Published online Nov 26, 2018. doi: 10.4330/wjc.v10.i11.210
Peer-review started: July 17, 2018
First decision: July 31, 2018
Revised: September 4, 2018
Accepted: October 9, 2018
Article in press: October 9, 2018
Published online: November 26, 2018
Processing time: 133 Days and 14.8 Hours
Cardiovascular diseases represent the leading cause of mortality and morbidity in the western world. Assessment of cardiac function is pivotal for early diagnosis of primitive myocardial disorders, identification of cardiac involvement in systemic diseases, detection of drug-related cardiac toxicity as well as risk stratification and monitor of treatment effects in patients with heart failure of various etiology. Determination of ejection fraction with different imaging modalities currently represents the gold standard for evaluation of cardiac function. However, in the last few years, cardiovascular magnetic resonance feature tracking techniques has emerged as a more accurate tool for quantitative evaluation of cardiovascular function with several parameters including strain, strain-rate, torsion and mechanical dispersion. This imaging modality allows precise quantification of ventricular and atrial mechanics by directly evaluating myocardial fiber deformation. The purpose of this article is to review the basic principles, current clinical applications and future perspectives of cardiovascular magnetic resonance myocardial feature tracking, highlighting its prognostic implications.
Core tip: Cardiac magnetic resonance feature tracking analysis is progressively establishing its role as an accurate tool to for quantitative evaluation of cardiovascular function by directly evaluating myocardial fiber deformation. Feature-tracking derived strain parameters are able to identify subtle myocardial abnormalities before overt clinical manifestation thus allowing early diagnosis of primitive cardiomyopathies, identification of cardiac involvement in systemic diseases, detection of drug-related cardiac toxicity as well as risk stratification and monitor of treatment effects in patients with heart failure of various etiology. The present article summarizes the basic principles, current applications and future perspectives of cardiovascular magnetic resonance myocardial feature tracking.
- Citation: Muser D, Castro SA, Santangeli P, Nucifora G. Clinical applications of feature-tracking cardiac magnetic resonance imaging. World J Cardiol 2018; 10(11): 210-221
- URL: https://www.wjgnet.com/1949-8462/full/v10/i11/210.htm
- DOI: https://dx.doi.org/10.4330/wjc.v10.i11.210
The evaluation of cardiac function has a pivotal role in diagnosis, risk stratification and assessment of treatment response in several cardiac disorders. Traditionally, left ventricular ejection fraction (LVEF) defined as the ratio between systolic output and end diastolic volume, assessed by various techniques including ventricular angiography, 2D- and 3D- echocardiography, cardiac single proton emission tomography, computed tomography and cardiac magnetic resonance (CMR), has represented the gold standard for evaluating cardiac function. Current international guidelines recommend the use of LVEF to assess the risk of sudden cardiac death (SCD) in patients with ischemic and non-ischemic cardiomyopathies, being patients with LVEF ≤ 35% at particular high risk and therefore referred for primary prevention implantable cardioverter defibrillator (ICD)[1]. Assessment of LVEF is also routinely recommended for familiar screening in patients affected by cardiomyopathies as well as to early detect cardiac involvement in systemic immune-mediated diseases or cardiac toxicity in patients undergoing cancer treatments[2-4]. However, in the last decade, new imaging modalities such as echo and CMR myocardial strain have progressively emerged as superior tools to evaluate global and regional myocardial mechanics, pointing out the limitations of LVEF especially in evaluating regional myocardial function and detecting early stage subclinical cardiac disorders[5]. In the present review we will focus on CMR feature tracking (CMR-FT) imaging of myocardial strain summarizing its basic principles, current clinical applications and future perspectives.
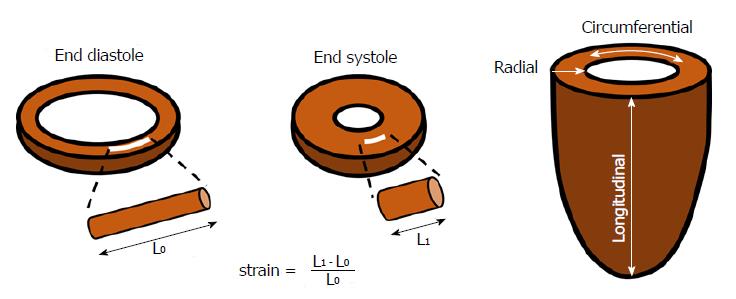
Myocardial strain is a deformation index defined as the percentage change in dimension from a resting state (end-diastole) to the one achieved after contraction (end-systole)[6]. Considering a myocardial fiber, and being L0 its initial length in end-diastole and L1 its final length in end-systole, myocardial strain (S) can be defined as follow:
S = (L1 - L0)/L0 (Figure 1)
Due to the complex 3D architecture of the left ventricular (LV) musculature, systolic deformation takes place along several different directions. The evaluation of strain along each of these axes leads to the definition of different types of strain: (1) longitudinal strain (LS) represents the longitudinal shortening of the cardiac muscle, from the base to the apex, it is mostly determined by the longitudinally oriented muscle fibers in the subendocardial layer and is conventionally represented by a negative value due to systolic shortening; (2) circumferential strain (CS) is an expression of cardiomyocytes shortening along the LV circular perimeter, it is calculated in a short-axis view, it is mostly influenced by circumferentially oriented muscle fibers in the mid-wall and as well as LS it has a negative value due to systolic shortening; and (3) radial strain (RS) represents myocardial deformation toward the center of the ventricular cavity, and it is a measure of the LV thickening during systole and therefore is conventionally represented by a positive value.
Several other mechanical deformation parameters can be derived from strain analysis, of note: (1) Strain rate representing the “velocity” or rate at which the deformation occurs; and (2) LV torsion defined by the angle generated by the clockwise rotation of the basal segments and the counterclockwise rotation of the apex relatively to a stationary reference point conventionally located at a mid-ventricular level.
Myocardial CMR strain was initially investigated using tagging techniques in which magnetic labels (tags) are superimposed to the LV during cine imaging acquisition by radiofrequency spatial modulation of magnetization able to saturate parallel planes throughout the image, allowing the analysis of deformation of those lines throughout the cardiac cycle by post-processing semi-automated methods[7,8]. Soon after those initial experiences, new methods to evaluate myocardial strain based upon post-processing tracking of myocardial “features” overcame the need for prospective time-consuming image acquisition protocols. Cardiac CMR-FT is able to detect and follow over time myocardial boundaries leading to a more automatized quantification of strain parameters[9]. From a technical point of view, the features tracked by CMR-FT are anatomic elements typically identified along the cavity-myocardial interface due to the high contrast resolution between blood pool and myocardium. Every feature is tracked across the cardiac cycle by specific algorithms searching the most similar image pattern on the following frame within a small window centered around the feature[10]. The endocardial and epicardial borders are usually manually traced in the end-diastolic phase, then the CMR-FT software automatically tracks the border across the whole cardiac cycle. Global longitudinal strain (GLS) is derived by 2 or 3 long-axis steady state free precession (SSFP) cine images while global circumferential strain (GCS) and global radial strain (GRS) are derived from the short axis cine image stack. Several CMR-FT softwares are commercially available and can be directly applied to all CMR routine scans, as they work on standard SSFP cine images[11].
Studies investigating CMR-FT strain values in healthy subjects have repeatedly demonstrated that global measurements are more reproducible compared to regional ones that are limited by partial volume effect with features fading/leaving the image plane between end diastole and end systole[12]. In particular, GCS seems to be less affected by inter-observer and inter-vendor variability compared to GRS and GLS in which the complex anatomy of the atrio-ventricular annular region may lead to poor tracking[11]. Strain values are affected by both gender and age but in general terms, values of circumferential and LS < -17% to -20% as well as values of RS > 25%-30% are considered within normal range[12-15].
Both global and regional strain parameters as well as dyssynchrony indices have been widely investigated in patients with ischemic heart disease. The main areas of interest include the correlation between regional strain parameters and infarct characteristics, the impact of strain parameters on long-term LV remodeling and the ability of strain to predict long-term clinical outcomes and to identify inducible ischemia[16].
All strain parameters are impaired in infarcted territories with strain values inversely related to the infarct size and infarct transmurality[17,18]. Compared to wall motion abnormalities, segmental strain values allows a more accurate discrimination between areas of subendocardial from transmural infarction and non-infarcted remote zones with only certain limitations in the early post-infarct phase due to the coexistence of edema, inflammatory cells, hemorrhage and viable and necrotic myocardial fibers[17,19-21]. Among all strain parameters, CS has shown to be the more accurate in assessing infarct extension and has also demonstrated its superiority to conventional LVEF determination in identifying subtle impairments of LV contractile function[19,22,23].
Data concerning the capability of strain analysis to predict adverse LV remodeling after acute myocardial infarction are debating[24-26]. In a study including 74 patients who underwent CMR within 4 d after successfully reperfused ST-elevation myocardial infarction (STEMI), a cut-off value of -19.3% for GCS appeared as accurate as late gadolinium enhancement (LGE) extension to identify patients with recovered LVEF (≥ 50%) at 6-mo follow-up while no significant correlation was determined using GLS[24]. In another study including 164 STEMI who underwent CMR a median of 3 d after the acute event, CS was not inferior to the area of LGE in predicting segmental functional improvement/normalization after a median of 9-mo[25]. Conversely, Shetye et al[26] have recently failed to demonstrate a correlation between baseline strain parameters and the development of adverse LV remodeling after 4-mo follow-up among 65 patients with STEMI who underwent CMR within 3 d post-reperfusion, even though a good correlation was found between strain parameters, baseline LVEF and infarct size.
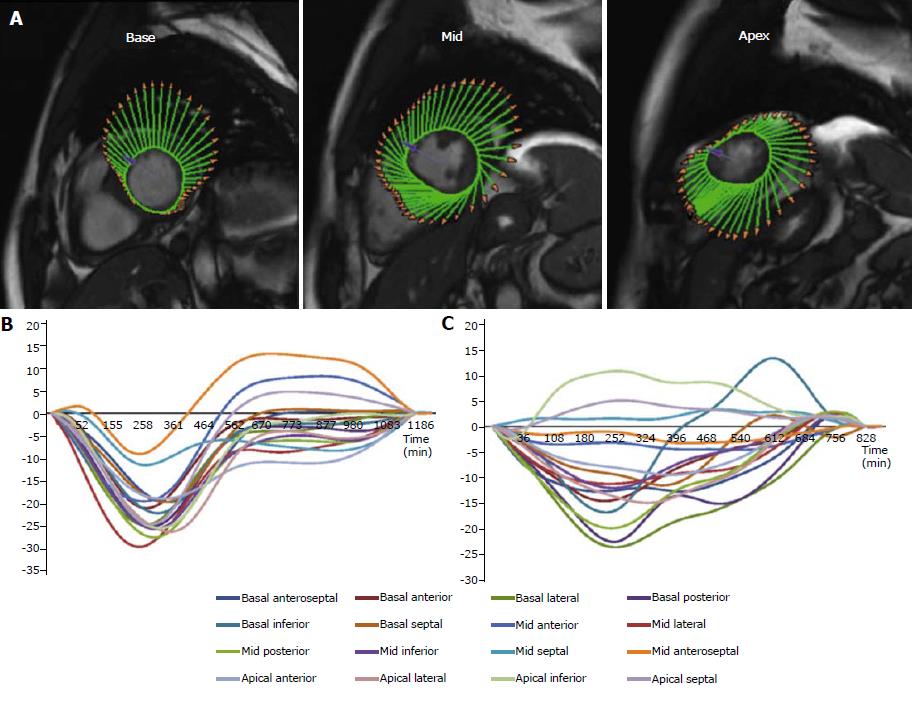
The correlation between scar extent determined by LGE imaging and long-term risk of major adverse cardiac events (MACE) after myocardial infarction is well known[27]. However, it has been recently pointed out how various CMR-FT derived parameters maybe able to accurately predict long-term clinical outcomes (Table 1). In a large study by Gavara et al[28], the prognostic value of CMR-FT was investigated among 323 patients who underwent CMR one week after a STEMI. During a median follow-up of 36-mo, all strain parameters were correlated to the incidence of a composite endpoint including cardiac death, readmission for heart failure and reinfarction. However, after adjustment for baseline and CMR variables, GLS (HR 1.21; 95%CI: 1.11-1.32; P < 0.001) was the only independent predictor of MACE. In particular, MACE rate was higher in patients with a GLS ≥ -11% (22% vs 9%; P = 0.001). In the same study, those results were also validated in an external cohort of 190 STEMI patients confirming both the higher incidence of MACE in patients with a GLS ≥ -11% (34% vs 9%; P < 0.001) and the independent predictive value of GLS (HR 1.18; 95%CI: 1.04-1.33; P = 0.008)[28]. These findings were subsequently confirmed and extended by our group. We have reported GCS to be significantly and independently associated to the occurrence of a composite endpoint including cardiovascular death, aborted SCD and hospitalization for heart failure (HR 1.16; 95%CI: 1.07-1.25; P < 0.001) during a median follow-up of 95 mo in a population of 180 patients admitted for a first STEMI and who underwent CMR imaging a median of 8 d following the index event[29]. We have also reported how early assessment (median of 7 d after STEMI) of LV mechanical dispersion defined as the standard deviation of the time-to-peak CS of the LV segments, is significantly and independently related to the occurrence of MACE (HR 1.39, 95%CI: 1.20-1.62, P < 0.001) (Figure 2)[30].
| Reference | No. of patients | Herat disease | Parameters analyzed | Outcome | Occurrence of outcome, % | Independent predictors of the outcome event (HR) | Follow-up duration |
| Gavara et al[28], 2017 | 323 | IHD (recent STEMI) | GLS GCS GRS n. segments with altered LS n. segments with altered CS n. segments with altered RS LVEF LGE MVO | Cardiac death, readmission for heart failure and reinfarction | 17 | GLS (1.21) | 36 mo (median) |
| Nucifora et al[29], 2018 | 180 | IHD (recent STEMI) | GCS LVEF LGE MVO | Cardiovascular death, aborted SCD and hospitalization for heart failure | 22 | GCS (1.16) | 95 mo (median) |
| Muser et al[30], 2017 | 130 | IHD (recent STEMI) | Mechanical dispersion LVEF LGE MVO | Cardiovascular death, aborted SCD and hospitalization for heart failure | 20 | Mechanical dispersion (1.39) | 95 mo (median) |
| Buss et al[35], 2015 | 210 | IDCM | GLS GCS GRS Mean LS Mean CS Mean RS LVEF LGE | Composite of cardiac death, heart transplant and aborted SCD | 12 | GLS (1.27) Mean LS (5.44) | 5.3 yr (median) |
| Riffel et al[36], 2016 | 146 | IDCM | Long axis strain LVEF LVEDV LGE | Composite of cardiac death, heart transplant and aborted SCD | 16 | Long axis strain (1.28) LVEDV/BSA (1.01) LGE (2.51) | 4.3 ± 2.0 yr |
| Romano et al[37], 2017 | 470 | IHD + IDCM | GLS LVEF LGE | All-cause death | 20 | GLS (2.35) LVEF (0.95) | 4 yr (median) |
| Romano et al[38], 2018 | 1012 | IHD + NICM | GLS LVEF LGE | All-cause death | 13 | GLS (1.89) | 4.4 yr (median) |
| Pi et al[39], 2018 | 172 | IDCM | GLS GCS GRS LVEF LGE | Composite of cardiac death and heart transplant | 25 | LGE (4.73) | 47 mo (median) |
Due to its high sensitivity in identifying contractile function compared to visual assessment of wall motion abnormalities or determination of changes in LVEF, CMR-FT has recently been proposed to evaluate inducible ischemia and establishing contractile reserve in patients with chronic ischemic heart disease[31,32]. Schneeweis et al[33] have demonstrated how in 25 patients with suspected or known coronary artery disease, undergoing dobutamine stress CMR, CS during high doses of dobutamine, was able to identify segments supplied by a vessel of > 70% narrowing with a sensitivity of 75% and specificity of 67% using a strain threshold of -33.2%[33]. In the same study, the authors also showed how impairment of CS already occurred at intermediate-doses of dobutamine allowing an earlier detection of inducible ischemia compared to visual assessment[33]. In another study including 15 patients undergoing viability assessment by low-dose dobutamine stress, there was no response to dobutamine in dysfunctional segments with scar transmurality > 75% while dysfunctional segments without scar showed improvement either in subendocardial and subepicardial GCS as well as in GRS. In particular, GCS improved in all segments up to a transmurality of 75% while GRS improved in segments with < 50% transmurality and remained unchanged above 50% transmurality[32].
The presence and extension of scar represents a negative prognostic factor in patients with idiopathic dilated cardiomyopathy (IDCM), as well[27]. Unfortunately, no clear evidence of LGE can be found in up to 60% of these patients making extremely important to identify new potential predictors to further stratify individual risk beyond simple LVEF[34]. A study including 210 patients with IDCM found GLS to be an independent predictor of MACE including cardiac death, heart transplantation and aborted SCD (HR 1.27, 95%CI: 1.06-1.52, P < 0.02) during a median follow-up of 5.3-years regardless to the baseline LVEF and the presence of LGE, while no significant association was found with GCS and GRS[35]. Another study including 146 patients affected by IDCM, investigated the value of “long axis strain” defined as the distance between the epicardial border of the LV apex and the midpoint of a line connecting the origins of the mitral valve leaflets in end-systole and end-diastole in predicting a combination of cardiac death, heart transplantation and aborted SCD and found that the ratio between LV end-diastolic volume/body surface area (HR 1.01, 95%CI: 1.00-1.02, P = 0.04), the presence of LGE (HR 2.51, 95%CI: 1.02–6.19, P = 0.04) as well as long axis strain (HR 1.28, 95%CI: 1.07-1.52, P < 0.01) were all independent predictors of MACE[36]. The incremental value of CMR-FT strain was subsequently confirmed in a series of 470 patients of whom 140 with IDCM, in which was described an independent correlation of GLS (HR 2.35, 95%CI: 1.81-3.06, P < 0.001) and LVEF (HR 0.95, 95%CI: 0.91-0.99, P = 0.038) with the risk of death during a median follow-up of 4-years regardless to the presence and extension of LGE[37]. In a large multicenter study including 1012 patients with both ischemic heart disease and non-ischemic cardiomyopathy, GLS was an independent predictor of all-cause mortality over LVEF and presence of LGE in the all cohort (HR 1.89, 95%CI: 1.55-2.07, P < 0.001) as well as in the ischemic (HR 1.95, 95%CI: 1.48-2.58, P < 0.001) and non-ischemic (HR 2.14, 95%CI: 1.56-2.91, P < 0.001) subgroups[38]. The above results have not been confirmed by a recent study including 172 patients with IDCM and moderately to severely reduced LVEF (<40%). In this study, neither GLS, GCS or GRS were correlated with the risk of death or heart transplant during a median follow-up of 47-mo, while presence of LGE and serum sodium were the only independent predictors of the outcome event (Table 1)[39].
Myocardial strain has proven to be a valuable tool in early identification, staging and risk stratification of several forms of non-ischemic cardiomyopathy. In patients with hypertrophic cardiomyopathy (HCM), a direct correlation between strain parameters and the presence of LGE has been repeatedly proven, being the presence of replacement fibrosis associated to a reduction in CS[40,41]. However, intramural strain has demonstrated to be reduced in more hypertrophied segments compared to the non-hypertrophied ones regardless to the presence of LGE, proving myocardial scarring not to be the only determinant of regional contractile dysfunction[42].
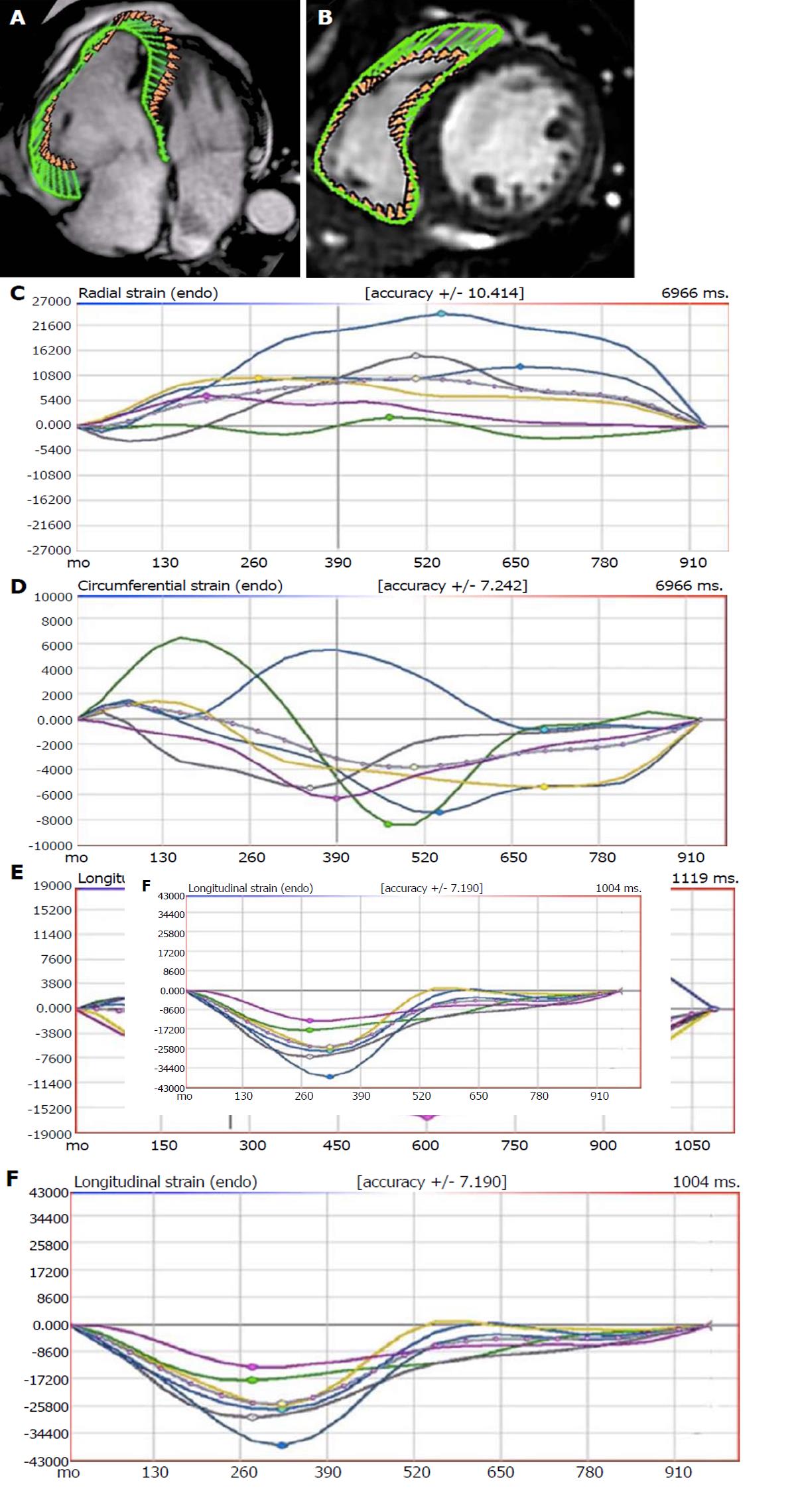
Global and regional right ventricular (RV) strain parameters have shown to be impaired in patients with overt arrhythmogenic right ventricular cardiomyopathy (ARVC) regardless to RV dimensions and function[43,44]. In a study comparing RV strain parameters in 32 patients matching the task force criteria for ARVC but with no or only minor CMR criteria, to 32 patients with idiopathic RV outflow tract premature ventricular contractions and 32 healthy volunteers, we found that RV GLS, GCS and GRS were all significantly reduced in the ARVC group. In particular, a RV GLS > -23.2% was able to identify 88% of ARVC patients without definite CMR criteria showing the incremental value of CMR-FT over conventional CMR imaging in early detection of the disease (Figure 3)[45].
In the same direction, strain parameters have shown to be impaired in patients with acute or previous myocarditis and preserved LVEF regardless to the presence of LGE confirming the higher sensitivity of CMR-FT in identifying contractile impairment at a subclinical stage[46,47]. In patients affected by LV non-compaction, we have found that subclinical impairment of myocardial deformation, occurs early in the natural history of the disease, being noticeable since the pediatric age with e reduction in all global strain parameters while overt LVEF reduction tends to manifest only later in adulthood[48]. In this regard, CMR-FT may be used to early detect cardiac involvement in systemic diseases or during administration of drugs with potential cardiotoxic effects. Monitoring of cardiotoxicity during cancer therapies is currently recommended by echocardiographic evaluation of LVEF and a decline in the LVEF is needed in order to decide to suspend/modify therapy[3]. Nakano et al[49] have demonstrated how both GLS and GCS were significantly reduced after 6 mo of therapy with trastuzumab in 9 women treated for breast cancer. Changes in global strain parameters were correlated with changes in LVEF, however their predictive value on the development of heart failure needs to be proven[49].
Some preliminary data have shown that parameters derived from CMR-FT such as LV rotational indices are disease specific and therefore maybe be used to differentiate between cardiomyopathies. We compared LV twist and untwist rates between 20 patients with cardiac amyloidosis (CA) to 20 patients with HCM and 20 healthy controls showing how both peak LV twist and peak LV untwist rates were significantly impaired in patients with CA while peak LV twist rate was significantly increased in patients with HCM compared to controls. Patients with HCM also presented a preserved peak LV untwist rate. The time to peak LV untwist rate was significantly prolonged both in CA and HCM compared to healthy subjects[50].
The follow-up of grown-up patients with congenital heart diseases (CHD) is usually based upon echocardiography, however, the poor acoustic window due to previous multiple surgical procedures or coexistence of other anatomical deformities as well the complex anatomy of repaired congenital defects represents some major limitations. For this reason, CMR imaging is increasingly becoming the imaging modality of choice for evaluation of CHD. In these terms CMR-FT derived parameters may have important clinical implications in evaluating surgical results and in early detection of complications. In patients with repaired tetralogy of Fallot (TOF), for example, a significant impairment of all global strain parameters has been described in those patients who experienced death or sustained ventricular tachycardia compared to those of similar age who did not experienced outcome events[51]. An improvement in LV GCS and GLS within 6 mo after transcatheter implantation of a pulmonic valve has also been documented in patients with repaired TOF and clinically relevant residual pulmonary regurgitation/stenosis after initial corrective surgery[52]. Moreover, RV GLS has been related to clinically relevant variables such as exercise capacity and oxygen consumption in 28 patients with repaired TOF[53]. Those findings have been further confirmed in a recent large prospective series of 372 repaired TOF patients, in which LV GCS and RV GLS independently predicted death, aborted SCD or documented ventricular tachycardia during a median follow-up of 7.4 years[54]. Similar findings have been found in 15 patients who underwent Fontan palliative intervention; GCS and GLS of the single ventricle both correlated with New York Heart Association class and peak oxygen uptake on cardiopulmonary exercise test[55]. Among patients with successful repaired coarctation of the aorta and preserved LVEF, impairment of GLS but not GCS or GRS has been associate to coexistence of LV hypertrophy[56].
A significant proportion of cardiac resynchronization therapy (CRT) patients may fail to reach an adequate response in terms of LVEF and HF status improvement and those patients are commonly addressed as “non-responders”. Increasing efforts have been made in the last years to better select optimal candidates to CRT but also to identify the best site for LV lead implantation in order to maximize the chances of response[57,58]. For this reason, CMR has emerged as a valuable technique been able to noninvasively evaluate LV activation patterns and dyssynchrony[30,45,59]. Taylor et al[60] have demonstrated that CRT implantation guided by determination of segments with latest mechanical activation (defined by time to peak systolic CS) and absence of LGE is able to improve LV reverse remodeling as well as long term survival and reduce the risk of hospitalizations for heart failure. From a practical point of view, CMR derived information on scar, dyssynchrony and coronary venous anatomy maybe integrated with fluoroscopy or 3D-electroanatomical mapping systems to real-time guide LV lead placement during interventional procedures[61,62].
The application of CMR-FT techniques to the left atrium (LA) have shown to accurately characterize LA physiology compared to simple global measures such as atrial volume, area and atrial ejection fraction[63]. It has been advocated that changes in LA function precede the development of heart failure in several cardiac disorders. The development of LA dysfunction due to increased LV stiffness associated to the presence of replacement or diffuse ventricular fibrosis has been described as a potential marker of early diastolic disfunction[64]. Recent findings highlighted how there may be disease specific patterns of LA dysfunction. Patients with HCM, for example, present an increased contractile function compared with healthy controls. On the other side, patients with diastolic dysfunction and preserved LVEF have reduced atrial contractility[65]. There is some evidence that quantitative measurement of LA function may also have prognostic implications as impairment in LA strain has shown to precede development of heart failure in the general population and to improve risk stratification for cerebrovascular events in patients with atrial fibrillation[66,67].
Analysis of CMR-FT strain parameters has been applied to patients with isolated bicuspid aortic valve (BAV) (i.e., without aortic stenosis, aortic regurgitation or aortic dilatation)[68]. Interestingly, patients with “clinically normal” BAV have significant impairment of LV systolic and diastolic myocardial mechanics; furthermore, the impairment of LV systolic mechanics observed in BAV patients is independently related to the congenital abnormality of aortic valve itself. The authors postulated that the observed intrinsic impairment of LV contractility may accelerate the ominous LV remodeling pathways occurring after the development of significant aortic valve dysfunction, possibly explaining the described premature occurrence of congestive heart failure in BAV patients compared with the general population[69].
CMR-FT strain analysis has been also applied to study the adult consequences of pre-term birth. Pre-term birth interferes with the normal in-utero development of the heart, potentially leading to abnormally remodeled left ventricles. In a large study, 102 subjects have been prospectively followed since pre-term birth (gestational age = 30.3 ± 2.5 wk) to the age of 20-39 years when they underwent a CMR study. Compared to 132 subjects born at term of similar age, preterm individuals had increased LV mass proportional to the degree of prematurity and short LVs with small internal diameters and a displaced apex. Interestingly, even if LVEF was similar to subjects born at term, both longitudinal systolic (peak strain, strain rate, and velocity) and diastolic (peak strain rate and velocity) function as well as rotational (apical and basal peak systolic rotation rate, net twist angle) parameters were significantly lower[70]. Similar findings have been described by the same group affecting the RV. Pre-term subjects had smaller RV with bigger RV mass with the severity of differences proportional to gestational age. Moreover, differences in RV function were greater than those reported for the LV as subjects born pre-term had significantly lower RV ejection fraction (RVEF) with 6% of them having clear RV systolic dysfunction. In agreement with the lower RVEF, RV GLS and peak systolic strain rate were also significantly lower compared to subjects born at term[71]. The clinical implications of these findings on the potential development of overt cardiomyopathies need further prospective evaluation.
In the last years a growing amount of evidence suggests that study of cardiac function by strain analysis can accurately detect cardiac disorders at a subclinical level, improve risk stratification of patients with various cardiac conditions and potentially monitor treatment effect. In this setting, the use of CMR-FT may represent an easy and fast tool due to its applicability to routinely acquired SSFP cine images by dedicated software without need for upfront use of specific protocols. Further technical improvements able to achieve a higher level of accuracy and reproducibility in the assessment of measures and more standardization between different vendors are still required to make CMR-FT ready to use in routine clinical practice.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A, A, A, A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Altarabsheh SE, Anan R, Lin GM, Montecucco F, Nurzynska D, Said SAM, Ueda H, Voon V S- Editor: Ji FF L- Editor: A E- Editor: Wu YXJ
| 1. | Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2017;72:S0735-1097(17)41306-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 812] [Article Influence: 116.0] [Reference Citation Analysis (0)] |
| 2. | Rapezzi C, Arbustini E, Caforio AL, Charron P, Gimeno-Blanes J, Heliö T, Linhart A, Mogensen J, Pinto Y, Ristic A. Diagnostic work-up in cardiomyopathies: bridging the gap between clinical phenotypes and final diagnosis. A position statement from the ESC Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:1448-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 287] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 3. | Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GYH, Lyon AR. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:2768-2801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1409] [Cited by in RCA: 1498] [Article Influence: 166.4] [Reference Citation Analysis (0)] |
| 4. | Caforio ALP, Adler Y, Agostini C, Allanore Y, Anastasakis A, Arad M, Böhm M, Charron P, Elliott PM, Eriksson U. Diagnosis and management of myocardial involvement in systemic immune-mediated diseases: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Disease. Eur Heart J. 2017;38:2649-2662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 5. | Schuster A, Hor KN, Kowallick JT, Beerbaum P, Kutty S. Cardiovascular Magnetic Resonance Myocardial Feature Tracking: Concepts and Clinical Applications. Circ Cardiovasc Imaging. 2016;9:e004077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 284] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 6. | Ibrahim el-SH. Myocardial tagging by cardiovascular magnetic resonance: evolution of techniques--pulse sequences, analysis algorithms, and applications. J Cardiovasc Magn Reson. 2011;13:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 187] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 7. | Zerhouni EA, Parish DM, Rogers WJ, Yang A, Shapiro EP. Human heart: tagging with MR imaging-a method for noninvasive assessment of myocardial motion. Radiology. 1988;169:59-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1132] [Cited by in RCA: 893] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 8. | Götte MJ, Germans T, Rüssel IK, Zwanenburg JJ, Marcus JT, van Rossum AC, van Veldhuisen DJ. Myocardial strain and torsion quantified by cardiovascular magnetic resonance tissue tagging: studies in normal and impaired left ventricular function. J Am Coll Cardiol. 2006;48:2002-2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 159] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 9. | Hor KN, Baumann R, Pedrizzetti G, Tonti G, Gottliebson WM, Taylor M, Benson DW, Mazur W. Magnetic resonance derived myocardial strain assessment using feature tracking. J Vis Exp. 2011;pii:2356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 10. | Claus P, Omar AMS, Pedrizzetti G, Sengupta PP, Nagel E. Tissue Tracking Technology for Assessing Cardiac Mechanics: Principles, Normal Values, and Clinical Applications. JACC Cardiovasc Imaging. 2015;8:1444-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 336] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 11. | Schuster A, Stahnke VC, Unterberg-Buchwald C, Kowallick JT, Lamata P, Steinmetz M, Kutty S, Fasshauer M, Staab W, Sohns JM. Cardiovascular magnetic resonance feature-tracking assessment of myocardial mechanics: Intervendor agreement and considerations regarding reproducibility. Clin Radiol. 2015;70:989-998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 148] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 12. | Kowallick JT, Morton G, Lamata P, Jogiya R, Kutty S, Lotz J, Hasenfuß G, Nagel E, Chiribiri A, Schuster A. Inter-study reproducibility of left ventricular torsion and torsion rate quantification using MR myocardial feature tracking. J Magn Reson Imaging. 2016;43:128-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Taylor RJ, Moody WE, Umar F, Edwards NC, Taylor TJ, Stegemann B, Townend JN, Hor KN, Steeds RP, Mazur W. Myocardial strain measurement with feature-tracking cardiovascular magnetic resonance: normal values. Eur Heart J Cardiovasc Imaging. 2015;16:871-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 191] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 14. | Augustine D, Lewandowski AJ, Lazdam M, Rai A, Francis J, Myerson S, Noble A, Becher H, Neubauer S, Petersen SE. Global and regional left ventricular myocardial deformation measures by magnetic resonance feature tracking in healthy volunteers: comparison with tagging and relevance of gender. J Cardiovasc Magn Reson. 2013;15:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 239] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 15. | Andre F, Steen H, Matheis P, Westkott M, Breuninger K, Sander Y, Kammerer R, Galuschky C, Giannitsis E, Korosoglou G. Age- and gender-related normal left ventricular deformation assessed by cardiovascular magnetic resonance feature tracking. J Cardiovasc Magn Reson. 2015;17:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 164] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 16. | Mangion K, McComb C, Auger DA, Epstein FH, Berry C. Magnetic Resonance Imaging of Myocardial Strain After Acute ST-Segment-Elevation Myocardial Infarction: A Systematic Review. Circ Cardiovasc Imaging. 2017;10:pii: e006498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 17. | Gao H, Allan A, McComb C, Luo X, Berry C. Left ventricular strain and its pattern estimated from cine CMR and validation with DENSE. Phys Med Biol. 2014;59:3637-3656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | McComb C, Carrick D, McClure JD, Woodward R, Radjenovic A, Foster JE, Berry C. Assessment of the relationships between myocardial contractility and infarct tissue revealed by serial magnetic resonance imaging in patients with acute myocardial infarction. Int J Cardiovasc Imaging. 2015;31:1201-1209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Khan JN, Singh A, Nazir SA, Kanagala P, Gershlick AH, McCann GP. Comparison of cardiovascular magnetic resonance feature tracking and tagging for the assessment of left ventricular systolic strain in acute myocardial infarction. Eur J Radiol. 2015;84:840-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 20. | Neizel M, Lossnitzer D, Korosoglou G, Schäufele T, Peykarjou H, Steen H, Ocklenburg C, Giannitsis E, Katus HA, Osman NF. Strain-encoded MRI for evaluation of left ventricular function and transmurality in acute myocardial infarction. Circ Cardiovasc Imaging. 2009;2:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Altiok E, Neizel M, Tiemann S, Krass V, Becker M, Zwicker C, Koos R, Kelm M, Kraemer N, Schoth F. Layer-specific analysis of myocardial deformation for assessment of infarct transmurality: comparison of strain-encoded cardiovascular magnetic resonance with 2D speckle tracking echocardiography. Eur Heart J Cardiovasc Imaging. 2013;14:570-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Miyagi H, Nagata M, Kitagawa K, Kato S, Takase S, Sigfridsson A, Ishida M, Dohi K, Ito M, Sakuma H. Quantitative assessment of myocardial strain with displacement encoding with stimulated echoes MRI in patients with coronary artery disease. Int J Cardiovasc Imaging. 2013;29:1779-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Kihlberg J, Haraldsson H, Sigfridsson A, Ebbers T, Engvall JE. Clinical experience of strain imaging using DENSE for detecting infarcted cardiac segments. J Cardiovasc Magn Reson. 2015;17:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Buss SJ, Krautz B, Hofmann N, Sander Y, Rust L, Giusca S, Galuschky C, Seitz S, Giannitsis E, Pleger S. Prediction of functional recovery by cardiac magnetic resonance feature tracking imaging in first time ST-elevation myocardial infarction. Comparison to infarct size and transmurality by late gadolinium enhancement. Int J Cardiol. 2015;183:162-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Khan JN, Nazir SA, Singh A, Shetye A, Lai FY, Peebles C, Wong J, Greenwood JP, McCann GP. Relationship of Myocardial Strain and Markers of Myocardial Injury to Predict Segmental Recovery After Acute ST-Segment-Elevation Myocardial Infarction. Circ Cardiovasc Imaging. 2016;9:pii: e003457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Shetye AM, Nazir SA, Razvi NA, Price N, Khan JN, Lai FY, Squire IB, McCann GP, Arnold JR. Comparison of global myocardial strain assessed by cardiovascular magnetic resonance tagging and feature tracking to infarct size at predicting remodelling following STEMI. BMC Cardiovasc Disord. 2017;17:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Ganesan AN, Gunton J, Nucifora G, McGavigan AD, Selvanayagam JB. Impact of Late Gadolinium Enhancement on mortality, sudden death and major adverse cardiovascular events in ischemic and nonischemic cardiomyopathy: A systematic review and meta-analysis. Int J Cardiol. 2018;254:230-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 28. | Gavara J, Rodriguez-Palomares JF, Valente F, Monmeneu JV, Lopez-Lereu MP, Bonanad C, Ferreira-Gonzalez I, Garcia Del Blanco B, Rodriguez-Garcia J, Mutuberria M. Prognostic Value of Strain by Tissue Tracking Cardiac Magnetic Resonance After ST-Segment Elevation Myocardial Infarction. JACC Cardiovasc Imaging. 2018;11:1448-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 29. | Nucifora G, Muser D, Tioni C, Shah R, Selvanayagam JB. Prognostic value of myocardial deformation imaging by cardiac magnetic resonance feature-tracking in patients with a first ST-segment elevation myocardial infarction. Int J Cardiol. 2018;271:387-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Muser D, Tioni C, Shah R, Selvanayagam JB, Nucifora G. Prevalence, Correlates, and Prognostic Relevance of Myocardial Mechanical Dispersion as Assessed by Feature-Tracking Cardiac Magnetic Resonance After a First ST-Segment Elevation Myocardial Infarction. Am J Cardiol. 2017;120:527-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Schuster A, Kutty S, Padiyath A, Parish V, Gribben P, Danford DA, Makowski MR, Bigalke B, Beerbaum P, Nagel E. Cardiovascular magnetic resonance myocardial feature tracking detects quantitative wall motion during dobutamine stress. J Cardiovasc Magn Reson. 2011;13:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 32. | Schuster A, Paul M, Bettencourt N, Morton G, Chiribiri A, Ishida M, Hussain S, Jogiya R, Kutty S, Bigalke B. Cardiovascular magnetic resonance myocardial feature tracking for quantitative viability assessment in ischemic cardiomyopathy. Int J Cardiol. 2013;166:413-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 33. | Schneeweis C, Qiu J, Schnackenburg B, Berger A, Kelle S, Fleck E, Gebker R. Value of additional strain analysis with feature tracking in dobutamine stress cardiovascular magnetic resonance for detecting coronary artery disease. J Cardiovasc Magn Reson. 2014;16:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | McCrohon JA, Moon JC, Prasad SK, McKenna WJ, Lorenz CH, Coats AJ, Pennell DJ. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 855] [Cited by in RCA: 813] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 35. | Buss SJ, Breuninger K, Lehrke S, Voss A, Galuschky C, Lossnitzer D, Andre F, Ehlermann P, Franke J, Taeger T. Assessment of myocardial deformation with cardiac magnetic resonance strain imaging improves risk stratification in patients with dilated cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2015;16:307-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 213] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 36. | Riffel JH, Keller MG, Rost F, Arenja N, Andre F, Aus dem Siepen F, Fritz T, Ehlermann P, Taeger T, Frankenstein L. Left ventricular long axis strain: a new prognosticator in non-ischemic dilated cardiomyopathy? J Cardiovasc Magn Reson. 2016;18:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 37. | Romano S, Judd RM, Kim RJ, Kim HW, Klem I, Heitner J, Shah DJ, Jue J, White BE, Shenoy C. Association of Feature-Tracking Cardiac Magnetic Resonance Imaging Left Ventricular Global Longitudinal Strain With All-Cause Mortality in Patients With Reduced Left Ventricular Ejection Fraction. Circulation. 2017;135:2313-2315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 38. | Romano S, Judd RM, Kim RJ, Kim HW, Klem I, Heitner JF, Shah DJ, Jue J, White BE, Indorkar R. Feature-Tracking Global Longitudinal Strain Predicts Death in a Multicenter Population of Patients with Ischemic and Nonischemic Dilated Cardiomyopathy Incremental to Ejection Fraction and Late Gadolinium Enhancement. JACC Cardiovasc Imaging. 2018;11:1419-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 210] [Article Influence: 30.0] [Reference Citation Analysis (1)] |
| 39. | Pi SH, Kim SM, Choi JO, Kim EK, Chang SA, Choe YH, Lee SC, Jeon ES. Prognostic value of myocardial strain and late gadolinium enhancement on cardiovascular magnetic resonance imaging in patients with idiopathic dilated cardiomyopathy with moderate to severely reduced ejection fraction. J Cardiovasc Magn Reson. 2018;20:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 40. | Bogarapu S, Puchalski MD, Everitt MD, Williams RV, Weng HY, Menon SC. Novel Cardiac Magnetic Resonance Feature Tracking (CMR-FT) Analysis for Detection of Myocardial Fibrosis in Pediatric Hypertrophic Cardiomyopathy. Pediatr Cardiol. 2016;37:663-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 41. | Nucifora G, Muser D, Gianfagna P, Morocutti G, Proclemer A. Systolic and diastolic myocardial mechanics in hypertrophic cardiomyopathy and their link to the extent of hypertrophy, replacement fibrosis and interstitial fibrosis. Int J Cardiovasc Imaging. 2015;31:1603-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 42. | Aletras AH, Tilak GS, Hsu LY, Arai AE. Heterogeneity of intramural function in hypertrophic cardiomyopathy: mechanistic insights from MRI late gadolinium enhancement and high-resolution displacement encoding with stimulated echoes strain maps. Circ Cardiovasc Imaging. 2011;4:425-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 43. | Heermann P, Hedderich DM, Paul M, Schülke C, Kroeger JR, Baeßler B, Wichter T, Maintz D, Waltenberger J, Heindel W. Biventricular myocardial strain analysis in patients with arrhythmogenic right ventricular cardiomyopathy (ARVC) using cardiovascular magnetic resonance feature tracking. J Cardiovasc Magn Reson. 2014;16:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 44. | Vigneault DM, te Riele AS, James CA, Zimmerman SL, Selwaness M, Murray B, Tichnell C, Tee M, Noble JA, Calkins H. Right ventricular strain by MR quantitatively identifies regional dysfunction in patients with arrhythmogenic right ventricular cardiomyopathy. J Magn Reson Imaging. 2016;43:1132-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 45. | Prati G, Vitrella G, Allocca G, Muser D, Buttignoni SC, Piccoli G, Morocutti G, Delise P, Pinamonti B, Proclemer A. Right Ventricular Strain and Dyssynchrony Assessment in Arrhythmogenic Right Ventricular Cardiomyopathy: Cardiac Magnetic Resonance Feature-Tracking Study. Circ Cardiovasc Imaging. 2015;8:e003647; discussion e003647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 46. | Baeßler B, Schaarschmidt F, Dick A, Michels G, Maintz D, Bunck AC. Diagnostic implications of magnetic resonance feature tracking derived myocardial strain parameters in acute myocarditis. Eur J Radiol. 2016;85:218-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 47. | Weigand J, Nielsen JC, Sengupta PP, Sanz J, Srivastava S, Uppu S. Feature Tracking-Derived Peak Systolic Strain Compared to Late Gadolinium Enhancement in Troponin-Positive Myocarditis: A Case-Control Study. Pediatr Cardiol. 2016;37:696-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 48. | Nucifora G, Sree Raman K, Muser D, Shah R, Perry R, Awang Ramli KA, Selvanayagam JB. Cardiac magnetic resonance evaluation of left ventricular functional, morphological, and structural features in children and adolescents vs. young adults with isolated left ventricular non-compaction. Int J Cardiol. 2017;246:68-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 49. | Nakano S, Takahashi M, Kimura F, Senoo T, Saeki T, Ueda S, Tanno J, Senbonmatsu T, Kasai T, Nishimura S. Cardiac magnetic resonance imaging-based myocardial strain study for evaluation of cardiotoxicity in breast cancer patients treated with trastuzumab: A pilot study to evaluate the feasibility of the method. Cardiol J. 2016;23:270-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 50. | Nucifora G, Muser D, Morocutti G, Piccoli G, Zanuttini D, Gianfagna P, Proclemer A. Disease-specific differences of left ventricular rotational mechanics between cardiac amyloidosis and hypertrophic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2014;307:H680-H688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 51. | Moon TJ, Choueiter N, Geva T, Valente AM, Gauvreau K, Harrild DM. Relation of biventricular strain and dyssynchrony in repaired tetralogy of fallot measured by cardiac magnetic resonance to death and sustained ventricular tachycardia. Am J Cardiol. 2015;115:676-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 52. | Harrild DM, Marcus E, Hasan B, Alexander ME, Powell AJ, Geva T, McElhinney DB. Impact of transcatheter pulmonary valve replacement on biventricular strain and synchrony assessed by cardiac magnetic resonance feature tracking. Circ Cardiovasc Interv. 2013;6:680-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 53. | Kempny A, Fernández-Jiménez R, Orwat S, Schuler P, Bunck AC, Maintz D, Baumgartner H, Diller GP. Quantification of biventricular myocardial function using cardiac magnetic resonance feature tracking, endocardial border delineation and echocardiographic speckle tracking in patients with repaired tetralogy of Fallot and healthy controls. J Cardiovasc Magn Reson. 2012;14:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 54. | Orwat S, Diller GP, Kempny A, Radke R, Peters B, Kühne T, Boethig D, Gutberlet M, Dubowy KO, Beerbaum P. Myocardial deformation parameters predict outcome in patients with repaired tetralogy of Fallot. Heart. 2016;102:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (1)] |
| 55. | Schmidt R, Orwat S, Kempny A, Schuler P, Radke R, Kahr PC, Hellige A, Baumgartner H, Diller GP. Value of speckle-tracking echocardiography and MRI-based feature tracking analysis in adult patients after Fontan-type palliation. Congenit Heart Dis. 2014;9:397-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 56. | Kutty S, Rangamani S, Venkataraman J, Li L, Schuster A, Fletcher SE, Danford DA, Beerbaum P. Reduced global longitudinal and radial strain with normal left ventricular ejection fraction late after effective repair of aortic coarctation: a CMR feature tracking study. Int J Cardiovasc Imaging. 2013;29:141-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 57. | Bertini M, Mele D, Malagù M, Fiorencis A, Toselli T, Casadei F, Cannizzaro T, Fragale C, Fucili A, Campagnolo E. Cardiac resynchronization therapy guided by multimodality cardiac imaging. Eur J Heart Fail. 2016;18:1375-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 58. | Sassone B, Nucifora G, Mele D, Valzania C, Bisignani G, Boriani G; for Task Force on Imaging of Italian Association of Arrhythmias and Cardiac Stimulation (AIAC). Role of cardiovascular imaging in cardiac resynchronization therapy: a literature review. J Cardiovasc Med (Hagerstown). 2018;19:211-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 59. | Selvanayagam JB, Nucifora G. Myocardial Deformation Imaging by Feature-Tracking Cardiac Magnetic Resonance in Acute Myocardial Infarction: Do We Need It? Circ Cardiovasc Imaging. 2016;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 60. | Taylor RJ, Umar F, Panting JR, Stegemann B, Leyva F. Left ventricular lead position, mechanical activation, and myocardial scar in relation to left ventricular reverse remodeling and clinical outcomes after cardiac resynchronization therapy: A feature-tracking and contrast-enhanced cardiovascular magnetic resonance study. Heart Rhythm. 2016;13:481-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 61. | Shetty AK, Duckett SG, Ginks MR, Ma Y, Sohal M, Bostock J, Kapetanakis S, Singh JP, Rhode K, Wright M. Cardiac magnetic resonance-derived anatomy, scar, and dyssynchrony fused with fluoroscopy to guide LV lead placement in cardiac resynchronization therapy: a comparison with acute haemodynamic measures and echocardiographic reverse remodelling. Eur Heart J Cardiovasc Imaging. 2013;14:692-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 62. | Laksman Z, Yee R, Stirrat J, Gula LJ, Skanes AC, Leong-Sit P, Manlucu J, McCarty D, Turkistani Y, Scholl D. Model-based navigation of left and right ventricular leads to optimal targets for cardiac resynchronization therapy: a single-center feasibility study. Circ Arrhythm Electrophysiol. 2014;7:1040-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 63. | Evin M, Cluzel P, Lamy J, Rosenbaum D, Kusmia S, Defrance C, Soulat G, Mousseaux E, Roux C, Clement K. Assessment of left atrial function by MRI myocardial feature tracking. J Magn Reson Imaging. 2015;42:379-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 64. | Imai M, Ambale Venkatesh B, Samiei S, Donekal S, Habibi M, Armstrong AC, Heckbert SR, Wu CO, Bluemke DA, Lima JA. Multi-ethnic study of atherosclerosis: association between left atrial function using tissue tracking from cine MR imaging and myocardial fibrosis. Radiology. 2014;273:703-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 65. | Kowallick JT, Kutty S, Edelmann F, Chiribiri A, Villa A, Steinmetz M, Sohns JM, Staab W, Bettencourt N, Unterberg-Buchwald C. Quantification of left atrial strain and strain rate using Cardiovascular Magnetic Resonance myocardial feature tracking: a feasibility study. J Cardiovasc Magn Reson. 2014;16:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 210] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 66. | Habibi M, Chahal H, Opdahl A, Gjesdal O, Helle-Valle TM, Heckbert SR, McClelland R, Wu C, Shea S, Hundley G. Association of CMR-measured LA function with heart failure development: results from the MESA study. JACC Cardiovasc Imaging. 2014;7:570-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 161] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 67. | Inoue YY, Alissa A, Khurram IM, Fukumoto K, Habibi M, Venkatesh BA, Zimmerman SL, Nazarian S, Berger RD, Calkins H. Quantitative tissue-tracking cardiac magnetic resonance (CMR) of left atrial deformation and the risk of stroke in patients with atrial fibrillation. J Am Heart Assoc. 2015;4:pii: e001844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 68. | Nucifora G, Miller J, Gillebert C, Shah R, Perry R, Raven C, Joseph MX, Selvanayagam JB. Ascending Aorta and Myocardial Mechanics in Patients with “Clinically Normal” Bicuspid Aortic Valve. Int Heart J. 2018;59:741-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 69. | Disha K, Espinoza A, Rouman M, Secknus MA, Kuntze T, Girdauskas E. Long-Term Recovery of Reduced Left Ventricular Ejection Fraction after Aortic Valve Replacement in Patients with Bicuspid Aortic Valve Disease. Thorac Cardiovasc Surg. 2016;64:418-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 70. | Lewandowski AJ, Augustine D, Lamata P, Davis EF, Lazdam M, Francis J, McCormick K, Wilkinson AR, Singhal A, Lucas A. Preterm heart in adult life: cardiovascular magnetic resonance reveals distinct differences in left ventricular mass, geometry, and function. Circulation. 2013;127:197-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 374] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 71. | Lewandowski AJ, Bradlow WM, Augustine D, Davis EF, Francis J, Singhal A, Lucas A, Neubauer S, McCormick K, Leeson P. Right ventricular systolic dysfunction in young adults born preterm. Circulation. 2013;128:713-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 206] [Article Influence: 17.2] [Reference Citation Analysis (0)] |