, QD). After 10 mo, isosorbide dinitrate patches (40 mg patch, QD) were added because her chest pain recurred four times a month. After that, however, she felt discomfort due to dermal isosorbide dinitrate, so the patches were discontinued and she was switched from diltiazem hydrochloride to benidipine hydrochloride (4 mg tablet, BID), which improved her anginal symptoms. After 4 mo, she switched from benidipine hydrochloride to a generic CCB. At that time, she began to have angina attacks four to five times a month. Therefore, we switched her back to the brand-name CCB, and she has not experienced chest pain since (Table 1).
| Time course | Frequency of chest pain | Cardiovascular drug regimen |
| Discharge | None | Diltiazem hydrochloride (brand-name) 100 mg capsule, BID |
| Pitavastatin calcium (brand-name) 2 mg tablet, QD | ||
| 10 mo later | 4/mo | Diltiazem hydrochloride (brand-name) 100 mg capsule, BID |
| Pitavastatin calcium (brand-name) 2 mg tablet, QD | ||
| Isosorbide dinitrate (brand-name) 40 mg tape, QD | ||
| 11 mo later | None, but nausea (+) | Benidipine hydrochloride (brand-name) 4 mg tablet, QD |
| Pitavastatin calcium (brand-name) 2mg tablet, QD | ||
| 12 mo later | None | Benidipine hydrochloride (brand-name) 4 mg tablet, QD |
| Pitavastatin calcium (brand-name) 2 mg tablet, QD | ||
| 14 mo later | None | Benidipine hydrochloride (generic) 4 mg tablet, QD |
| Pitavastatin calcium (brand-name) 2 mg tablet, QD | ||
| 15 mo later | 4-5/mo | Benidipine hydrochloride (brand-name) 4 mg tablet, QD |
| Pitavastatin calcium (brand-name) 2 mg tablet, QD | ||
| Thereafter | None | Benidipine hydrochloride (brand-name) 4 mg tablet, QD |
| Pitavastatin calcium (brand-name) 2 mg tablet, QD |
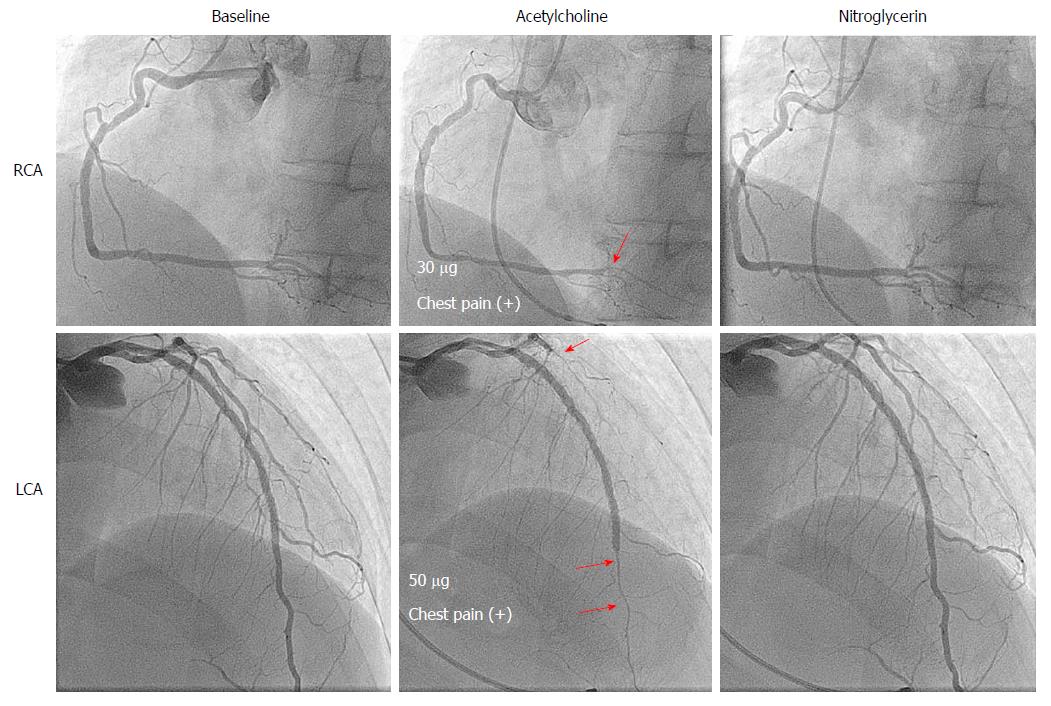
A 50-year-old man, who had previously undergone percutaneous coronary artery stenting for unstable angina and was also diagnosed as having VSA, was treated with many kinds of vasodilators. He was admitted to our institution to undergo CAG due to increased frequency of chest pain at night and during early mornings, with an overall frequency of three or four times per week over several months. His coronary risk factors included smoking (40/d × 30 years) and hypercholesterolemia. On admission, his vitals were stable. ECG, chest radiography, and echocardiography showed no specific findings. CAG showed no significant coronary stenosis, including stented segments. SPT, performed when taking vasodilators, revealed diffuse spasms in the three major coronary arteries (at doses of 30 µg of ACh for the RCA and 50 µg of ACh for the left coronary artery). Intracoronary infusions of nitroglycerin relieved the bilateral coronary spasms (Figure 2). We questioned him in detail about his past medications to determine treatment options. He had been on medication [isosorbide dinitrate (20 mg tablet, BID and 40 mg patch, QD), diltiazem hydrochloride (100 mg tablet, BID), verapamil hydrochloride (80 mg tablet, at bedtime), sarpogrelate hydrochloride (100 mg tablet, BID), ezetimibe (10 mg tablet, QD), aspirin (100 mg tablet, QD), rosuvastatin calcium (20 mg tablet, QD), ethyl icosapentate (900 mg capsule, BID), and nitroglycerin (0.3 mg tablet, prn)] before admission, but the frequency of angina attacks had been increasing ever since he switched from a brand-name CCB to a generic CCB several months earlier. After we switched back from the generic CCB to the brand-name CCB, the frequency of angina attacks decreased to once per week (Table 2).
| Time course | Frequency of chest pain | Cardiovascular drug regimen |
| Several months ago | Diltiazem hydrochloride (brand-name) 100 mg capsule, BID | |
| Isosorbide dinitrate (brand-name) 20 mg capsule, BID | ||
| Isosorbide dinitrate (brand-name) 40 mg tape, QD | ||
| Verapamil hydrochloride (brand-name) 80 mg tablet, at bedtime | ||
| Sarpogrelate hydrochloride (generic) 100mg tablet, BID | ||
| 1/wk | Rousvastatin calcium (brand-name) 20 mg tablet, QD | |
| Ezetimibe (brand-name) 10 mg tablet, QD | ||
| Ethil isosapentate (generic) 900 mg capsule, QD | ||
| Several months ago, admission | Diltiazem hydrochloride (generic) 100 mg capsule, BID | |
| Isosorbide dinitrate (brand-name) 20 mg capsule, BID | ||
| Isosorbide dinitrate (brand-name) 40 mg tape, QD | ||
| Verapamil hydrochloride (brand-name) 80 mg tablet, at bedtime | ||
| Sarpogrelate hydrochloride (generic) 100 mg tablet, BID | ||
| Rousvastatin calcium (brand-name) 20 mg tablet, QD | ||
| Ezetimibe (brand-name) 10 mg tablet, QD | ||
| Ethil isosapentate (generic) 900 mg capsule, QD | ||
| 3-4/wk | ||
| Thereafter | Diltiazem hydrochloride (brand-name) 100 mg capsule, BID | |
| Isosorbide dinitrate (brand-name) 20 mg capsule, BID | ||
| Isosorbide dinitrate (brand-name) 40 mg tape, QD | ||
| Verapamil hydrochloride (brand-name) 80 mg tablet, at bedtime | ||
| Sarpogrelate hydrochloride (generic) 100 mg tablet, BID | ||
| Rousvastatin calcium (brand-name) 20 mg tablet, QD | ||
| Ezetimibe (brand-name) 10 mg tablet, QD | ||
| 1/wk | Ethil isosapentate (generic) 900 mg capsule, QD |
We describe two cases of VSA with an increase in the frequency of angina attacks after switching from a brand-name CCB to a generic CCB. In both cases of VSA, chest pain was under control to some extent while taking the brand-name CCB. However, switching to a generic CCB increased the frequency of angina attacks. Switching back to the brand-name CCB regimen reduced the frequency of angina attacks. These cases suggest that switching of coronary vasodilators from a brand-name drug to a generic drug has could worsen anginal symptoms in some patients with VSA.
One advantage of using generic drugs is the reduction in medical expenditure because of their low cost. However, brand-name drugs and generic drugs differ in terms of their dosage, additives, and production methods. A comparative study of the amount of CCB-related substances identified in a purity test reported that a generic CCB contains twice as much analog of the active ingredient than a brand-name CCB[8]. On the other hand, according to meta-analyses, generic and brand-name drugs used in cardiovascular disease are clinically equivalent: there is no evidence supporting the superiority of brand-name drugs over generic drugs[6,7]. Specifically, the difference in the effects of brand-name and generic vasodilators used for cardiovascular diseases, especially for VAS, is not known.
Variation in the severity of VSA is seasonal, and it cannot be denied that the worsening and improvement of angina attacks in these two cases may be because of the natural course of VSA symptoms. However, we think that the dramatic changes in the frequency of angina attacks immediately after switching of CCBs-between generic and brand-name drugs-was because of the lower efficacy of the generic CCBs used. Although similar effects can be expected in the majority of patients treated for VSA with either generic or brand-name drugs, it is possible that the effects of these two drugs differ based on patients’ disease severity and/or the nature of their disease. Our two cases of VSA demonstrated a positive SPT including multi-vessel spasms and spasm provocation induced by low doses of ACh, which is characteristic of intractable VSA[9]. In addition, the patient described in case 2 had a positive SPT even while on a treatment regimen that included several vasodilators, demonstrating highly severe VSA. Accordingly, even a minor change in the effect of CCBs when switching between brand-name drugs and generic drugs may influence the VSA attack threshold. Thus, switching from a brand-name drug to a generic drug may worsen angina attacks in patients with highly severe VSA.
CCBs are generally recommended as the first line of therapy to prevent angina attacks in VSA patients, and some patients have angina attacks even while taking CCB medications[3]. In such cases, we follow several courses of action. First, we must consider the type of CCB, because CCBs may differ in their ability to prevent angina attacks[10,11]. Second, we must consider the dosing regimen, such as whether a submaximal or maximal dose or medication once or twice a day would be appropriate. We sometimes encounter patients on a once-a-day CCB regimen who have angina attacks just before they are due to take their medication. Third, we must consider the medication timing. In general, angina attacks often occur between midnight and the early morning[1,2]. Thus, taking a CCB at bedtime is usually recommended. However, for some VSA patients, taking a CCB at the time of rising may be effective. In addition, we may add another vasodilator such as long-acting nitrates, nicorandil and other types of CCBs (dihydropyridine CCB vs non-dihydropyridine CCB)[12]. Finally, as shown in the present case reports, we must check whether the prescribed vasodilators are brand-name vasodilators or not. This may also prevent a redundant increase in the numbers of vasodilators prescribed to such patients.
There were several limitations in the present case reports. We did not observe any ST-T changes on ECG during anginal attacks. Furthermore, we did not perform a repeat coronary angiography and spasm provocation test to confirm the effect of brand-name vs generic coronary vasodilators. Thus, the severity of anginal symptoms was based only on self-assessment.
In conclusion, switching of CCBs from brand-name drugs to generic ones may worsen angina attacks in some patients with VSA. Therefore, when treating VSA patients with medication-refractory anginal symptoms, it is important that cardiologists confirm the type (brand-name or generic) of vasodilator drugs used.
The frequency of angina attacks increased after switching a brand-name vasodilator to a generic vasodilator in two patients with vasospastic angina (VSA).
VSA.
Organic coronary stenosis, microvascular angina, chest pain syndrome and gastroesophageal reflux disease.
Both patients had dyslipidemia, but the laboratory tests for lipid levels were within normal limits because they were being treated for dyslipidemia.
Coronary angiography and the spasm provocation test showed bilateral coronary spasm in both cases. These findings are indicative of severe VSA.
After switching back from the generic vasodilators to the brand-name vasodilators, the frequency of angina attacks decreased to baseline in both patients.
According to meta-analyses, the clinical effects of generic and brand-name drugs used in cardiovascular disease are similar, but it is unclear whether this is the case for patients with higher severity of VSA.
VSA is characterized by the transient vasoconstriction of the epicardial coronary artery, leading to myocardial ischemia. VSA causes not only rest angina but also exertional angina, acute coronary syndrome and ischemic cardiac arrest.
Generic vasodilators may not sufficiently prevent angina attacks in patients with a high severity of VSA. Thus, when treating VSA patients with medication-refractory anginal symptoms, it is important that cardiologists confirm the type (brand-name or generic) of vasodilator drugs used.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Avanzas P S- Editor: Cui LJ L- Editor: A E- Editor: Li RF
| 1. | Yasue H, Kugiyama K. Coronary spasm: clinical features and pathogenesis. Intern Med. 1997;36:760-765. [PubMed] |
| 2. | Yasue H, Nakagawa H, Itoh T, Harada E, Mizuno Y. Coronary artery spasm--clinical features, diagnosis, pathogenesis, and treatment. J Cardiol. 2008;51:2-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 378] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 3. | JCS Joint Working Group. Guidelines for diagnosis and treatment of patients with vasospastic angina (Coronary Spastic Angina) (JCS 2013). Circ J. 2014;78:2779-2801. [PubMed] |
| 4. | Sueda S, Kohno H, Ochi T, Uraoka T, Tsunemitsu K. Overview of the pharmacological spasm provocation test: Comparisons between acetylcholine and ergonovine. J Cardiol. 2017;69:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Sueda S, Miyoshi T, Sasaki Y, Sakaue T, Habara H, Kohno H. Safety and optimal protocol of provocation test for diagnosis of multivessel coronary spasm. Heart Vessels. 2016;31:137-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Manzoli L, Flacco ME, Boccia S, D’Andrea E, Panic N, Marzuillo C, Siliquini R, Ricciardi W, Villari P, Ioannidis JP. Generic versus brand-name drugs used in cardiovascular diseases. Eur J Epidemiol. 2016;31:351-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 7. | Kesselheim AS, Misono AS, Lee JL, Stedman MR, Brookhart MA, Choudhry NK, Shrank WH. Clinical equivalence of generic and brand-name drugs used in cardiovascular disease: a systematic review and meta-analysis. JAMA. 2008;300:2514-2526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 306] [Cited by in RCA: 270] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 8. | Ichihara E, Okumra M, Yoshida F, Kawano Y, Ookura K, Iwakiri T, Yasuda K, Shiotsuki S, Matsuda M, MIzumoto K. Generics should be evaluated form an overall perspective -characteristics of benidipine hydrochroride tablets whose dosage form has been changed and comparison of branded and generic products regarding drug information supplied. Jpn J Pharm Health Care Sci. 2008;34:366-373. |
| 9. | Teragawa H, Fujii Y, Oshita C, Ueda T. Importance of the spasm provocation test in diagnosing and clarifying the activity of vasospastic angina. Interv Cardiol J. 2017;3:58. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Nishigaki K, Inoue Y, Yamanouchi Y, Fukumoto Y, Yasuda S, Sueda S, Urata H, Shimokawa H, Minatoguchi S. Prognostic effects of calcium channel blockers in patients with vasospastic angina--a meta-analysis. Circ J. 2010;74:1943-1950. [PubMed] |
| 11. | Oikawa Y, Matsuno S, Yajima J, Nakamura M, Ono T, Ishiwata S, Fujimoto Y, Aizawa T. Effects of treatment with once-daily nifedipine CR and twice-daily benidipine on prevention of symptomatic attacks in patients with coronary spastic angina pectoris-Adalat Trial vs Coniel in Tokyo against Coronary Spastic Angina (ATTACK CSA). J Cardiol. 2010;55:238-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Rodríguez-Mañero M, Oloriz T, le Polain de Waroux JB, Burri H, Kreidieh B, de Asmundis C, Arias MA, Arbelo E, Díaz Fernández B, Fernández-Armenta J. Long-term prognosis of patients with life-threatening ventricular arrhythmias induced by coronary artery spasm. Europace. 2017; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |