Published online Dec 26, 2018. doi: 10.4330/wjc.v10.i12.267
Peer-review started: August 25, 2018
First decision: October 5, 2018
Revised: October 23, 2018
Accepted: November 26, 2018
Article in press: November 27, 2018
Published online: December 26, 2018
Processing time: 123 Days and 0.7 Hours
Coronary angiography is considered to be the gold standard in the morphological evaluation of coronary artery stenosis. The morphological assessment of the severity of a coronary lesion is very subjective. Thus, the invasive fractional flow reserve (FFR) measurement represents the current standard for estimation of the hemodynamic significance of coronary artery stenosis. The FFR-guided revascularization strategy was initially classified as a Class-IA-recommendation in the 2014 European Society of Cardiology/European Association for Cardio-Thoracic Surgery guidelines on myocardial revascularization. Both the Deferral vs Performance of Percutaneous Coronary Intervention of Functionally Non-Significant Coronary Stenosis and Flow Reserve vs Angiography for Multivessel Evaluation studies showed no treatment advantage for hemodynamically insignificant stenoses. With the help of FFR (and targeted interventions), clinical results could be improved; however, the use in clinical practice is still limited due to the need of adenosine administration and a significant prolongation of the length of the procedure. Instantaneous wave-free ratio (iFR®) is a new innovative approach for the determination of the hemodynamic significance of coronary stenosis, which can be obtained at rest without the use of vasodilators. Regarding the periprocedural complications as well as prognosis, iFR® showed non-inferiority to FFR in the SWEDEHEART and DEFINE-FLAIR trials. Furthermore, iFR®, enhanced by iFR®-pullback, provides the possibility to display the iFR®-change over the course of the vessel to create a hemodynamic map.
Core tip: Invasive fractional flow reserve measurement represents the current standard for estimation of the hemodynamic significance of coronary artery stenosis and was initially classified as a Class-IA-recommendation in the 2014 European Society of Cardiology/European Association for Cardio-Thoracic Surgery guidelines on myocardial revascularization. Instantaneous wave-free ratio (iFR®) is a new innovative approach for the functional evaluation of a coronary stenosis, which can be obtained at rest without the use of vasodilators. The diagnostic value of iFR® showed non-inferiority compared to fractional flow reserve. It can be enhanced by iFR®-pullback, which provides the possibility to display the iFR®-change over the course of the vessel to create a hemodynamic map.
- Citation: Baumann S, Chandra L, Skarga E, Renker M, Borggrefe M, Akin I, Lossnitzer D. Instantaneous wave-free ratio (iFR®) to determine hemodynamically significant coronary stenosis: A comprehensive review. World J Cardiol 2018; 10(12): 267-277
- URL: https://www.wjgnet.com/1949-8462/full/v10/i12/267.htm
- DOI: https://dx.doi.org/10.4330/wjc.v10.i12.267
The optimal strategy for revascularization of hemodynamically significant coronary stenosis is an important therapeutic option in patients with coronary heart disease (CHD)[1]. Despite being the gold standard in the diagnosis of coronary stenosis, coronary angiography has a few limitations. Sometimes, the angiographic demonstration of the correct anatomy is limited due to morphologic deviations; additionally, visual evaluation of the coronary lesion is subjective and is associated with large inter-observer variability[2,3].
The current standard for invasive assessment of a coronary lesion with hemodynamic significance is the fractional flow reserve-(FFR)-measurement[4]. This was initially adopted as a Class-IA-recommendation in the European Society of Cardiology/European Association for Cardio-Thoracic Surgery (ECS/EACTS) guidelines of 2014 on myocardial revascularization[5]. Especially with intermediate coronary stenoses and in patients with a multivessel disease, FFR can help the clinician to assess the severity of the lesion and to formulate the required treatment[5]. Other than the angiographic imaging, FFR provides a direct functional assessment of coronary stenoses.
Both the Deferral vs Performance of Percutaneous Coronary Intervention of Functionally Non-Significant Coronary Stenosis and the FAME (Flow Reserve vs Angiography for multivessel Evaluation) studies could not prove a prognostic benefit of treating hemodynamically insignificant coronary stenosis through percutaneous coronary intervention (PCI)[6-8]. Furthermore, long-term analysis of the Deferral vs Performance of Percutaneous Coronary Intervention of Functionally Non-Significant Coronary Stenosis study showed that the use of FFR improved the clinical outcome and lowered the procedural costs[9].
Patients with stable CHD who received FFR-guided PCI along with an adequate medication appeared to be more convalescent compared to patients on the medication-only therapy and were subjected to an emergency revascularization less frequently (FAME-II-study[8]). Additionally, patients with hemodynamically insignificant coronary stenoses (FFR > 0.80) who received optimal medical treatment alone showed a very good long-term outcome.
The FFR utilizes the linear relationship between pressure and flow at a point of an increased intracoronary resistance[10]. Assuming intracoronary pressure is proportional to the flow, a pressure gradient could indicate a lowered blood flow caused by a coronary stenosis. However, the intracoronary resistance changes periodically during a cardiac cycle. The periodic variations in resistance emerge from the interaction between the myocardium and the microvasculature during systole (high intracoronary resistance, compression of the microvasculature) and diastole (low intracoronary resistance, decompression of the microvasculature[11]). To perform the FFR-measurement, adenosine is administered to the patient to induce a hyperemic condition in order to achieve a constant blood flow, and FFR can be calculated and averaged over several cardiac cycles.
Although the clinical and economical benefits of FFR have been proven[7,9], it is only used in about 6% of patients undergoing PCI for intermediate coronary stenoses (40%-70% diameter stenosis)[12]. This is due to the high price for a single FFR-wire (600-800€[13]) as well as the use of adenosine, which is an additional expense. Furthermore, with each coronary assessment there exists a certain risk of a perforation or dissection[14] whilst applying the wire. In addition, the assessment time is longer, and adenosine administration could lead to adverse effects like dyspnea, chest pressure and discomfort, hypotension, and even atrioventricular blocks. However, vasodilators offer a pragmatic solution to achieve a constant blood flow and stable perfusion. Although FFR delivers accurate results and provides valuable information for the clinician assessing a single stenosis, the process of estimating the severity of each single stenosis in vessels with multiple lesions is difficult and time consuming[15]. The hemodynamic effect of removing a single stenosis in complex CHD is not easily predictable. The reason for this is an interdependence between multiple lesions in continuous coronary arteries under hyperemia, leading the examiner to overestimate a distal lesion and underestimate a proximal lesion. Inconveniently, after the treatment of each stenosis, the segment has to be reassessed by the clinician[16,17]. Therefore, new methods like iFR® (“instantaneous wave-free ratio”, Volcano Corporation, Koninklijke Philips N.V., Amsterdam, The Netherlands) offer a different approach. iFR® is based on the hypothesis that a specific time interval during the cardiac cycle, the diastolic “wave-free” period, can be identified when microvascular resistance is naturally minimized without the need of hyperemia induced by the administration of a vasodilator[18]. Next to the two large multicenter studies Functional Lesion Assessment of Intermediate Stenosis to Guide Revascularization (DEFINE-FLAIR)[19] and Swedish Web-Based System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART)[20], which proved the non-inferiority of iFR® towards FFR, the first meta-analysis with 23 studies including 6300 coronary lesions was just recently published. This study verified a significant correlation between iFR® and the gold standard FFR and a good performance of iFR® identifying FFR-positive stenoses[21]. Besides FFR, iFR® was just recently adopted as a Class-IA-recommendation in the ECS/EACTS guidelines of 2018 on myocardial revascularization[22].
iFR®-measurement is based on the physical law outlined by the Hagen-Poiseuille equation, which describes a laminar flow of an incompressible viscous fluid flowing through a cylindrical pipe of a constant cross section, which depends on the type of fluid and the consistency of the pipe[23]. This law is a deviation of Ohm’s Law (U = R × I).
P = Q × R
Pressure = Flow × Resistance
ΔP ≈ ΔQ × R
Pressure change ≈ Flow change × Constant resistance
At a constant resistance, pressure changes are proportional to change of flow. When administering a vasodilator, the FFR-measurement utilizes this constant resistance proportionality, and the iFR®-index is obtained during a period of the cardiac cycle (diastole) when the resistance is minimal and naturally stable. The unique qualities of coronary blood flow result from the proximal pressure changes through pulsatile blood ejection as well as peripheral variations in coronary microcirculation[11]. It is not adequate to assess a stenosis severity by simply measuring the drop in maximum or intermediate pressure of the vessel, since the distal predominant pressure is affected by several components and does not necessarily reflect the proximal aortic pressure. The distal predominant pressure is primarily influenced by the pressure changes in the coronary microcirculation but can significantly affect the (instantaneous) proportion of pressure and blood flow as an index of intracoronary resistance. Wave intensity analysis helps to differentiate between distal and proximal variations[11].
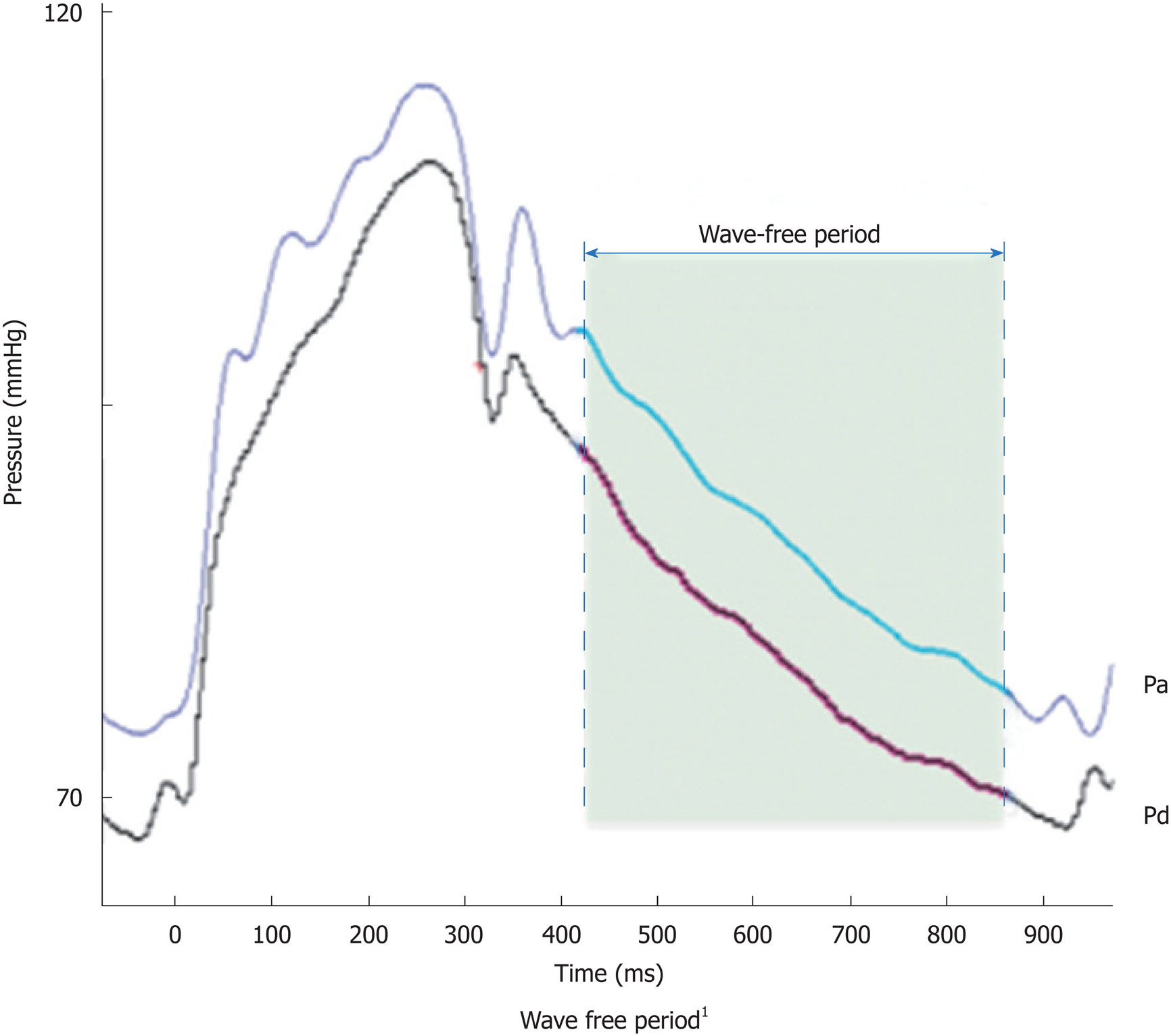
In early systole, pressure rises rapidly without an increase in flow velocity (Figure 1). Accordingly, the index of intracoronary resistance rises as well. The rapid increase in pressure (without the flow acceleration) develops from adaption of the ejection wave within the aorta and the compression wave from the coronary microcirculation.
Quite the opposite happens in early diastole: Pressure decreases while flow accelerates, which leads to a rapidly decreasing intracoronary resistance and absorption of blood into the coronary microcirculation. After this short period of pressure decrease, the index of coronary resistance is almost minimal and stable, since neither from the proximal nor from the distal coronary end wave activity is emitted. This wave-free period prevails over most of the diastole and is the basis for iFR®-measurement.
Pressure-derived flow indices like FFR refer to a proportional correlation between pressure and flow when resistance is constant[24], which only applies to a specific period of the cardiac cycle. Manipulations with vasodilators primarily reduce the systolic component of the resistance and can thus be used to achieve a minimal and stable value.
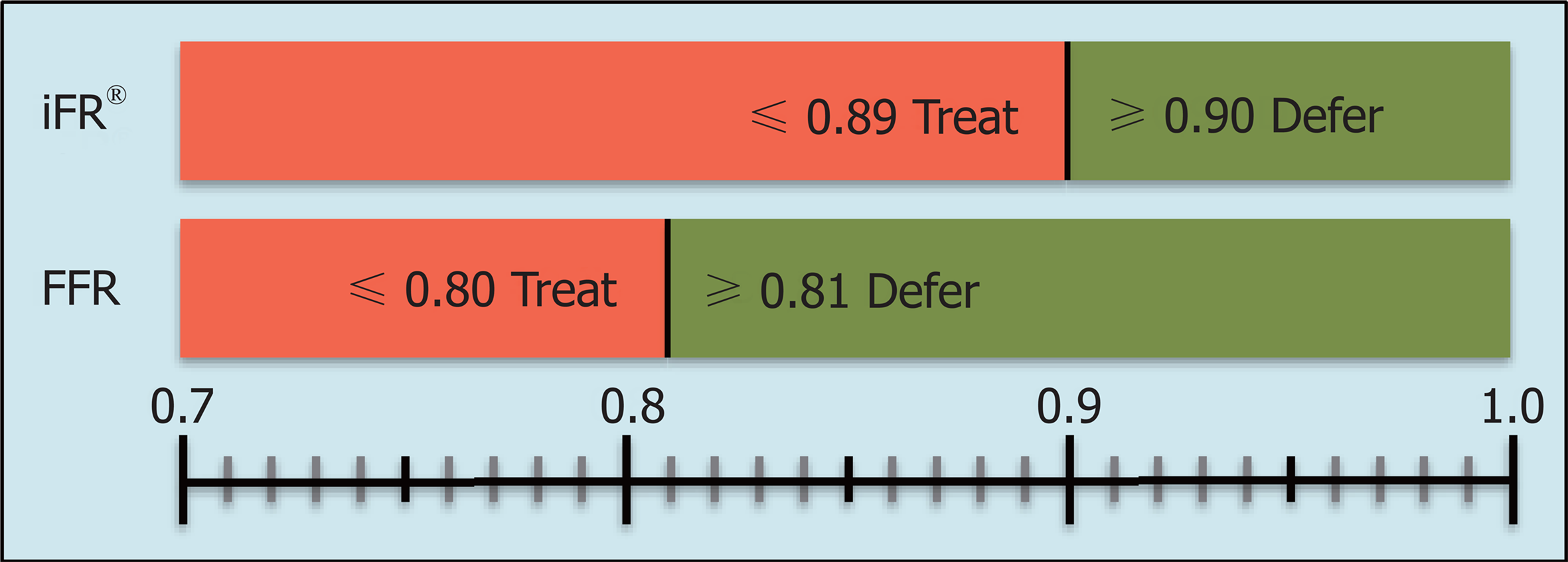
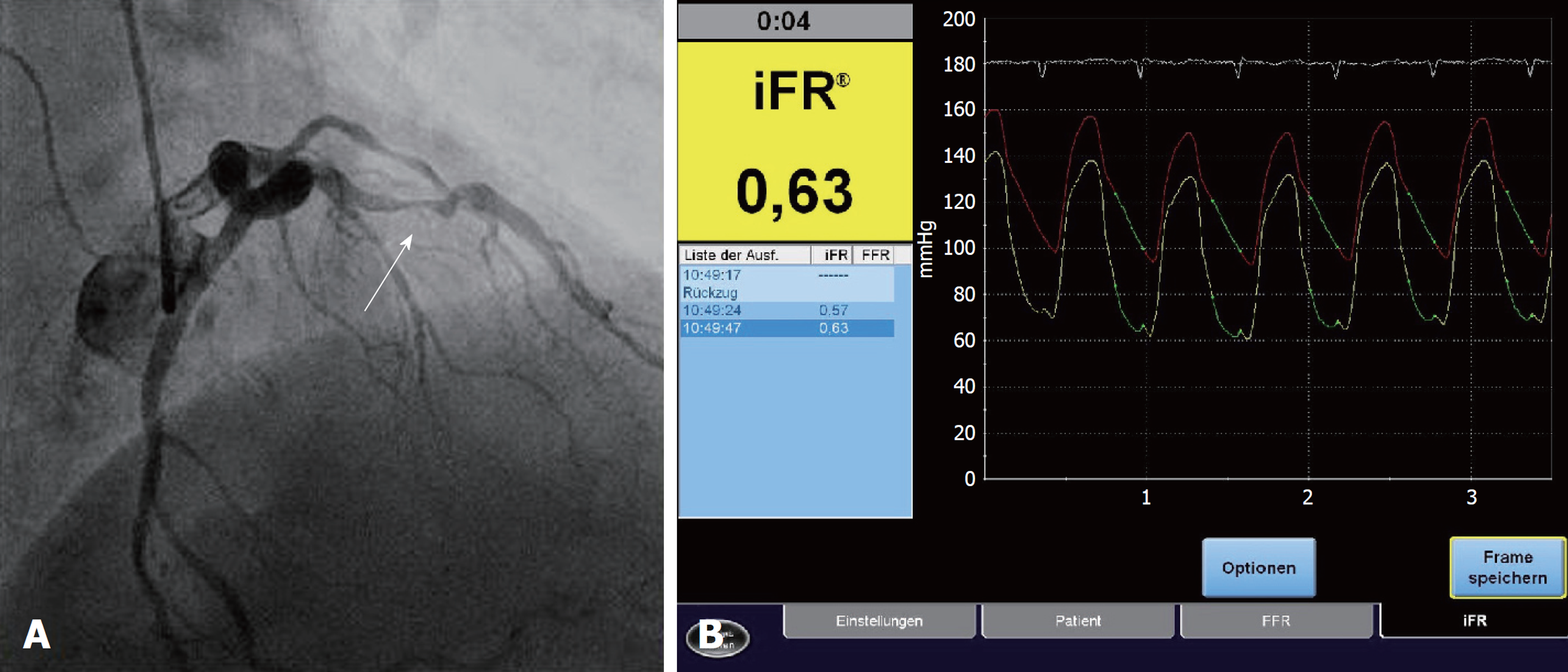
Among the advantages of iFR® are a drug-free approach, as well as the ability to reach a higher flow velocity during the measurement, which allows a better discrimination of hemodynamically significant stenoses. A series of measured and reproducible data are generated during a period of five consecutive heartbeats. Indices of 0.89 or less generated by iFR® are equivalent to the common limit of 0.80 or less in FFR[4] and serve as an indicator for ischemia (Figure 2). A clinical example is presented in Figure 3.
To detect the specific time during diastole for the calculation of iFR®, it is necessary to acquire electrocardiographic signals of the patient. To simplify the assessment process it has recently become possible to calculate the index by only using the pressure signals, thus allowing the process to be run independent of electrocardiography (ECG). Specific end-systolic and end-diastolic waveform characteristics are identified to receive the accurate proximal (Pa) and distal (Pd) coronary pressure[25].
Regarding the assessment of vessels with multiple lesions, iFR®-pullback offers a technique to create a hemodynamic map of a coronary artery, which allows an individual estimation of each stenosis. The pullback itself is conducted manually and detects continuously pressure changes per millimeter for a given length[16,17]. Since the iFR® is obtained under resting conditions, whereby the autoregulatory mechanisms in the vessel ensure a stable and constant baseline-flow, serial lesions are not affected by each other[26].
Baseline physiology offers the opportunity to quantify the impact of each single stenosis and can, therefore, predict the effect of a treatment of an individual stenosis within a vessel with multiple lesions. A hemodynamic map via pullback can simultaneously display iFR®-changes over the whole vessel and track down the lesion with the predominant pressure-loss[16]. Additionally, it can be overlaid with angiographic imaging in order to locate the exact physiologically significant anatomical site of the narrowings (co-registration)[17].
Other diastolic resting indices, such as the diastolic pressure ratio (dPR) obtained in different phases of the diastole like dPR25–75 (25% to 75% of diastole) or dPRmid (midpoint of diastole) along with Matlab calculated iFR® (iFRmatlab) and iFR®-like indices shortening the length of the wave-free period by 50 and 100 ms (iFR-50 ms and iFR-100 ms), were compared to the iFR® and found to be numerically identical. Therefore, all guidelines and cut-off values as well as clinical recommendation can be applied to these indices[27].
During the course of the initial pilot study Adenosine Vasodilator Independent Stenosis Evaluation (ADVISE), a wave-free period during the cardiac cycle was identified for the first time, enabling to determine stenosis severity without the administration of vasodilators. It was an international, multicenter, non-randomized study (Table 1), in which the flow and pressure data from 157 stenoses were collected. The study revealed a good correlation between the FFR- and iFR®-measurements. However, with only 131 patients, the population was relatively small. In this population, the iFR®-index 0.83 showed the best correlation with the FFR-index of 0.80. A subgroup analysis in patients with multivessel disease, similar to the FAME-study collective, confirmed an excellent diagnostic correlation of 93% between iFR® and FFR.
| Advise | Verify | Clarify | Park et al[39] | Resolve | Advise in practice | Indolfi et al[42] | ADVISE II | Harle et al[44] | Van de Hoef et al[45] | DEFINE-FLAIR | iFR®-SWEDHEART | |
| First author journal and year of Publication | Sen et al[18]. J Am Coll Cardiol 2012 | Berry et al[37]. J Am Coll Cardiol 2013 | Sen et al[38]. J Am Coll Cardiol 2013 | Park et al[39]. Int J Cardiol 2013 | Jeremias et al[40]. J Am Coll Cardiol 2014 | Petraco et al[41]. Am Heart J 2014 | Indolfi et al[42]. Int J Cardiol 2015 | Escaned et al[43]. J Am Coll Cardiol-Intv 2015 | Harle et al[44]. Int J Cardiol 2015 | Van de Hoef et al[45]. Euro-Intervention 2015 | Davies et al[19]. N Engl J Med 2017 | Götberg et al[20]. N Engl J Med 2017 |
| Study design | PC, multicenter, non-randomized | PC, multicenter, non-randomized | PC, multicenter | PC, multicenter, non-randomized | RS, multicenter, non-randomized | PC, multicenter, non-randomized | PC, monocenter, non-randomized | PC, multicenter, non-randomized | PC, monocenter, non-randomized | PC, multicenter, non-randomized | PC, multicenter, randomized | PC, multicenter, randomized |
| Countries (centers) | 2 (3) | 6 (6) | 2 (3) | 1 (2) | 7 (15) | 101 (16) | 1 (1) | 8 (45) | 1 (1) | 3 (7) | 19 (49) | 3 (14) |
| Included patients | 131 | 206 | 51 | 238 | 1768 | 313 | 82 | 598 | 109 | 228 (iFR® = 66) | 2492 (iFR® = 1242) | 2037 (iFR® = 1019 |
| Stenoses | 157 | 206 | 51 | 238 | 1974 | 392 | 123 | 690 | 151 | 299 (iFR® = 85) | 3183 (iFR® = 1575) | 3004 (iFR® = 1568) |
| Hemodynamic relevant stenoses (%) | N/A | 134 (65) | N/A | 103 (43.3) | N/A | 153 (39) | 37 (30.1) | 248 (35.9) | N/A | N/A | 451 (28.6) | 457 (29.1) |
| Age in years ± SD | 62.6 ± 10.2 | 65.2 ± 10.2 | 66.2 ± 9.2 | 62.8 ± 0.6 | 63.4 ± 10.3 | 67 ± 11 | 64 ± 9 | 63.6 ± 10.8 | 67 ± 11 | 58 ± 11 | 65.5 ± 10.8 | 67.6 ± 9.6 |
| Men (%) | 83.5 | 71 | 82.4 | 68 | 74.9 | 79 | 81.7 | 68.9 | 63.9 | 68 | 77.5 | 74.2 |
| Diabetes mellitus (%) | 54 (34.4) | 50 (24) | 14 (27.4) | 66 (28) | 497 (28.1) | 94 (30) | 14 (17.1) | 209 (35) | N/A | 10 (15) | 382 (30.8) | 232 (22.8) |
| Hypertonia (%) | 88 (56.1) | 137 (67) | 18 (35.2) | 133 (56) | N/A | 232 (74) | 61 (74.4) | 471 (78.8) | N/A | 25 (38) | 873 (70.3) | 730 (71.6) |
| Smoking (%) | 34 (21.7) | 64 (31) | 15 (29.4) | 64 (27) | 520 (29.4) | 160 (51) | 49 (59.8) | 135 (22.6) | N/A | 21 (32) | 243 (19.6) | 159 (15.6) |
| One-vessel CAD (%) | 108 (68.8) | 85 (41) | N/A | N/A | N/A | 113 (36) | 50 (61) | N/A | 75 (69.4) | N/A | N/A | 452 (44.3) |
| Multi-vessel CAD (%) | 49 (31.2) | 105 (51) | N/A | N/A | 951 (53.8) | 197 (63) | 32 (39) | N/A | 33 (30.6) | N/A | 505 (40.7) | 364 (35.7) |
| Stable angina (%) | 151 (96.2) | 140 (68) | N/A | 151 (63) | 1216 (68.6) | 228 (73) | 29 (35) | 320 (53.5) | N/A | N/A | 986 (79.4) | 632 (62.0) |
| Unstable angina (%) | 6 (3.8) | 46 (22) | N/A | 84 (36) | 255 (14.4) | 85 (27) | 53 (65) | 151 (25.3) | N/A | N/A | 186 (15.0) | 211 (20.7) |
| iFR® cut-off | 0.83 | ≤ 0.83 | 0.86 | 0.9 | 0.9 | 0.9 | 0.92 | 0.89 | 0.896 | 0.9 | 0.89 | 0.89 |
| MACE-rate after 1 yr (iFR®vs FFR, P-value) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 6.8 vs 7.0 (P = 0.003) | 6.7 vs 6.1 (P = 0.007) |
| Adverse events (iFR®vs FFR, P-value) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 3.1 vs 30.8 (P < 0.001) | 3.0 vs 68.3 (P < 0.0001) |
| Diagnostic accuracy in % (iFR®vs FFR) | 93 | 68 | 92.3 | 82 | 80.4 | 80 | 81.3 | 82.5 | 83.4 | N/A | N/A | N/A |
The Multicenter Core Laboratory Comparison if Instantaneous Wave-Free Ratio and Resting Pd/Pa with Fractional Flow Reserve (RESOLVE)-study tried to examine the diagnostic accuracy of iFR®vs FFR. In the course of this retrospective, multicenter, non-randomized study, 1593 (81%) out of 1974 lesions were analyzed, as 381 lesions had to be excluded due to the inadequate image quality. Despite this, the result showed a moderate correlation between iFR® and FFR, with a diagnostic precision of 80.4%.
DEFINE-FLAIR, a leading multicenter, international, randomized, blinded study designed to prove the non-inferiority of iFR®, reiterated the findings of the previously mentioned studies. As of now, the available data are based on a 1-year-analysis. The ongoing study is conducted in 49 places in over 19 countries. Patients were included if they had at least one angiographically confirmed coronary disease, in which there was at least one stenosis of a questionable hemodynamic severity. Suitable patients were randomly assigned to a particular arm at a ratio of 1:1 FFR towards iFR®. The primary endpoint was the 1-year risk for major adverse cardiac events like cardiovascular death, nonfatal myocardial infarction, or unplanned revascularization. From January 2014 to December 2015, 2492 patients were included, 1242 in the iFR®- and 1250 in the FFR-group. The 1-year analysis showed comparable results regarding the endpoints, confirming the non-inferiority of iFR® towards FFR. The length of the procedure time was significantly shorter in the iFR®-group (iFR® 40.5 min, FFR: 45.0 min; P < 0.001), and less patients suffered from adverse effects like angina pectoris and dyspnea (3.1% vs 30.8%, P < 0.001), mainly because adenosine was not administered. In addition, when compared to FFR, this method was identified as more economically advantageous.
Published at about the same time, Instantaneous Wave-Free Ratio versus Fractional Flow Reserve to Guide PCI (iFR®-SWEDEHEART) also examined the non-inferiority of iFR® in the course of a multicenter, randomized, clinical study. The inclusion of eligible patients was based on the Swedish Coronary Angiography and Angioplasty Registry. Two thousand thirty-seven patients with a stable angina pectoris or an acute coronary syndrome were included and randomly allocated in a particular arm (iFR®vs FFR). Primary endpoint was the 1-year risk for major adverse cardiac effects like cardiovascular death, nonfatal myocardial infarction, or unplanned revascularization. Information about myocardial infarction or unplanned revascularization was gathered from the web-based register SWEDEHEART. The study was conducted in 15 places (13 in Sweden, one in Denmark, one in Iceland). The patients were recruited from May 2014 to October 2015. Of these 2037 patients, 1019 received iFR® and 1018 received FFR. Final analysis included 2019 patients, as 18 participants had to be excluded because of the adverse effects under adenosine or technical problems.
The 1-year analysis of endpoints confirmed the non-inferiority of the iFR®-method. Especially in uncertain cases, where iFR® and FFR results differ, the data indicate that iFR® provides more accurate results. FFR-measurement tends to overrate the severity, since the vasodilator dependent hyperemia leads to a pressure decrease. The number of hemodynamically significant stenoses in this trial was much lower than in the FAME-study population, which only included patients with multivessel diseases. The iFR®-SWEDEHEART population is a better representation of the reality in clinical practice, since every patient with the indication for invasive coronary assessment could be included, independent of coronary status. Additionally, as described by Tonino and de Bruyne[7], an improvement in the clinical outcome of FFR-guided PCI was shown.
To compare ECG-independent iFR® calculation and the current method using ECG and pressure signals, Petraco et al[25] tested the only pressure-dependent iFR® algorithm in 320 coronary hemodynamically significant stenoses that were already included in multicenter studies (ADVISE[18], ADVISE Registry study[28], and a study by Nijjer et al[17]). The iFR®-indices of both methods correlated highly (r = 0.9997), which makes the ECG-independent iFR® applicable to the recent results of DEFINE-FLAIR and SWEDEHEART[25].
Based on the RESOLVE and ADVISE studies, Nijjer et al[17] have conducted a study (Pre-Angioplasty Instantaneous Wave-Free Ratio Pullback Provides Virtual Intervention and Predicts Hemodynamic Outcome for Serial Lesions and Diffuse Coronary Artery Disease) to create a hemodynamic map using the motorized pullback with iFR®, questioning if it helps to predict the stent impact in tandem and diffusely diseased vessels[17]. Thirty-two coronary arteries with two or more stenoses in 29 patients were assessed and underwent PCI. After physiological mapping, a computer-aided simulation calculated the best-case PCI effect. First, the virtual and real-world stents were compared to examine the predictive capability of iFR®-pullback. Second, the length of virtual stents, only positioned in areas with a high iFR®-intensity loss, was compared to the length of real world stents. ΔiFR®(exp) and ΔiFR®(obs) showed a strong relationship (r = 0.97, P < 0.001), and post-PCI iFR® was predicted with a 2% ± 1% error. Furthermore, the hereby examined physiological lesion length was significantly shorter than the anatomical length obtained by QCA (12.6 ± 1.5 mm vs 23.3 ± 1.3 mm, P < 0.001) and the length of the stent that was implanted in reality (27.5 ± 2.3 mm, P < 0.001).
Another study, published in 2017 by Kawase et al[29] (Residual pressure gradient across the implanted stent: An important factor of post-PCI physiological results) evaluated the accuracy of the predicted iFR®-value compared to the iFR® result, which was observed in reality after PCI. Additionally, they tried to discover potential factors for a failed prediction. iFR® ratios of 73 lesions in 71 patients were compared retrospectively before and after the coronary intervention. Pullback was conducted manually, anatomic lesion length was obtained by QCA, and the cut-off value of a difference between iFR®(pre) and iFR®(obs) was set at 0.036. The cut-off point was slightly missed, with a calculated mean difference of 0.036 ± 0.037, although the values correlated adequately (r = 0.756). In the course of a multivariate regression analysis, only a residual pressure gradient remained as an independent risk factor, leading to a failed prediction. After subtraction of the residual pressure gradient, the correlation between iFR®(pre) and iFR®(obs) improved. The only risk factors for a residual pressure gradient appeared to be a small diameter of the implanted stent and a high Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease-score[30], a score that calculates the amount of blood supplied to the myocardium by the targeted vessel. Kawase et al[29] noted that a larger cohort study could identify additional factors that have caused a failed prediction. An overview about the most important current publications is composed in Table 1. Our manuscript is based on the review of previous published articles and did not involve animal or human subjects. Therefore, neither an ethical approval nor a patient consent was necessary.
The process of advancing the coronary-pressure guide wire in FFR-measurement is still occasionally criticized and potentially accompanied by complications, which similarly constitutes a limitation of iFR®-measurement. This could hinder the regular clinical use of FFR- or iFR®-measurement.
It is not completely clarified how to proceed in uncertain cases and whether a stress test with adenosine is indicated. If hyperemia cannot be achieved through adequate doses, it is possible that the calculated value does not reflect the real FFR[31]. First, adenosine leads to peripheral vasoconstriction transmitted by pulmonary receptors, followed by its immediate effect on larger arteries that leads to a drop in blood pressure. This circumstance makes the ratio dependent on the time of measurement[32]. There is a small number of cases where not truly flow-limiting stenoses have led to acceptable iFR®-gradients but at the same time false positive hyperemic pressure gradients (FFR)[31]. High incidents of patient related discomfort, like dyspnea, chest pain, hypotension, and AV-blocks, or in one recorded case even ventricular fibrillation[33], still remain a limitation of the application of adenosine[34]. This limitation can be overcome by an adenosine free assessment like the iFR®.
In the analysis about the accuracy of the prediction of post-PCI iFR®, Kawase identified the residual pressure gradient as a risk factor for a the failed prediction and mentioned that its consideration might help the examining clinician[29].
Regarding microvascular diseases, studies could not prove a correlation between FFR and the index of microvasculatory resistance, an index for the microvascular status measured by the thermodilution technique[35]. This must not be seen as a shortcoming of the FFR method since it might rather show that micro- and macrovascular diseases are caused by different disease processes[36]. These findings can be employed on iFR®, since its non-inferiority towards FFR was proven.
Finally, there are currently new studies expected in which the iFR®-technique is to be subjected to the specific questioning, i.e., the sequential assessment of stenosis. A reduction in costs is to be expected due to no administration of adenosine and shortened procedural time.
The current standard of cardiac invasive ischemic diagnostic is invasive FFR-measurement, which was initially adopted as a Class-IA-recommendation to the ECS/EACTS guidelines of 2014 on myocardial revascularization. Despite good existing evidence, the performance of pressure-derived functional assessment in daily routine is still limited. Here, iFR® provides a new innovative approach to assess coronary stenosis severity without administering vasodilators. Besides FFR, iFR® was just recently adopted as a Class-IA-recommendation in the ECS/EACTS guidelines of 2018 on myocardial revascularization[22]. Additionally, the eliminated necessity to record the electrocardiographic signals simplifies the procedure of the invasive functional assessment.
iFR®, extended by iFR®-pullback, can help achieve a better physiological result in treating vessels with multiple lesions by creating a hemodynamic map. Since implanting potentially larger stents to prevent a geographical miss is currently the standard in treating multivessel disease, a physiologically justified stent length might therefore be more hemodynamically beneficial for the vessel[16]. Therefore, factors like a residual pressure gradient and other potential not yet discovered influences that have led to an inaccurate prediction of post-PCI iFR® ratio have to be considered. Large-scale multicenter, randomized studies demonstrated the non-inferiority of iFR® to FFR, whilst requiring less procedural time, having lower costs, and having a lower number of patients who suffer from adverse effects due to a spared use of adenosine.
We would like to thank Volcano Corporation, Koninklijke Philips N.V. (Amsterdam, The Netherlands) for providing the image copyrights of Figure 1.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Barik R, Dai X, Lai S, Lin GM, Vermeersch P S- Editor: Ji FF L- Editor: Filipodia E- Editor: Song H
| 1. | Nef H, Renker M, Hamm CW; European Society of Cardiology; European Association for Cardio-thoracic Surgery. [ESC/EACTS guidelines on myocardial revascularization: Amendments 2014]. Herz. 2014;39:913-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Zir LM, Miller SW, Dinsmore RE, Gilbert JP, Harthorne JW. Interobserver variability in coronary angiography. Circulation. 1976;53:627-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 541] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 3. | Kleiman NS, Rodriguez AR, Raizner AE. Interobserver variability in grading of coronary arterial narrowings using the American College of Cardiology/American Heart Association grading criteria. Am J Cardiol. 1992;69:413-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek J Koolen JJ, Koolen JJ. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med. 1996;334:1703-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1629] [Cited by in RCA: 1614] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 5. | Authors/Task Force members, Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Jüni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541-2619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3295] [Cited by in RCA: 3380] [Article Influence: 307.3] [Reference Citation Analysis (0)] |
| 6. | Pijls NH, van Schaardenburgh P, Manoharan G, Boersma E, Bech JW, van’t Veer M, Bär F, Hoorntje J, Koolen J, Wijns W. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J Am Coll Cardiol. 2007;49:2105-2111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1099] [Cited by in RCA: 1166] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 7. | Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’ t Veer M, Klauss V, Manoharan G, Engstrøm T, Oldroyd KG. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2974] [Cited by in RCA: 3058] [Article Influence: 191.1] [Reference Citation Analysis (0)] |
| 8. | De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, Jagic N, Möbius-Winkler S, Rioufol G, Witt N. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367:991-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1837] [Cited by in RCA: 1993] [Article Influence: 153.3] [Reference Citation Analysis (0)] |
| 9. | Fearon WF, Bornschein B, Tonino PA, Gothe RM, Bruyne BD, Pijls NH, Siebert U; Fractional Flow Reserve Versus Angiography for Multivessel Evaluation (FAME) Study Investigators. Economic evaluation of fractional flow reserve-guided percutaneous coronary intervention in patients with multivessel disease. Circulation. 2010;122:2545-2550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 282] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 10. | Spaan JA, Piek JJ, Hoffman JI, Siebes M. Physiological basis of clinically used coronary hemodynamic indices. Circulation. 2006;113:446-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 173] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 11. | Davies JE, Whinnett ZI, Francis DP, Manisty CH, Aguado-Sierra J, Willson K, Foale RA, Malik IS, Hughes AD, Parker KH. Evidence of a dominant backward-propagating “suction” wave responsible for diastolic coronary filling in humans, attenuated in left ventricular hypertrophy. Circulation. 2006;113:1768-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 298] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 12. | Kleiman NS. Bringing it all together: integration of physiology with anatomy during cardiac catheterization. J Am Coll Cardiol. 2011;58:1219-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Dale M. The Pressure Wire fractional flow reserve measurement system for coronary artery disease. Briefing. 2014; Available from: https://www.nice.org.uk/advice/mib2/resources/the-pressurewire-fractional-flow-reserve-measurement-system-for-coronary-artery-disease-1763862133957. |
| 14. | Tavakol M, Ashraf S, Brener SJ. Risks and complications of coronary angiography: a comprehensive review. Glob J Health Sci. 2012;4:65-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 15. | Kim HL, Koo BK, Nam CW, Doh JH, Kim JH, Yang HM, Park KW, Lee HY, Kang HJ, Cho YS. Clinical and physiological outcomes of fractional flow reserve-guided percutaneous coronary intervention in patients with serial stenoses within one coronary artery. JACC Cardiovasc Interv. 2012;5:1013-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 16. | Nijjer SS, Sen S, Petraco R, Mayet J, Francis DP, Davies JE. The Instantaneous wave-Free Ratio (iFR) pullback: a novel innovation using baseline physiology to optimise coronary angioplasty in tandem lesions. Cardiovasc Revasc Med. 2015;16:167-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 17. | Nijjer SS, Sen S, Petraco R, Escaned J, Echavarria-Pinto M, Broyd C, Al-Lamee R, Foin N, Foale RA, Malik IS. Pre-angioplasty instantaneous wave-free ratio pullback provides virtual intervention and predicts hemodynamic outcome for serial lesions and diffuse coronary artery disease. JACC Cardiovasc Interv. 2014;7:1386-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 18. | Sen S, Escaned J, Malik IS, Mikhail GW, Foale RA, Mila R, Tarkin J, Petraco R, Broyd C, Jabbour R. Development and validation of a new adenosine-independent index of stenosis severity from coronary wave-intensity analysis: results of the ADVISE (ADenosine Vasodilator Independent Stenosis Evaluation) study. J Am Coll Cardiol. 2012;59:1392-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 534] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 19. | Davies JE, Sen S, Dehbi HM, Al-Lamee R, Petraco R, Nijjer SS, Bhindi R, Lehman SJ, Walters D, Sapontis J. Use of the Instantaneous Wave-free Ratio or Fractional Flow Reserve in PCI. N Engl J Med. 2017;376:1824-1834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 679] [Cited by in RCA: 717] [Article Influence: 89.6] [Reference Citation Analysis (0)] |
| 20. | Götberg M, Christiansen EH, Gudmundsdottir IJ, Sandhall L, Danielewicz M, Jakobsen L, Olsson SE, Öhagen P, Olsson H, Omerovic E. Instantaneous Wave-free Ratio versus Fractional Flow Reserve to Guide PCI. N Engl J Med. 2017;376:1813-1823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 705] [Article Influence: 88.1] [Reference Citation Analysis (1)] |
| 21. | De Rosa S, Polimeni A, Petraco R, Davies JE, Indolfi C. Diagnostic Performance of the Instantaneous Wave-Free Ratio: Comparison With Fractional Flow Reserve. Circ Cardiovasc Interv. 2018;11:e004613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 22. | Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2722] [Cited by in RCA: 4498] [Article Influence: 899.6] [Reference Citation Analysis (0)] |
| 23. | Nijjer S, Davies J. Physiologic assessment in the cardiac catheterization laboratory: CFR, FFR, iFR®, and beyond. Dangas GD, Di Mario C, Kipshidze NN. Interventional cardiology: principles and practice, second edition. Wiley Blackwell 2017; 59-70. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 24. | Pijls NH, van Son JA, Kirkeeide RL, De Bruyne B, Gould KL. Experimental basis of determining maximum coronary, myocardial, and collateral blood flow by pressure measurements for assessing functional stenosis severity before and after percutaneous transluminal coronary angioplasty. Circulation. 1993;87:1354-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 807] [Cited by in RCA: 802] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 25. | Petraco R, Sen S, Nijjer S, Malik IS, Mikhail GW, Al-Lamee R, Cook C, Baker C, Kaprielian R, Foale RA. ECG-Independent Calculation of Instantaneous Wave-Free Ratio. JACC Cardiovasc Interv. 2015;8:2043-2046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Uren NG, Melin JA, De Bruyne B, Wijns W, Baudhuin T, Camici PG. Relation between myocardial blood flow and the severity of coronary-artery stenosis. N Engl J Med. 1994;330:1782-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 594] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 27. | Van’t Veer M, Pijls NHJ, Hennigan B, Watkins S, Ali ZA, De Bruyne B, Zimmermann FM, van Nunen LX, Barbato E, Berry C. Comparison of Different Diastolic Resting Indexes to iFR: Are They All Equal? J Am Coll Cardiol. 2017;70:3088-3096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 165] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 28. | Petraco R, Escaned J, Sen S, Nijjer S, Asrress KN, Echavarria-Pinto M, Lockie T, Khawaja MZ, Cuevas C, Foin N. Classification performance of instantaneous wave-free ratio (iFR) and fractional flow reserve in a clinical population of intermediate coronary stenoses: results of the ADVISE registry. EuroIntervention. 2013;9:91-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 29. | Kawase Y, Kawasaki M, Kikuchi J, Hirata T, Okamoto S, Tanigaki T, Omori H, Ota H, Okubo M, Kamiya H. Residual pressure gradient across the implanted stent: An important factor of post-PCI physiological results. J Cardiol. 2018;71:458-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Graham MM, Faris PD, Ghali WA, Galbraith PD, Norris CM, Badry JT, Mitchell LB, Curtis MJ, Knudtson ML; APPROACH Investigators (Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease. Validation of three myocardial jeopardy scores in a population-based cardiac catheterization cohort. Am Heart J. 2001;142:254-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 122] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 31. | Nijjer SS, Sen S, Petraco R, Davies JE. Advances in coronary physiology. Circ J. 2015;79:1172-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Tarkin JM, Nijjer S, Sen S, Petraco R, Echavarria-Pinto M, Asress KN, Lockie T, Khawaja MZ, Mayet J, Hughes AD. Hemodynamic response to intravenous adenosine and its effect on fractional flow reserve assessment: results of the Adenosine for the Functional Evaluation of Coronary Stenosis Severity (AFFECTS) study. Circ Cardiovasc Interv. 2013;6:654-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Buccheri D, Sorce S, Piraino D, Andolina G. Fractional flow reserve: A useful tool for interventionists which should be used with caution! Int J Cardiol. 2016;221:404-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 34. | Götberg M, Cook CM, Sen S, Nijjer S, Escaned J, Davies JE. The Evolving Future of Instantaneous Wave-Free Ratio and Fractional Flow Reserve. J Am Coll Cardiol. 2017;70:1379-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 154] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 35. | Lee JM, Layland J, Jung JH, Lee HJ, Echavarria-Pinto M, Watkins S, Yong AS, Doh JH, Nam CW, Shin ES. Integrated physiologic assessment of ischemic heart disease in real-world practice using index of microcirculatory resistance and fractional flow reserve: insights from the International Index of Microcirculatory Resistance Registry. Circ Cardiovasc Interv. 2015;8:e002857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 36. | Yong AS, Ho M, Shah MG, Ng MK, Fearon WF. Coronary microcirculatory resistance is independent of epicardial stenosis. Circ Cardiovasc Interv. 2012;5:103-108, S1-S2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 37. | Berry C, van ‘t Veer M, Witt N, Kala P, Bocek O, Pyxaras SA, McClure JD, Fearon WF, Barbato E, Tonino PA. VERIFY (VERification of Instantaneous Wave-Free Ratio and Fractional Flow Reserve for the Assessment of Coronary Artery Stenosis Severity in EverydaY Practice): a multicenter study in consecutive patients. J Am Coll Cardiol. 2013;61:1421-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 181] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 38. | Sen S, Asrress KN, Nijjer S, Petraco R, Malik IS, Foale RA, Mikhail GW, Foin N, Broyd C, Hadjiloizou N. Diagnostic classification of the instantaneous wave-free ratio is equivalent to fractional flow reserve and is not improved with adenosine administration. Results of CLARIFY (Classification Accuracy of Pressure-Only Ratios Against Indices Using Flow Study). J Am Coll Cardiol. 2013;61:1409-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 187] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 39. | Park JJ, Petraco R, Nam CW, Doh JH, Davies J, Escaned J, Koo BK. Clinical validation of the resting pressure parameters in the assessment of functionally significant coronary stenosis; results of an independent, blinded comparison with fractional flow reserve. Int J Cardiol. 2013;168:4070-4075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 40. | Jeremias A, Maehara A, Généreux P, Asrress KN, Berry C, De Bruyne B, Davies JE, Escaned J, Fearon WF, Gould KL. Multicenter core laboratory comparison of the instantaneous wave-free ratio and resting Pd/Pa with fractional flow reserve: the RESOLVE study. J Am Coll Cardiol. 2014;63:1253-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 280] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 41. | Petraco R, Al-Lamee R, Gotberg M, Sharp A, Hellig F, Nijjer SS, Echavarria-Pinto M, van de Hoef TP, Sen S, Tanaka N. Real-time use of instantaneous wave-free ratio: results of the ADVISE in-practice: an international, multicenter evaluation of instantaneous wave-free ratio in clinical practice. Am Heart J. 2014;168:739-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 42. | Indolfi C, Mongiardo A, Spaccarotella C, Torella D, Caiazzo G, Polimeni A, Sorrentino S, Micieli M, Sabatino J, Curcio A. The instantaneous wave-free ratio (iFR) for evaluation of non-culprit lesions in patients with acute coronary syndrome and multivessel disease. Int J Cardiol. 2015;178:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | Escaned J, Echavarría-Pinto M, Garcia-Garcia HM, van de Hoef TP, de Vries T, Kaul P, Raveendran G, Altman JD, Kurz HI, Brechtken J. Prospective Assessment of the Diagnostic Accuracy of Instantaneous Wave-Free Ratio to Assess Coronary Stenosis Relevance: Results of ADVISE II International, Multicenter Study (ADenosine Vasodilator Independent Stenosis Evaluation II). JACC Cardiovasc Interv. 2015;8:824-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 161] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 44. | Härle T, Bojara W, Meyer S, Elsässer A. Comparison of instantaneous wave-free ratio (iFR) and fractional flow reserve (FFR)--first real world experience. Int J Cardiol. 2015;199:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 45. | van de Hoef TP, Petraco R, van Lavieren MA, Nijjer S, Nolte F, Sen S, Echavarria-Pinto M, Henriques JP, Koch KT, Baan J Jr, de Winter RJ, Siebes M, Spaan JA, Tijssen JG, Meuwissen M, Escaned J, Davies JE, Piek JJ. Basal stenosis resistance index derived from simultaneous pressure and flow velocity measurements. EuroIntervention. 2016;12:e199-e207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |