Published online Oct 26, 2018. doi: 10.4330/wjc.v10.i10.165
Peer-review started: March 28, 2018
First decision: May 2, 2018
Revised: May 21, 2018
Accepted: August 26, 2018
Article in press: August 27, 2018
Published online: October 26, 2018
Processing time: 212 Days and 19.2 Hours
To statistically examine the released clinical trials and meta-analyses of polymeric bioresorbable scaffolds resuming the main accomplishments in the field with a translation to the routine clinical practice.
The statistical power in clinical trials such as ABSORB Japan, ABSORB China, EVERBIO II, AIDA, and few meta-analyses by the post hoc odds ratio-based sample size calculation, and the patterns of artery remodeling published in papers from ABSORB A and B trials were evaluated.
The phenomenal admiration from the first ABSORB studies in 2006-2013 was replaced by the tremendous disappointment in 2014-2017 due to reported relatively higher rates of target lesion failure (a mean prevalence of 9.16%) and device thrombosis (2.38%) in randomized controlled trials. Otherwise, bioresorbable vascular scaffold (BVS) performs as well as the metallic drug-eluting stent (DES) with a trend toward some benefits for cardiac mortality [risk ratio (RR), 0.58-0.94, P > 0.05]. The underpowered design was confirmed for some studies such as ABSORB Japan, ABSORB China, EVERBIO II, AIDA trials, and meta-analyses of Polimeni, Collet, and Mahmoud with some unintentional bias (judged by the asymmetrical Funnel plot). Scaffold thrombosis rates with Absorb BRS were comparable with DES performed with a so-called strategy of the BVS implantation with optimized pre-dilation (P), sizing (S) and post-dilation (P) (PSP) implantation (RR, PSP vs no PSP 0.37) achieving 0.35 per 100 patient-years, which is comparable to the RR 0.49 with bare-metal stents and the RR 1.06 with everolimus DES. Both ABSORB II and ABSORB III trials were powered enough for a five-year follow-up, but the results were not entirely conclusive due to the mostly non-significant fashion of data. The powered meta-analyses were built mostly on statistically poor findings.
The misunderstanding of the pathology of transient scaffolding drives the failures of the clinical trials. More bench studies of the vascular response are required. Several next-generation BVS including multifunctional electronic scaffold grant cardiology with a huge promise to make BVS technology great again.
Core tip: The high rates of target lesion failure and device thrombosis in randomized controlled trials caused confusion in the international interventional community with a decline of the technology in routine practice. The provided statistical analysis confirms the underpowered design of some clinical studies and meta-analyses of bioresorbable scaffolds. The misunderstanding of the pathobiology of the transient scaffolding drives the failures of the clinical trials. More bench studies of the vascular response are required to verify the leading mechanism of the device failure. Several next-generation scaffolds including multifunctional electronic scaffold grant cardiology with a huge promise to make this technology great again.
- Citation: Kharlamov AN. Undiscovered pathology of transient scaffolding t1remains a driver of failures in clinical trials. World J Cardiol 2018; 10(10): 165-186
- URL: https://www.wjgnet.com/1949-8462/full/v10/i10/165.htm
- DOI: https://dx.doi.org/10.4330/wjc.v10.i10.165
The groundbreaking news of September 8, 2017 delivered by Abbott Vascular (Santa Clara, CA; https://http://www.vascular.abbott/) outlined that “due to low commercial sales, Abbott will stop selling the first-generation Absorb bioresorbable vascular scaffold (BVS)”. The reassuring point was that Abbott will continue work on a next-generation 99 μm bioresorbable device. This was the end of the device thrombosis story that reached its climax this year when the United States Food and Drug Administration (FDA) on March 18, 2017 (and then on October 31, 2017 after release of ABSORB II 4-year, ABSORB III 3-year, and ABSORB IV 30-d results at TCT 2017 in Denver, CO, United States) informed[1] health care providers who are managing patients with Absorb GT1 BVS that there is a high rate of major adverse cardiac events (MACE) or correctly target lesion failure (TLF) registered in patients receiving BVS when compared to metallic XIENCE drug-eluting stent (DES). This alert was initially provoked by the release of the two-year data from the ABSORB III trial (a paper was not published yet) showing[2] an 11% rate of MACE in patients treated with BVS (n = 1322) at two years compared with 7.9% (n = 686) in XIENCE DES (P = 0.03). This study also demonstrated a 1.9% rate of scaffold thrombosis (ST) within the BVS vs 0.8% within the XIENCE stent at two years (P > 0.05, NS). These observed higher MACE rates in BVS patients were more likely when the device was implanted in small heart vessels (< 2.25 mm). Almost immediately after the United States, the European Union restricted BVS[3] to the sites of clinical registries (valid as of May 31, 2017). The CE Mark of approval remains in place, but only centers participating in formal registries (about 12 registries across Europe covering 114 hospitals) should be using BVS for now. Furthermore, the Australian Therapeutic Goods Administration (TGA) on May 2, 2017[4] issued a hazard alert as well and recalled all BVS from medical centers not studying the device. These reactions[5-15] of the healthcare authorities amid few recently published meta-analyses[16-25] wreck confusion[26-37] among physicians[38-45] about when and if they will ever be using the device again, jeopardizing the future of BVS and further development of bioresorbable devices. Notwithstanding, did we clear the situation with causes of these trends toward increased MACE and ST in the case of BVS? What is going on with cardiac mortality after BVS? Can we trust these data? Can we propose a solution and optimize our approaches for BVS implantation to prevent these complications and secure the technology? Do we understand the pathology of transient scaffolding? Should we first complete the bench studies and clear the vascular pathobiology of the BVS implantation?
Certainly, we face a real phenomenon of the elevated TLF and ST after implantation of BVS, which is very confusing and disappointing for physicians even for the fathers of technology such as Professor Patrick W Serruys, MD, PhD (Erasmus MC, Rotterdam, The Netherlands; Imperial College, London, United Kingdom) who said that “It’s not what we were expecting…That’s the reality!...Of course, we’re still in the first generation…” (a report to TCTMD on Oct 30, 2016 at TCT2016, Washington, DC, United States). We must mind the fact that this is a first-generation technology, and as Professor Gregg Stone, MD (Columbia University, NY, United States) said once, “If science stopped with every challenge for first gen devices, we would have no PCI devices!” (Twitter, @GreggWStone, Apr 16th, 2017). Meanwhile, the Colombo’s concept of the plastic jacket (a team of Professor Antonio Colombo, MD, San Raffaele Scientific Institute, Milan, Italy)[10], recently reported positive results of the European registries (German GABI-R, Italian IT-DISAPPEARS, Italian RAI, Swedish SCAAR, British Absorb United Kingdom, and French France Absorb) and Asian randomized controlled trials (RCT)[11] (ABSORB China, ABSORB Japan) are very reassuring and promising with relatively low rates of both TLF (3.9%-9.9% by one year) and ST (< 1.7%) even in complex lesions due to optimized accurate technique of the implantation (a high-pressure post-dilation in some registries was performed in 96.8% of cases with full PSP strategy in > 65.8% of lesions).
The running fourth industrial revolution[23-38] supports us with the remarkable idea of the transient scaffolding[39-51] and a dream of the vascular restoration therapy[25] of coronary atherosclerosis that has a potential to revolutionize cardiology providing physicians with a tackle for both to revitalize a circulation[20-36] in the coronary pool tailoring atheroregression[37-52]. Since 2006 the multimodality imaging studies of ABSORB[31] and ABSORB B[53-56] clinical trials that examined BVS have crashed to perform significant regression of atherosclerosis but with a trend toward absolute reduction of the percent atheroma volume (PAV) at 60 mo (at least -2.62%), which claims further research efforts.
The goal of this systematic review was to evaluate the accomplishments of the clinical trials, and patterns of pathology with trends of the artery remodeling in patients who underwent transient scaffolding with polymeric BVS within 60 mo after implantation with the grey-scale (GS) and virtual histology (VH) intravascular ultrasound (IVUS) imaging, quantitative coronary angiography (QCA), multislice computed tomography (MSCT) angiography, and optical coherence tomography (OCT). This review must answer the question about both the reasons for unsuccessful experience in clinical trials and the potential of BVS for regression of atherosclerosis with its further introduction into the routine clinical practice.
The available sources of the information published in PubMed/MEDLINE (primary electronic database), Google Scholar, ResearchGate, ClinicalTrials.Gov, and SCOPUS with the key words BVS, BRS, regression of atherosclerosis, imaging, artery remodeling, and atherosclerosis through the last 20 years with a focus on polymeric BVS and particularly ABSORB trials were investigated. The estimated observations comprised mean and/or median numbers of the variables reported by the authors of the original papers without access to the detailed patient-by-patient matrix of the evaluated studies. This systematic review (without meta-analysis) was accomplished by two reviewers who independently analyzed abstracts, extract data, and calculate the risk of bias. The received data have a different quality and potential of comparability but provide the most recent information. The risk of bias of each included trial was tested with the Cochrane Collaboration’s tool and elaborated with the information about expert imaging analysis and a methodology of the statistical estimation.
We have selected some trials for the further in-depth analysis including special subanalysis of the published results from ABSORB A and B trials. The study design of ABSORB A (NCT00300131) and B (NCT00856856) trials with a description of the study population, study device, study procedure, and definitions[31-43] were previously reported[44-56]. Briefly, the Absorb BVS device (Abbott Vascular, Santa Clara, CA, United States) was examined in the single-group, prospective, open-label study (A), with safety and imaging endpoints, 30 patients were enrolled at four participating sites between March 7, and July 18, 2006. The BVS was tested in 101 patients of the ABSORB Cohort B study, which was subdivided into two subgroups of patients: the first group (B1) underwent invasive imaging with QCA, IVUS grey-scale, and OCT at 6, 24, and 60 mo (n = 45), whereas the second group (B2) underwent invasive imaging at 12, 36, and 60 mo (n = 56).
For binary variables, percentages were calculated. Continuous variables are performed as the mean and standard deviation (SD) with a median (m) and 95% confidential interval (CI). The overall comparison of serial measurement was assessed by applying the Friedman test, and pairwise comparisons between post-procedure, and follow-up was calculated by a Wilcoxon signed-rank test adjusted by the Bonferroni method. The unpaired t-test was applied in cases when the matrix of datasets was unavailable. To assess the changes of imaging variables over time, the longitudinal repeated measurement analysis using a mixed effect model with five follow-up visits (at post-procedure, 6 mo, 1 year, 2 years, and 3 years) was performed in the SAS procedure PROC MIXED by pooling two cohorts (B1 and B2), as these two groups of patients were comparable in baseline characteristics. Compound symmetry covariance structure was used in the mixed model. In fact, there is no additional random effect beyond the residual error in this analysis. As no formal hypothesis examination was scheduled for assessing the success of the study, no statistical adjustment was proceeded. P values presented are exploratory analyses only and should therefore be interpreted cautiously. The estimated P value for the plaque burden (PB) in the ABSORB cohort A and B trials was adjusted by the maximum P value calculated originally for numerator or denominator at the formula of the PAV due to unavailability of the datasets with a frame-by-frame and patient-by-patient analysis. The P value was interpreted as non-significant (NS, > 0.05) in the case if historically assessed P value of either numerator or denominator was above 0.05. Statistical analysis was completed with SPSS 20.0 software (SPSS Inc., Chicago, IL, United States).
Current RCTs are being conducted with an underpowered design. In our previous analysis[12], we concluded that the meta-analyses were built on the non-significant data. Only a combo analysis of ABSORB III and ABSORB IV has a chance to clear those trends with ST in the near future (one-year results of ABSORB IV trial are expected before Spring 2018). We intentionally conducted the brief analysis (Table 1) in order to calculate a posthoc sample size by the odds ratio (OR) and estimate the statistical power for each available RCT comparing BVS with DES. The documented high resemblance between ad hoc and post hoc sample size calculations confirms the presence of bias due to the very confusing tailored fashion of their appearance. The OR-based sample size calculation that we used in our analysis is one of the most simple and reliable. It is sensitive to a relative precision, a prevalence, an OR, and a presence to absence ratio. Both ABSORB II and ABSORB III were specifically designed and powered for a five-year supervision. However, RESOLUTE All-Comers[13] became dramatically underpowered beyond the primary end-point by the five-year follow-up. Both ABSORB China and ABSORB Japan were not powered enough either. ABSORB China lost statistical power by the second year of the trial, and ABSORB Japan lost statistical power at three years. Regretfully, EVERBIO II and AIDA RCTs, available registries with DES, and few meta-analyses[6,7,36], were not optimally powered either (Table 1). Regretfully, ABSORB II[42] and ABSORB III[43] became underpowered by four years and three years, respectively. Moreover, in cases with truly powered research, all the outcomes with P values > 0.05 must be interpreted as false, which means that we can only trust the higher rates of TLF driven by the target vessel myocardial infarction (TV-MI).
| Trial | No. patients with BVS (ad hoc sample size) | No. patients with metallic DES (ad hoc sample size) | TLF, RR (BVS/DES), P value | ST (definite/probable), RR, P value | ST (definite/probable), rate in BVS patients | Estimated (post hoc) minimal sample size for groups BVS:DES (by TLF), (eOR) | Estimated (post hoc) minimal sample size for groups BVS:DES (by ST), (eOR) | Post-dilation, percent of patients | Full PSP, estimated maximum, percent of patients | Small vessels, QCA RVD (< 2.25 mm), percent of patients | ||||
| Total TLF | TLF, rate in BVS patients | Cardiac death | TV-MI | ID-TLR | ||||||||||
| BVS randomized controlled trials | ||||||||||||||
| ABSORB II, by 3 yr[31] | 335 | 166 | 2.11SP = 0.04 | 10.5% | 0.5 P = 0.40 | 5.20SP = 0.01 | 1.65 P = 0.56 | Nul P = 0.03 | 2.8% | 324:162 eOR= 2.23 | NA | Approximately 60% | NA | NA |
| ABSORB II, by 4 yr UD[42] | 289 | 139 | 2.04SP = 0.05 | 11.1% | NA | NA | NA | NA | 3.0% | 293:141UD eOR= 2.04 | NA | NA | NA | NA |
| ABSORB III, by 1 yr (13 mo)[2] | 1313 | 677 | 1.28 P = 0.16 | 7.8%2 | 4.12 P = 0.29 | 1.31 P = 0.18 | 1.21 P = 0.5 | 2.08 P = 0.13 | 1.5% | 1236:637 eOR = 1.31 | 1285:666 eOR= 2.09 | 66% | 66% | 19% |
| ABSORB III, by 2 yr (25 mo)[2] | 1322 | 686 | 1.39SP = 0.03 | 11.0% | 1.83 P = NS | 1.49SP = 0.04 | 1.23 P = NS | 2.38 P = NS | 1.9% | 1291:669 eOR= 1.45 | 1215:630 eOR= 2.52 | 66% | 66% | 19% |
| ABSORB III, by 3 yr UD[43] | 1322 | 686 | 1.31 P = 0.06 | 13.4% | 1.17 P = 0.71 | 1.47SP = 0.03 | 1.23 P = 0.27 | 3.12SP = 0.01 | 2.3% | 1262:655 eOR= 1.23 | 1375:714UD eOR= 3.16 | NA | NA | 18.8% |
| ABSORB IV, by 1 yr (13 mo)[2] | 1273 (1500)1 | 1273 (1500)1 | NA | NA | NA | NA | NA | NA | 0.5% | NA | NA | 83% | 83% | 4% |
| ABSORB Japan, by 3 yrUD[11] | 258 | 128 | 1.62 P = 0.23 | 8.9% | Nul P = 1.00 | 1.74 P = 0.31 | 1.79 P = 0.23 | 2.25 P = 0.35 | 3.6% | 260:130UD eOR= 1.69 | 248:124 eOR= 2.28 | Approximately 80% (low pressure) | NA | NA |
| ABSORB China, by 3 yrUD[11,37] | 234 | 229 | 1.17 P = 0.71 | 5.5% | 0.33 P = 0.31 | 2.97 P = 0.16 | 1.64 P = 0.33 | Nul P = 0.16 | 0.9% | 231:231UD eOR= 1.19 | NA | 16.9% | 13.5% | 18.1% |
| EVERBIO II, by 9 moUD[28] | 78 | 160 | 1.33 P = 0.60 | 12% | Nul P = 0.33 | NA | 1.11 P = 0.83 | Nul P = 0.33 | 1% | 83:170UD eOR= 1.26 | NA | 34% | 34% | NA |
| AIDA, by 2 yrUD[29] | 924 | 921 | 1.17 P= 0.31 | 10.3% | 0.78 P = 0.43 | 1.60SP = 0.04 | 1.33 P = 0.15 | 3.87SP < 0.001 | 3.5% | 941:941UD eOR= 1.17 | 898:898 eOR= 4.09 | 74% | 74% | 19% |
| DES trials and BVS registry comparators | ||||||||||||||
| RESOLUTE All-Comers, by 1 yrUD[13] | 1140 | 1152 | 1.01 P = 0.94 | 8.3%2 | 1.75 P = 0.08 | 0.98 P = 0.92 | 0.87 P = 0.50 | 2.29 P = NS | 1.6% | 1132:1132 eOR= 1.00 | 1152:1152UD eOR= 2.26 | NA | NA | NA |
| RESOLUTE All-Comers, by 5 yr UD[13] | 1140 | 1152 | 1.05 P = 0.61 | 17.0% | 1.14 P = 0.48 | 1 P = 1.00 | 1.09 P = 1.58 | 1.41 P = 0.24 | 2.4% | 2632:2632UD eOR= 0.43 | 1133:1133 eOR= 2.03 | NA | NA | NA |
| SCAAR Registry, by 2 yrUD[11] | 810 | 67099 | NA | NA | NA | NA | NA | 2.5 P = 0.006 | 1.5%2 | NA | 826:68308UD eOR= 2.47 | NA | NA | Nul |
| Recent meta-analyses of BVS | ||||||||||||||
| Polimeni et al[6], at 2 yr3UD | 3079 | 2140 | 1.33SP = 0.01 | 9.4% | 0.94 P = 0.80 | 1.66SP < 0.01 | 1.32 P = 0.05 | 3.22SP < 0.0001 | 2.3% | 2708:1881 eOR= NA | 3214:2234UD eOR= NA | > 61% | 61% | NA |
| Collet et al[7], at 2 yr3UD | 996 | 696 | 1.48 P = 0.09 | 8.2% | 0.69 P = 0.35 | 2.25 P = 0.09 | 1.89SP = 0.02 | 2.93SP = 0.01 | 2.2% | 616:437 eOR= NA | 2759:1930UD eOR= NA | NA | NA | NA |
| Ha et al[8], mostly at 2 yr3 | 1379 | 1095 | 1.31 P = 0.12 | 7.7% | 0.58 P = 0.23 | 2.59SP = 0.02 | 1.70SP = 0.04 | 2.35SP = 0.02 | 2.6% | 1233:987 eOR= NA | 1374:1082 eOR= NA | > 36% | 36% | NA |
| Mahmoud et al [5], at 2 yrUD | 3166 | 2226 | 1.32SP < 0.01 | 10.90% | 0.75 P = 0.21 | 1.65 SP < 0.001 | 1.39 SP = 0.01 | 3.22SP < 0.0001 | 2.40% | 3206:2258UD eOR= 1.4 | 3174:2235UD eOR= 3.58 | > 34% | 34% | NA |
| Sorrentino et al[9], mostly at 2 yr | 3261 | 2322 | 1.32 SP < 0.01 | 9.60% | 0.89 P = 0.63 | 1.62 SP < 0.001 | 1.40 SP = 0.007 | 3.15SP < 0.0001 | 2.40% | 3118:2227 eOR= 1.38 | 3241:2315 eOR= 3.49 | > 34% | 34% | NA |
| Ali et al[35], at 2 yr5 | 3261 | 2322 | 1.29 SP < 0.01 | 9.40% | 0.9 P = 0.90 | 1.64 SP < 0.001 | 1.39 SP = 0.009 | 2.99 SP < 0.0001 | 2.30% | 3054:2183 eOR= 1.32 | 3207:2296 eOR= 3.32 | 67% | 56% | < 51.8% |
| Zhang et al[36], at > 1 yr3UD | 3237 | 2303 | 1.37 SP < 0.01 | 9.96% (7.3%)4 | 0.92 P = 0.711 | 1.63 SP < 0.001 | 1.31 SP = 0.027 | 3.40 SP < 0.001 | 2.5% (1.8%)4 | 2968:2116 eOR= NA | 3470:2469UD eOR= NA | NA | NA | NA |
| Ali et al[39], at 3 yr5UD | 2096 | 1189 | 1.37 SP = 0.01 | 11.70% | 0.9 P = 0.77 | 1.68 SP = 0.001 | 1.41 SP = 0.03 | 2.83 SP = 0.01 | 2.40% | 2083:1177UD eOR= 1.5 | 2068:1162 eOR= 4.1 | NA | NA | NA |
| Kang et al [45], at > 2 yr3UD | 3179 | 2239 | 1.39 SP < 0.001 | 12.50% | 0.86 P = 0.49 | 1.67 SP < 0.001 | 1.46 SP = 0.004 | 3.59 SP < 0.001 | 2.60% | 2957:2083 eOR= NA | 3297:2322UD eOR= NA | NA | NA | NA |
Results of the recently published (Mahmoud et al[5]) meta-analysis (six RCTs with 5392 patients including ABSORB III, ABSORB China, ABSORB Japan, AIDA, ABSORB II, EVERBIO II; mean follow-up was 25 mo) were rather ambiguous. BVS had a higher rate of TLF [risk ratio (RR), 1.32, P = 0.002] with the worst results in ABSORB II (34/335 vs 8/166) compared with metallic DES run by the higher rates of TV-MI (RR, 1.65, P < 0.0001) and ischemia-driven target lesion revascularization (ID-TLR; RR, 1.39, P = 0.01). The risk of definite or probable ST (RR, 3.22, P < 0.0001) and very late ST (> one year; RR, 4.78, P = 0.004) was higher with BVS. This is a very tricky meta-analysis with a sophisticated statistical adjustment, which is totally undermining benefits of BVS for cardiac (RR, 0.75; P = 0.21) and all-cause mortality (RR, 0.79, P = 0.36). The better outcomes with BVS were observed in ABSORB China (cardiac mortality RR = 0.33, all-cause mortality RR = 0.17) and ABSORB II (cardiac mortality RR = 0.50, all-cause mortality RR = 0.66). The worst performance of BVS was documented in ABSORB Japan. Six meta-analyses from the teams of Collet et al[7], Ha et al[8], Sorrentino et al[9], Ali et al[35,39], Zhang et al[36], and Kang et al[45] released in 2017 with the latest findings demonstrate very similar numbers. The high-quality seven-study meta-analysis of Ali et al[35,39] demonstrated the inferiority of Absorb BVS vs metallic DES in randomized trials where BVS consorted with elevated rates of composite device-oriented and patient-oriented adverse events at both two- and three-year follow-up compared with DES, with a certain risk of adverse events between one and three years. The pooled meta-analysis of Zhang et al[36] with the longest follow-up findings from both observational and randomized trials exposed the incidence of definite or probable ST after BVS of 1.8% (95%CI: 1.5% to 2.2%, n = 21884), and the incidence of TLF of 7.3% (CI: 5.9% to 8.8%, n = 19998).
BVS is as good as DES by the rates of cardiac mortality with a trend toward more benefits from BVS for survival. Statistically, it is quite a challenging scenario to draw any conclusions from the data with no strong evidence that the intervention has an effect (with P > 0.05). The best evidence synthesis with only methodologically sound studies must be included in a meta-analysis to avoid any kind of bias. If the RCTs alone failed to demonstrate statistically significant data for ST, sources of bias by the method in such a meta-analysis of the weak studies cannot be controlled. Clinically, it is of paramount importance to detect any number of severe adverse events associated with the intervention in order to protect patients, but we must be honest with the correct interpretation of the findings and further conclusions.
Our previous analysis[12] with a Funnel plot of the existing observational and randomized trials demonstrated that there is an unintentional bias with a lack of data from patients with metallic DES in order to compare and judge BVS properly. The meta-analysis of Polimeni et al[6] (five RCTs with 5219 patients) and Sorrentino et al[9] (seven RCTs with 5583 patients) observed similar trends of clinical outcomes and another Funnel plot analysis. Unfortunately, the authors ignored clear asymmetry in the Funnel plots especially in the case of TV-MI, cardiac death, and ST where we can exclude a publication bias (due to negative Egger or Harbord test with P > 0.05). There are at least three studies with small sample sizes, which means it produces less precise estimated effects[14]. The ultimate assessment is very subjective but difficult because the number of trials is not large (less than ten). This “asymmetrical” bias can occur because of the poor methodological design in the trials, which is typical for a relatively small sample size trial because it can lead to spuriously inflated estimated treatment effects. It is challenging to specify the nature of the bias, and the only way to overcome it is to conduct a well-powered statistically harmonized RCT.
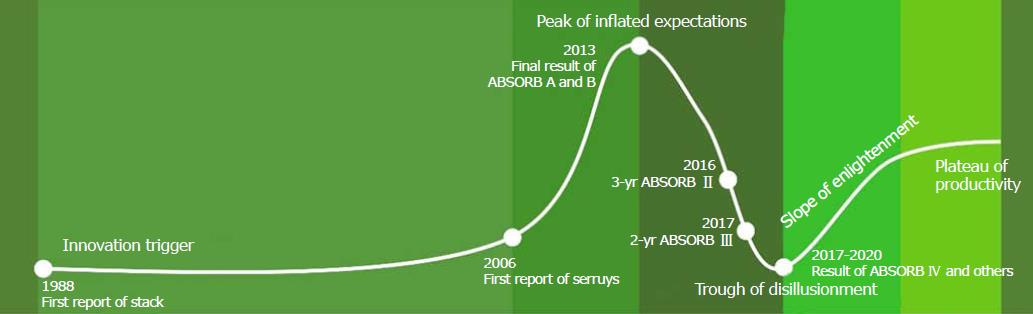
The story of BVS looks like a curve of the Gartner Hype Cycle[34] (Figure 1). The innovation trigger of BVS technology began in 1988 when the team of Professor Richard S Stack from Duke University (Durham, NC) first reported in the American Journal of Cardiology about a PLLA bioabsorbable stent. There were no usable products, but it brought us to the next stage[34], Gartner’s peak of inflated expectations. This stage started in 2006 with the first publication in the Journal of Catheterization and Cardiovascular Interventions from the team of Professor Patrick W Serruys from Erasmus MC (Rotterdam, The Netherlands). The third scientific compendium of 2013 included 72 articles (mostly pre-clinical studies, clinical ABSORB cohorts A and B trial, ABSORB EXTEND) covering all the challenging options related to transient scaffolding. This was a stage according to Gartner when early publicity was producing a number of success stories accompanied by scores and minor failures. The story became different with a new stage of the so-called trough of disillusionment in 2013 when the first paper with a design of ABSORB II trial was released in the American Heart Journal. The three-year (TCT 2016, Washington, DC) and four-year (TCT 2017, Denver, CO) results of ABSORB II, and then the two-year (ACC 2017, Washington, DC) and three-year (TCT 2017, Denver, CO) reports of ABSORB III trial destroyed all the previous illusions warning about concerns of a higher prevalence of TLF and ST in BVS patients. It is standard at this stage to continue investments to improve the products to the satisfaction of early adopters. In the future, a slope of enlightenment with more clearance about how technology can be beneficial and become more widely understood. Likely, the results of the ABSORB IV trial and further meta-analyses will resolve the situation with trends during 2017-2020. We predict that the second- and third-generation BVS (with thinner struts below 100 µm) will result in a plateau of productivity and finally the adoption of the technology.
ST rates with Absorb BRS were comparable with DES[44] performed with a lesion preparation or so-called PSP implantation[24] (RR, PSP vs no PSP 0.37) achieving 0.35 per 100 patient-years, which is comparable to the RR 0.49 with bare-metal stents, the RR 1.06 with everolimus DES, the RR 0.44 with paclitaxel DES, the RR 0.60 with sirolimus DES, and the RR 0.70 with zotarolimus DES.
The pathobiological mechanism responsible for failures of BVS in clinical trials and to assess patterns of the arterial remodeling have been investigated. A total of 30 patients were enrolled in the ABSORB A trial (a revision 1.0 of BVS) between March 7 and July 28, 2006[31,57], and 101 patients were assigned to the ABSORB Cohort B study (a revision 1.1 of BVS) from March 19 to November 6, 2009[53,55,56]. Baseline characteristics were published elsewhere. The main clinical outcomes and adverse events were previously reported[31,53,55-57].
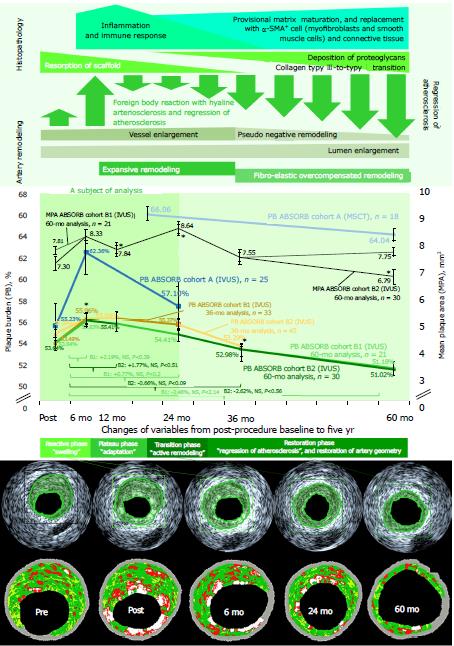
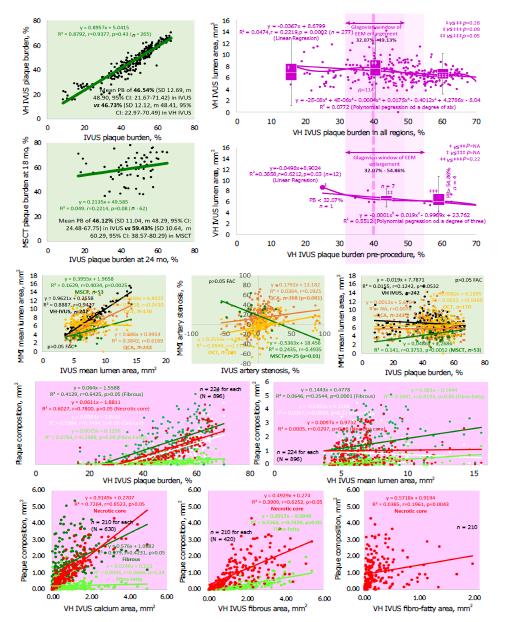
Figure 2 and Table 2 show the pooled data of PB and mean plaque area (MPA) collected with IVUS and MSCT. Between 6 and 60 mo of follow-up, there were 25 (A), 21 (B1), and 30 (B2) serial IVUS measurements. At five years, 18 patients took MSCT as an optional investigation that was performed at three of four centers. Quantitative analysis of the scaffolded segment was feasible in all patients. The insignificant increase of PB was documented in ABSORB cohort A and B1 trials at 6, 24, and 60 mo (if applicable). The absolute growth of PB was +7.52% (P value is not applicable, NA; n = 25) at 6 mo (a +13.72% relative increase), and +1.63% (P value is NA) at 24 mo (a relative +2.97% from baseline) in ABSORB A trial (n = 29), and +2.47% (P < 0.02, n = 33) at 6 mo (a +4.62% relative increase), +1.89% (NA, n = 33) at 24 mo (+3.53%) with a - 2.46% (estimated P value < 0.06, n = 21) reduction at 60 mo (a -4.59% relative decrease) in ABSORB cohort B1 trial (n = 45). A +1.60% increase of PB (P value is NA, n = 45) registered at 12 mo (a +2.94% relative increase) with a further - 1.10% reduction at 36 mo (a -2.02% relative decrease from baseline, P = 0.05, n = 45), and a -2.62% decrease at 60 mo (a -4.88% relative decrease, estimated P value < 0.22, n = 30) in ABSORB cohort B2 trial (n = 56).
| Variable | Post-procedure (baseline) | 6 mo | 12 mo | 24 mo | 36 mo | 60 mo | FriedmanP value |
| Intravascular ultrasound, 60-mo results of ABSORB cohort B (B1, n = 21, and B2, n = 30) trial | |||||||
| Mean vessel area in mm2, mean ± SD (n), BL-to-FUP P value | 14.56 ± 3.82 (21) | 14.92 ± 3.78 (21), 0.3925 | 15.88 ± 4.02 (21), 0.0014S | 15.28 ± 4.53 (21), 2.1371 | 0.0193S | ||
| 13.61 ± 2.40 (30) | 14.15 ± 2.61 (30), 0.2140 | 14.25 ± 2.57 (30), 0.0788 | 13.23 ± 2.70 (28), 0.2701 | 0.0337S | |||
| Mean lumen area in mm2, mean ± SD (n), BL-to-FUP P value | 6.75 ± 1.19 (21) | 6.59 ± 1.20 (21), 0.0610 | 7.24 ± 1.91 (21), 0.1995 | 7.46 ± 2.45 (21), 0.0851 | 0.0626 | ||
| 6.31 ± 0.86 (30) | 6.31 ± 1.01 (30), 0.5131 | 6.70 ± 1.48 (30), 0.0858 | 6.48 ± 1.50 (30), 0.5666 | 0.2221 | |||
| Plaque burden in %, mean ± estimated SD (n)1, BL-to-FUP P value | 53.64 ± 14.08 (21) | 55.83 ± 14.15 (21), < 0.392 | 54.41 ± 14.35 (21), < 0.202 | 51.18 ± 16.81 (21), < 2.142 | < 0.062 | ||
| 53.64 ± 9.46 (30) | 55.41 ± 10.22 (30), < 0.512 | 52.98 ± 11.70 (30), < 0.092 | 51.02 ± 11.81 (30), < 0.562 | < 0.222 | |||
| Mean plaque area in mm2, mean ± SD (n), BL-to-FUP P value | 7.81 ± 2.98 (21) | 8.33 ± 2.88 (21), 0.0660 | 8.64 ± 2.85 (21), 0.0004S | 7.75 ± 2.62 (21), 4.4007 | 0.0025S | ||
| 7.30 ± 1.85 (30) | 7.84 ± 1.92 (30), 0.0220S | 7.55 ± 1.58 (30), 0.8121 | 6.79 ± 1.90 (28), 0.0108S | < 0.0001S | |||
| Intravascular ultrasound, 36-mo results of ABSORB cohort B (B1, n = 33, and B2, n = 45) trial | |||||||
| Mean vessel area in mm2, mean ± SD (n), BL-to-FUP P value | 14.04 ± 3.80 (33) | 14.44 ± 3.82 (33), 0.008S | 15.35 ± 4.05 (33), < 0.001S | NA | NA | ||
| 13.79 ± 2.37 (45) | 14.43 ± 2.64 (45), 0.03S | 14.58 ± 2.67 (45), 0.002S | NA | 0.18 | |||
| Mean lumen area in mm2, mean ± SD (n), BL-to-FUP P value | 6.53 ± 1.24 (33) | 6.36 ± 1.18 (33), 0.02S | 6.85 ± 1.78 (33),0.35 | NA | NA | ||
| 6.29 ± 0.90 (45) | 6.35 ± 1.17 (45), NS | 6.81 ± 1.62 (45), 0.05S | NA | 0.007S | |||
| Plaque burden in %, mean ± estimated SD (n)1, BL-to-FUP P value | 53.49 ± 14.48 (33) | 55.96 ± 14.80 (33), < 0.02S | 55.37 ± 14.61 (33), < 0.35 | NA | NA | ||
| 54.39 ± 9.35 (45) | 55.99 ± 10.32 (45), NS | 53.29 ± 12.68 (45), < 0.05S | NA | NA | |||
| Mean plaque area in mm2, mean ± SD (n), BL-to-FUP P value | 7.52 ± 2.84 (33) | 8.08 ± 2.87 (33), < 0.001S | 8.49 ± 2.89 (33), < 0.001S | NA | NA | ||
| 7.50 ± 1.82 (45) | 8.08 ± 1.94 (45), < 0.001S | 7.77 ± 1.73 (45), NS | NA | 0.004 | |||
| Intravascular ultrasound, 24-mo (n = 25), and MSCT, 60 mo (n = 18), results of ABSORB cohort A trial | |||||||
| Mean vessel area in mm2, mean ± SD (n), BL-to-FUP P value | 13.49 ± 3.74 (25) | 13.79 ± 3.84 (25), 0.98 | NA | 12.75 ± 3.43 (19), 0.68 | NA | NA | NA |
| NA | NA | NA | 13.17 (18) (18 mo baseline) | NA | 11.93 (18), 0.26 | NA | |
| Mean lumen area in mm2, mean ± SD (n), BL-to-FUP P value | 6.04 ± 1.12 (25) | 5.19 ± 1.33 (25), < 0.0001S | NA | 5.47 ± 2.11 (19), 0.12 | NA | NA | NA |
| NA | NA | NA | 4.47 (18) (18 mo baseline) | NA | 4.29 (18), 0.11 | NA | |
| Plaque burden in %, mean ± estimated SD (n)1, BL-to-FUP P value | 55.23 ± 15.31 (25) | 62.36 ± 17.37 (25), < 0.98 | NA | 57.10 ± 22.02 (19), < 0.68 | NA | NA | NA |
| NA | NA | NA | 66.06 (18) (18 mo baseline) | NA | 64.04 (18), < 0.26 | NA | |
| Mean plaque area in mm2, mean ± SD (n), BL-to-FUP P value | 7.44 ± 2.83 (25) | 8.60 ± 2.85 (25), < 0.0001S | NA | 7.10 ± 2.02 (19), 0.80 | NA | NA | NA |
| NA | NA | NA | 8.23 (18) (18 mo baseline) | NA | 7.10 (18), 0.23 | NA | |
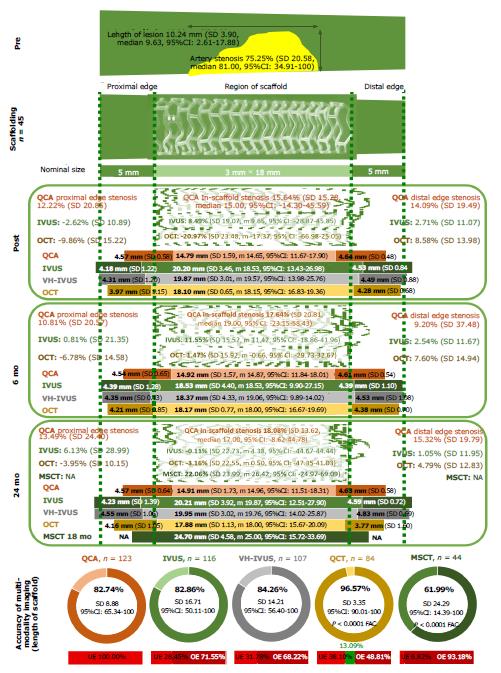
In order to evaluate the quality of the data, the accuracy of the imaging modalities in ABSORB cohort B1 trial was estimated. The length of the scaffolded region was measured in patients who underwent QCA, IVUS, VH-IVUS, MSCT, and OCT and then compared with the nominal size of the scaffold, which was equal to 18 mm in all cases (Figure 3). The accuracy (a trueness by ISO 57251) was highest for OCT imaging (96.6%) with very good precision (estimated by 95%CI of a trueness; the length of the scaffold was correct in 13.1% of cases). The lowest accuracy assessed in MSCT analysis with a 62.0% trueness and poor precision (the length was overestimated in 93.2% of observations). The moderate accuracy with relatively poor precision attested for QCA (82.7%, totally underestimated), IVUS, and VH-IVUS (82.9% and 84.3%, respectively, the length was mostly overestimated).
The GS IVUS analysis of 32 patients (72.73% of the cohort) with serial imaging examination established the statistically significant increase in PB at 6 mo (a +2.73% absolute increase, P = 0.05) with a further light decrease between 6 mo and 2 years (a +2.54% absolute increase from baseline, P = 0.08) with similar dynamic edges (Table 2 and Figure 4). Eight patients proved a -2.67% absolute abatement of PB at 24 mo (P = 0.28). The VH-IVUS assessments (Figure 4) substantiated a 0.12-fold increase in the number of VH-thin-cap fibroatheromas (VH-TCFA) from post-procedure to 24-mo follow-up as a manifestation of the natural history of atherosclerosis and significant area enlargement of the fibrous tissue (+48.52%, P = 0.02, n = 32) in the lesions without any powered (P > 0.05) distinction of the other plaque components. The patients with reduction of PB (n = 8) had a composition of the lesion at the scaffold region pre-procedure (one observation) with less pronounced depositions of calcium (P > 0.05) and a higher value of fibrous tissue (P > 0.05), which was very similar to the composition of the lesions that were documented in both edges (P > 0.05) compared to 24 patients (three observations) with increased PB at 24 mo. Post-procedure, the general tendency for reduction of the dense calcium was shown (P = 0.10) in all observations, which was related to the degradation of the scaffold that sometimes imitates calcium in VH-IVUS. A -0.11-fold decrease in the number of VH-TCFA per patient with a trend toward change of the percentages of necrotic core (a -11.10% relative decrease, P = 0.13) and fibrous tissue (a +20.12% relative increase, P = 0.11) with a significant increase of fibro-fatty tissue (a 0.91-fold increase, P = 0.04) at 24 mo was argued in observations (P = 8) to be “regression of atherosclerosis”.
Currently, it is unclear whether the plaque regression is a true phenomenon due to the disappearance of the scaffold, which is ultimately replaced by connective tissue[56]. The documented trend toward a reversal of atherosclerosis, lumen enlargement, and progressive hyaline arteriosclerosis[58] (mediating so-called OCT-phenomenon of the “golden tubes”[59]) after placement of BVS resembles the previously delineated histologic findings in animals[59-62] and human autopsies[63].
The curve of the PB draws a four-phase regression of atherosclerosis. First, the polymeric struts are covered by a fibromuscular neointima (first 6-12 mo, or “reactive” phase) with an inflammatory “swelling” (a foreign body reaction, including multinucleated giant cells, macrophages, and lymphocytes) of the artery wall (slowly shrinking inflammatory infiltrate with macrophages and lymphocytes mostly observed between 12 and 36 mo, maximum of 42 mo)[60,61]. The increase of the necrotic area post-procedure in VH-IVUS definitely consorted with the fibrinoid necrosis in the scaffolded lesion, but this fact demands a special investigation to discern the hyalinosis whereas the association of the necrotic core is based on the value of the cholesterol deposits (VH-IVUS fibro-fatty component). Second, struts and artery are of stable morphology through 18 mo (a “plateau phase” with an adaptation of the artery wall to the altered hemodynamics/shear stress and a foreign body in the grip of inflammation and a “frame” of the scaffold). Third, the struts become replaced by proteoglycan matrix (arterial hyalinosis)[58] at 24 mo (a “transition” phase with an active compensatory artery remodeling that starts at 12 mo with a peak at 18 mo)[60,61], which corresponds to resorption of BVS (morphological changes in the scaffold resorption sites begin at 18 mo) with a light calcification of the vessel wall and increasing eosinophilia (30 mo). These changes are linked to the absorption and inspissation of proteins (presence of proteinaceous material including albumin being a manifestation of the Vroman effect[64,65]). The precipitation of calcium phosphate might be the result of a benign, localized drop in pH caused by acidic polymer degradants at the strut-tissue interface. Fourth, the strut sites are eventually composed of a provisional matrix (mostly, proteoglycan) that matures from collagen type III integration (36 mo) to eventual replacement by α-SMA+ cells (myofibroblasts and smooth muscle cells) and collagen type I at 42 to 48 mo (hyaline-associated arteriosclerosis with an excess of the dense connective tissue at the sites of the struts replacement, which looks like a fibrous tissue at VH-IVUS), demonstrating an augmenting integration of scaffold into the arterial tissue (a “restoration” phase). Strictly speaking, the process of “bioresorption” finishes by 24 mo with the consequent “integration” of the struts within the arterial wall. The debris of the scaffold can be pinpointed until 36 mo. The drawbacks (increased stenosis and greater neointimal thickness) of the BVS seen at early time points were no longer pertinent at 36 mo[61]. Meanwhile, the MPA in IVUS exhibits a biphasic change with an increase until 12-24 mo and a plaque reduction until 24-36 mo[55],which is relevant to the above-mentioned findings, but different from the dynamics of PB.
The process of the PB reduction or “regression of atherosclerosis” starts at 12 mo and turns substantial with a relatively slow pace after 36 mo. However, the histopathologic signs of the excessive extracellular matrix production (including VH-IVUS signs of the fibrous metamorphosis in the lesion) with the “hardening” hyaline arteriosclerosis cause debate for a potential of the transient scaffolding for the “restoration” of the artery wall. Frankly, a hyaline deposition in the middle-size arteries is typical for aging and very benign in elderly patients[22,23]. The nature and structure of hyaline (nonfibrillar glycoprotein) in the case of the transient scaffolding is not entirely clear. However, the accumulation of the extracellular matrix in tissue might be evidence for a reversal of atherogenesis. Moreover, such a phenomenon with a lumen enlargement (as a result of the transient scaffolding) could be the only way to settle on atherosclerosis protecting the arteries, which necessitates further long-term studies.
The scaffold-induced hyaline arteriosclerosis managed by the complex immune response is typical for the foreign body (biomaterial-induced) reaction[60,61,64,65]. The mTOR-inhibition with everolimus devaluates the immune response to the scaffold. However, in human autopsies[62], macrophage and granulocyte activation where the limus drugs are not effective enough are observed. The complement system, a major host defense system, conserves clotting and inflammation, which is the most critical for the rate of ST and any adverse events[64,65]. Such an immune hurricane amid the post-intervention healing of the vessel wall has a potential to catalyze specific artery remodeling with a lumen enlargement, certain atheroprotective patterns, and hyaline arteriosclerosis as an outcome of the foreign body reaction to BVS partly adjusted with mTOR-inhibition.
The decrease in the number of TCFA lesions in the tested cohort of patients with “regression of atherosclerosis” is further evidence of the BVS benefits promising the prevention of MACE merely because it is known[59,66,67] that VH-IVUS can identify plaques at increased risk of subsequent events, and VH-TCFA are strongly linked with MACE (VIVA trial, 2011). Historically BVS (since 2006 in at least five studies: ABSORB II, ABSORB-Japan, ABSORB-China, ABSORB III, and ABSORB-Extend) substantiated the excellent clinical profile[20-54] with the proper safety (relatively low rate of TLF) that with the reduction of PB is able to significantly shift the management of atherosclerosis.
The benign adaptive artery remodeling with the lumen enlargement and vessel wall thinning of atherogenesis (the ABSORB B trial confirmed the strong trend toward reduction of PB through 60 mo without signs of the “natural” progression of atherosclerosis) with a potential to become a magic bullet in order to ultimately overpower atherosclerosis.
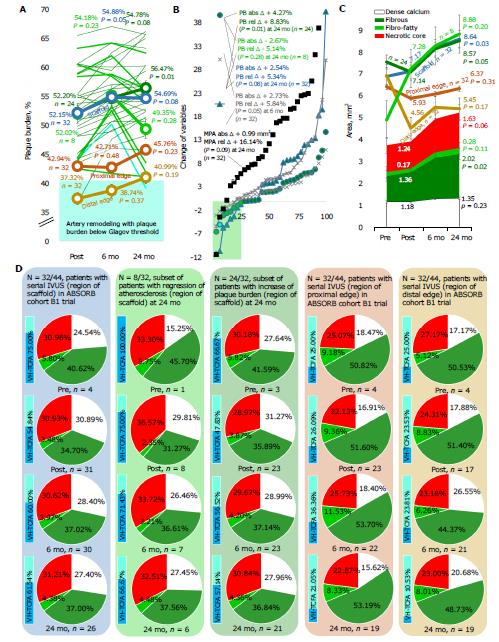
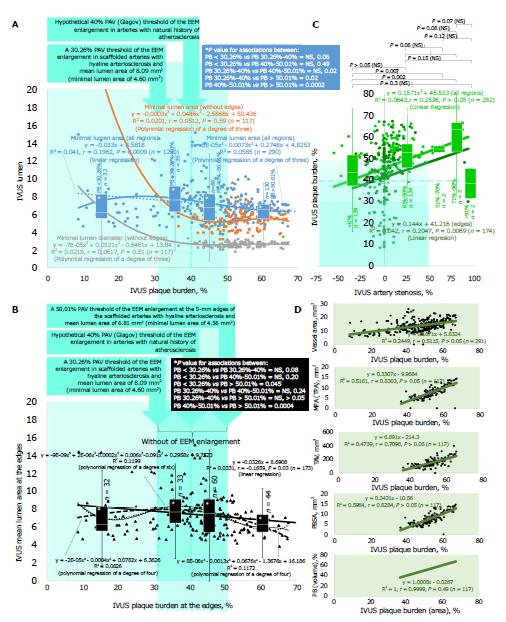
The multiple linear and polynomial regression analysis of the different degrees (Figure 5) in both scaffold (Figure 5A) and edge (Figure 5B) regions (290 observations) in ABSORB cohort B1 trial authenticated the existence of the window of external elastic membrane (EEM) enlargement with the boundaries between 30.26% and 50.01% where EEM slows down without compromised lumen geometry. The number of observations with PB below 30.26% was not high enough to ultimately clarify the lower limit (P = 0.06), but box-and-whisker analysis certified the statistically significant difference between three distributions above 30.26%, in particular between 30.26%-40.00% and 40.00%-50.01% (P = 0.02), which denotes that the Glagov threshold of a 40% PB is a real phenomenon but with the broader boundaries when lumen becomes narrow if PB achieves 50.01% (P < 0.02). These findings were corroborated at the analysis of associations between IVUS PB and artery stenosis. The artery stenosis was below 0% until the threshold of a 45.76% PB in all regions (including scaffold and edges), and a 40.34% PB in edges (beyond the scaffold). The upper boundary of a 50.01% PB was associated with a 33.85% IVUS artery stenosis in all regions and a 45.09% in edges that is relevant to data of the box-and-whisker analysis that did not reveal any significant difference in PB if match artery stenosis was below 0% and 0%-50%. The VH-IVUS examination (Figure 6, top right panel) of PB supported a 32.07%-49.13% window of EEM enlargement for all regions (P > 0.05 for all comparisons, FAC) vs a 32.07%-54.86% window for 12 observations in naïve pre-procedure arteries (P > 0.05, FAC).
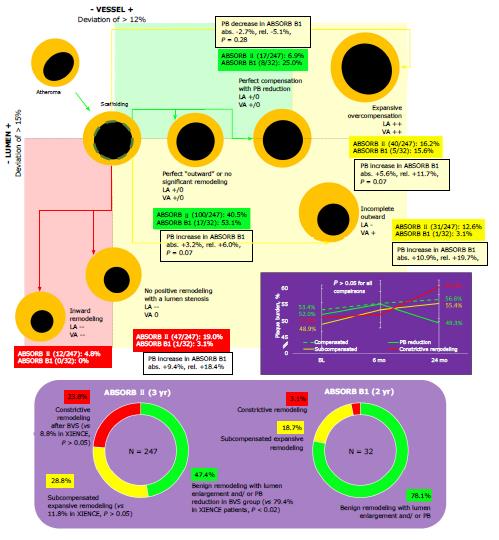
Eight BVS patients in the ABSORB B1 trial had a 2.67% decrease of PB at 24 mo (P = 0.28). The phenomenon of the window of EEM enlargement with a PB of 30.26%-54.86% was disclosed in the population, but only one patient had a PB reduction below a 40% Glagovian threshold. At 24 mo, 15/32 patients achieved PB within a window of EEM enlargement a vs 19 patients at baseline without cases below a lower end of the interval. Implantation of BVS in ABSORB II trial[17,68,69] was defined by more pronounced sub- and decompensated arterial remodeling when compared with stenting (Figure 7). There was documented positive remodeling with lumen enlargement and plaque decrease (OR, 0.23, 95%CI: 0.14, 0.38, P < 0.02) with more stable arterial geometry in the metallic jacket, subcompensated expansive remodeling (OR, 3.13, 95%CI: 1.74, 5.65, NS), and constrictive remodeling (OR, 3.24, 95%CI: 1.67, 6.28, NS). Transient scaffolding was labeled by tremendous regression of atherosclerosis in 6.9% of BVS patients vs 1.5% after stenting (OR, 4.95, 95%CI: 1.13, 21.77, P = 0.02) when compared with DES XIENCE.
The evaluated patterns of the Glagovian artery remodeling[50,51] play a role in the estimation of the existing CV risks in real clinical practice. A large PB of > 70% is related to higher risk of MACE being a predictor of events. Half of these events were related to nonculprit lesions (PROSPECT trial, 2011)[22,23]. The knowledge about the window of the EEM enlargement (“Glagov threshold”[23,50,51]) might be pivotal in the selection of the optimal strategy as a target for the reduction of PB. The decrease of PAV below that threshold or upper boundary bonds the restoration of the artery geometry and local circulation amid atheroprotective reorganization of the lesion and benign progressive hyalinosis. This was validated in 4/8 patients (one patient committed PB reduction below a 40% PB) of the ABSORB cohort B1 trial with a reversed atherogenesis at 24 mo with a similar trend in the entire population of the ABSORB B trial at 60 mo. Meanwhile, the PB in the Cath Lab might be assessed with the moderate accuracy by IVUS or VH-IVUS (to evaluate a composition of the lesion) and poor accuracy by MSCT[57]. However, accuracy analysis in the study was impaired by the progress of bioresorption with discontinuation and dismantling[16] of the scaffold as well as technical difficulties of the imaging[19-35], which were released previously[36-52,59].
The PB by the artery dimensions can be evaluated by correlation (Pearson’s R2 and r) between PAV, artery stenosis, lumen area and others (Figure 5C and D, and Figure 6) with the available multi-modality imaging tools. A low heterogeneity of data was appropriate for assessment of correlation and regression (P > 0.05 if not mentioned) to see how two variables vary together. The moderate correlation was validated (P > 0.05, FAC) for associations between PB and artery stenosis (r = 0.25 in all regions; r = 0.20 in edges, P = 0.007), vessel area (r = 0.51), and total atheroma volume (TAV) (r = 0.71) with high correlation between PB, plaque behind stent area (PBSA) (r = 0.83), and MPA (r = 0.83). There was no difference (P > 0.05 FAC) between PB calculated by either area or volume in IVUS (R2 = r = 1.0) with the minimal distinction between PB measured by IVUS and VH-IVUS (r = 0.94). MSCT was less accurate (Figure 6, top left panel) and overestimated PB when compared to IVUS (r = 0.22, P = 0.08).
To test comparability of the imaging modalities (Figure 6, middle panel), the variables were performed in IVUS with QCA, VH-IVUS, and OCT at the post-procedure baseline, 6 mo, and 24 mo. Then they were compared with the MSCT measurements at 18 mo, which were received at the inflammatory phase of the BVS resorption and resemble those at 24 mo. The IVUS mean lumen area (MLA) had the highest correlation (Figure 6, middle panel) with VH-IVUS (r = 0.94), moderate with QCA (r = 0.62) and MSCT (r = 0.40, P = 0.003), and lowest surprisingly with OCT (r = 0.04) with similar associations for artery stenosis (P > 0.05, FAC, if not mentioned). Measuring a degree of the association between IVUS PB and the lumen area, which was evaluated with other imaging modalities, we revealed a moderate correlation with MSCT (r = 0.38, P = 0.005), but not with others (r < 0.12, P > 0.05).
The estimation of the lesion’s components (Figure 6, bottom panel) verified a trend toward correlation between VH-IVUS PB and the size of necrotic core (r = 0.78) as well as deposits of dense calcium (r = 0.75, P > 0.05 FAC). However, MLA had the highest but moderate correlation with fibrous (r = 0.25, P = 0.0001) and fibro-fatty tissue (r = 0.42, P > 0.05). The strongest association between different components vindicated for necrotic core and calcium (r = 0.85, P > 0.05), fibrous and fibro-fatty tissue (r = 0.75, P > 0.05), necrotic core and fibrous tissue (r = 0.63, P > 0.05), and necrotic core and fibro-fatty tissue (r = 0.20, P = 0.004).
The most critical point for the first-generation BVS remains unpredictable prognosis. The modern-day transient scaffolding means a 36-48-mo biodegradation with compromised local shear stress[15], a risk of malapposition, uncovered struts, fractures, and discontinuation[16]. The immune and inflammatory response, hyaline arteriosclerosis (sometimes with abundance of the connective tissue which appears as an OCT-phenomenon of the “golden tubes”)[17,34,35], and a foreign body-like reaction drive the biological retaliation of the vessel to the implanted scaffold with a pronounced risk of thrombosis and decompensated artery remodeling. At least 19% of the lesions after BVS develop progressive atherosclerosis but without a clear impact on clinical outcomes[17,34,35].
Lower affordability (with budget constraints especially in case of PSP scenario with advanced multi-modality imaging), poor availability of BVS sizes, clinical concerns, and awareness due to confusing results from RCT tremendously restrict a broad utilization of BVS in real-world cardiology despite the phenomenal success of BVS in the first observational studies (ABSORB A, ABSORB B, and others since 2006).
BVS has a good clinical and research profile with a tremendous number of patients[5-9,17,19-21]. More than 150000 patients were treated, and more than 30000 patients were studied in 12 RCTs and 20 registries in more than 100 countries. The procedural success was tested in REPARA and GABI-R trials. Healing success comparable to the most advanced modern-day DES was confirmed in TROFI, II, and ESTROFA-BVS trials. Effectiveness and safety comparable to DES was documented in ABSORB III, ABSORB Japan, ABSORB China, ABSORB FIRST, and GHOST-EU trials. Event rates comparable to DES were observed in ABSORB II and ASSURE trials. Low long-term event rates were shown in the ABSORB EXTEND trial. Stable lumen area (OCT-documented compensated lumen enlargement) including a partial restoration of the vasomotor function was performed in the ABSORB cohort B trial[17,23,25]. A phenomenon of the vessel wall thinning was also shown. This is a kind of regression of atherosclerosis below a threshold of the 40% PB in accordance with the concepts of artery remodeling developed by Glagov and Pasterkamp[17,22,23]. Only 23.1% of patients had expansive remodeling of the artery with lumen enlargement and plaque decrease[11,17,24].
Transient scaffolding is challenging because the scaffold failure remains an Achilles’ heel of the technology. It is mostly associated with extensive malapposition and further secondary evaginations (9%), late discontinuity (8%), peri-strut low intensity area (5%), uncovered strut with delayed endothelialization (4%), under-deployment (4%), incomplete lesion coverage (4%), recoil with a decrease of radial strength including scaffold fracture and collapse, acute disruption of struts and overlapping struts (3%), restenosis (2%), neoatherosclerosis with severe inflammation (1%), bifurcation concerns (1%), and non-specific imaging findings including acute and chronic inflammatory responses, and increased thrombogenicity (1%)[26,27]. These points require some clinical awareness despite confusing statistics (an underpowered fashion at least for some variables in RCTs such as ABSORB China and Japan, EVERBIO II[28], and AIDA[29] and no optimized design of a few meta-analyses with a lack of statistical power from the teams of Polimeni, Collet, Mahmoud, Ali, Zhang, and Kang; Table 1) and relevant efforts to prevent the aforementioned complications.
There are several concerns to consider in real clinical practice with BVS (according to the ESC-EAPCI Task Force, not yet published, announced in May 2017 at EuroPCR, Paris, France)[30] and therefore must overcome to protect patients and harmonize intervention remain: (1) optimization of the implantation by the PSP scheme (pre-dilation with a residual stenosis < 30% (under IVUS imaging ideally), correct sizing (excluding patients with a vessel size < 2.25 mm and > 3.5 mm), post-dilation (balloon diameter/scaffold diameter = 1:1; balloon diameter < scaffold diameter + 0.5 mm) with a non-compliant balloon pressure > 18 atm (under OCT imaging ideally) to prevent malapposition and injury of BVS); (2) fractures and discontinuations (could be managed by the harmonized implantation); (3) duration of the anti-thrombotic therapy (> 18 mo) until the disappearance of the uncovered struts, which means hypothetically that some protection (for instance, with a prophylactic dose of oral anticoagulants) is necessary even for 48 mo[6]; and (4) optimal sites of intervention avoiding complex lesions (left main, bifurcations, long lesions, chronic total occlusions, calcification) and small vessels (< 2.25 mm)[7] excluding CHIP patients (at least for the moment due to absence of the strong evidence for any clinical benefit of the strategy). However, according to the ESC/EAPCI Task Force report[30] and an academic collaboration analysis[40], pre-dilation in ABSORB studies (ABSORB II[31], ABSORB III[2], ABSORB China, ABSORB Japan, ABSORB EXTEND[11]; pooled 2973 patients) was performed in 99.8% of cases, but high-pressure post-dilation in only 12.7%. Taking into account a number of patients (82.3%)[40] with optimal sizing (2.25 mm < RVD < 3.5 mm), a percentage of cases with full PSP were not higher than 10.4%[30], which means it is difficult to judge clinical outcomes in these RCTs.
The serial multi-imaging approach of the prospective, single-group, open-label study displayed that the transient scaffolding with BVS can beget a reduction of PB below the baseline in coronary arteries at 24 mo. Atherosclerosis was first described when Leonardo da Vinci autopsied a centenarian man. Leonardo theorized that the cause of the degeneration of the vessels were very close to the understanding of the role of cholesterol in atherosclerosis[46]. However, it was not described again until 1904 when Marchand first introduced the term, and in 1912 when Anichkov et al[47] effectuated lesions by adding pure cholesterol to rabbit food.
The latter part of the century was spent understanding the pathology and the reversal of atherosclerosis[47]. The first interventional study was published by Friedman et al[48] in 1957. Then the angiographic paradox was studied in the 1960s[49,50]. The first retrospective analysis with statins that found the benefits of low-density lipoprotein-cholesterol (LDL-C) in reducing coronary calcium-volume score was reported in 1998[51] with a series of further intravascular imaging studies of the lipid-lowering strategies[19,22] manifesting the strides of the third industrial revolution in biomedicine with a maximum 1.22% absolute reduction of PB under intensive statin therapy (ASTEROID trial, 2006; SATURN, 2011; IBIS-4, 2015; and STABLE, 2016)[22]. Four Japanese trials (ESTABLISH, 2004; JAPAN-ACS, 2009; COSMOS, 2009; and TWINS, 2009) demonstrated a dramatic 10.4% absolute reduction of PAV. However, statin therapy did not reduce mortality[22,66-69]. Unfortunately, statin trials such as REVERSAL (2004), CAMELOT (2004), A-PLUS (2004), ACTIVATE (2006), PERISCOPE (2008), ILLUSTRATE (2007), STRADIVARIUS (2008), IBIS-2 (2008), TOGETHAR (2010), and YELLOW (2013) did not successfully reduce atherosclerosis[22]. Novel lipid-lowering agents (combination of ezetimibe and statins) in ZEUS (2014) and PRECISE-IVUS (2015) showed a 2.39% decrease of PB, but results of the trials testing the PCSK9 (proprotein convertase subtilisin kexin 9) inhibitors are not yet available[22].
Fully bioresorbable BVS is a novel approach for the treatment of coronary stenosis that supplies a transient vessel support with drug delivery advocating the increases of the fourth industrial revolution with the potential to pave the way for a reduction in atherosclerosis[19-25].
The analyzed ABSORB and some other BVS studies, with a small number of patients and moderate accuracy were unpowered to detect changes in the imaging endpoints from baseline to follow-up. It is infeasible to determine the impact of both transient scaffolding and statin therapy on atherosclerosis in the absence of any baseline or follow-up data with LDL-C. More than 60% of patients enrolled in ABSORB A and B trials have had hyperlipidemia-requiring medication, but the levels of LDL-C were never reported, which does not allow us to estimate a contribution of statin drugs or other interventions to the size of the coronary atherosclerotic lesions.
Unfortunately, all BVS in development today are of the same first generation. Due to smaller struts, which are the key and the only point of the modern-day innovators that can optimize re-endothelialization and prevent the phenomenon of the uncovered struts attenuating biological response and slightly reducing the rate of complications. Notwithstanding, it is not solving mechanical concerns including options of integrity, radial strength, durability, duration of bioresorption, and implantation-associated troubles causing malapposition and ST. Both the optimization of the BVS implantation[24,44,68] and introduction of drug-coated balloons[38] after the implantation have the potential to dramatically harmonize short- and long-term outcomes. Recently published results of the BIOFLOW V randomized trial[32] demonstrated superiority of the ultrathin (60 µm) bioresorbable polymer sirolimus-eluting stent over the durable polymer everolimus-eluting stent, which means that this direction is quite promising. These findings are totally relevant to the BIONICS study (not yet published, TCT 2016 in Washington, DC, United States) of the ridaforolimus-eluting cobalt-chromium stent with the narrowest struts made of a combination of 40 µm struts with 72 µm supportive struts (a rate of ST was low at 0.4%). At least two BVS with thinner struts below 100 µm are in translational development today as well as early phase clinical trials (a courtesy of Amaranth Medical and Abbott Vascular on October 31, 2017 at TCT 2017 in Denver, CO, United States). Meanwhile, the biology and pathology of the transient scaffolding (including specific options of acute fracture, chronic recoil, and late intraluminal scaffold dismantling) remain largely unknown. That is why we cannot expect any breakthrough in this field until success in translational research including biology and pathology of arterial remodeling, immune response, foreign body reaction, and inflammatory feedback of the coronary, adventitial and perivascular adipose tissue (the last one is feasible with a PET/CT). The absence of the broad financial support for in-depth science and an expectant strategy of the industry including a pressure over editors and editorial content at the leading journals[41] compromise scientific findings and discourage progress in the field. A decision of Boston Scientific that halted the project of the Renuvia scaffold in July 2017 (courtesy of TCTMD, August 1, 2017) 3 mo prior to the release of the positive clinical outcomes at TCT 2017 jeopardized the future of the bioresorbable medical device technology. Potentially, the development of new polymers, exploration of the polymer biodegradation, and examination of the vascular biology and pathology of the transient scaffolding as well as immune response to the BVS implantation could shift BVS to a new level. The possibilities for BVS are exciting with the development of an electronic multi-tasking BVS[33] that has the potential to revolutionize this technology with an optimized mechanical profile and applied nanotechnologies able to measure blood flow (including shear stress) and temperature, release drugs, and dissolve when it is necessary.
Furthermore, large-scale randomized trials are necessary to evaluate associations between documented patterns of the artery remodeling (lumen enlargement with a PB reduction) within the Glagovian concept of atherogenesis and long-term clinical outcomes (including adverse events, in particular, MACE, restenosis, device thrombosis, complications of the device discontinuation, etc) of the transient scaffolding. The histopathological study of the hyaline arteriosclerosis in the middle-size coronary arteries could shed light on the physiology of transient scaffolding, clinical importance, and prognosis of patients who underwent implantation of BVS.
The pathology of transient scaffolding remains largely unknown. What is known has prevented further development of the technology because the controversial results are confusing, and therefore it has destroyed optimization of clinical approaches. The current study uncovered some of the flaws of the clinical trials. Many of these observations are clinically relevant and have to potential to advance the strategies for both imaging and treatment of progressive atherosclerosis.
Bioresorbable vascular scaffold (BVS) initially had incredible success in the first ABSORB studies in 2006-2013, but was consequently deemed a failure due to reported relatively higher rates of target lesion failure (TLF) and device thrombosis in randomized controlled trials. However, BVS performs as well as the metallic drug-eluting stent (DES) with a trend toward some benefits for cardiac mortality.
The exploration of the insights in statistics of the relevant studies has a potential to discover the main obstacles of the BVS research and development preventing major cardiovascular events and therefore improving clinical outcomes adapting the technology in routine clinical practice.
The overarching objective was to supplant the perils of the current standard of care with the polymeric bioresorbable scaffolds.
We evaluated the statistical power in clinical trials such as ABSORB Japan, ABSORB China, EVERBIO II, AIDA, and meta-analyses by the post hoc OR-based sample size calculation, and the patterns of artery remodeling published in papers from ABSORB A and B trials.
The underpowered design was confirmed for some studies such as ABSORB Japan, ABSORB China, EVERBIO II, AIDA trials, and meta-analyses of Polimeni, Collet, and Mahmoud with some unintentional bias (judged by the asymmetrical Funnel plot). ST rates with Absorb BRS were comparable with DES performed with a strategy of the BVS implantation with optimized pre-dilation (P), sizing (S), and post-dilation (P) (PSP) implantation achieving 0.35 per 100 patient-years, which is comparable to the RR 0.49 with bare-metal stents and the RR 1.06 with everolimus DES. Both ABSORB II and ABSORB III trials were powered enough for a five-year follow-up, but the results were not entirely conclusive due to the mostly non-significant fashion of data. The powered meta-analyses were built mostly on statistically poor findings.
The misunderstanding of the pathology of transient scaffolding drives the failures of the clinical trials. More bench studies of the vascular response are required. Several next-generation BVS including multifunctional electronic scaffold grant cardiology with a huge promise to make BVS technology great again.
The biology of transient scaffolding remains mostly unknown. The thin-strut scaffolds and stents with advanced mechanical features are able to partly solve the problem of device thrombosis. Nevertheless, the unclear mechanism of related complications challenges the further development of the technology. Future research must be focused on both bench and bedside studies of vascular biology and pathology of the transient scaffolding in order to understand which mechanism is a leading source of the troubles that we face in routine clinical practice with bioresorbable scaffolds.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: The Netherlands
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kin T, Ueda H S- Editor: Ji FF L- Editor: Filipodia E- Editor: Wu YXJ
| 1. | U.S. Food and Drug Administration. MedWatch Safety Alerts for Human Medical Products. [cited June 1 2017]. Available from: https://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/default.htm. |
| 2. | Ellis SG, Kereiakes DJ. A Bioresorbable Everolimus-Eluting Scaffold Versus a Metallic Everolimus-Eluting Stent III - ABSORB III. Presented by Ellis SG and Kereiakes DJ. American College of Cardiology Annual Scientific Session (ACC 2017). [cited June1 2017]. Available from: http://www.acc.org/latest-in-cardiology/clinical-trials/2015/10/10/21/09/absorb-iii#sthash.GJeEbjNK.dpuf. |
| 3. | O’Riordan M. Absorb in Europe: When, How, and by Whom the Beleaguered BVS Is Being Used After Restrictions. [cited June 1 2017]. Available from: https://www.tctmd.com/news/absorb-europe-when-how-and-whom-beleaguered-bvs-being-used-after-restrictions. |
| 4. | Therapeutic Goods Administration. Absorb Bioresorbable Vascular Scaffold System. [cited June 1 2017]. Available from: https://www.tga.gov.au/alert/absorb-bioresorbable-vascular-scaffold-system. |
| 5. | Mahmoud AN, Barakat AF, Elgendy AY, Schneibel E, Mentias A, Abuzaid A, Elgendy IY. Long-Term Efficacy and Safety of Everolimus-Eluting Bioresorbable Vascular Scaffolds Versus Everolimus-Eluting Metallic Stents: A Meta-Analysis of Randomized Trials. Circ Cardiovasc Interv. 2017;10:pii: e005286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Polimeni A, Anadol R, Münzel T, Indolfi C, De Rosa S, Gori T. Long-term outcome of bioresorbable vascular scaffolds for the treatment of coronary artery disease: a meta-analysis of RCTs. BMC Cardiovasc Disord. 2017;17:147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Collet C, Asano T, Miyazaki Y, Tenekecioglu E, Katagiri Y, Sotomi Y, Cavalcante R, de Winter RJ, Kimura T, Gao R. Late thrombotic events after bioresorbable scaffold implantation: a systematic review and meta-analysis of randomized clinical trials. Eur Heart J. 2017;38:2559-2566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Ha FJ, Nerlekar N, Cameron JD, Bennett MR, Meredith IT, West NE, Brown AJ. Midterm Safety and Efficacy of ABSORB Bioresorbable Vascular Scaffold Versus Everolimus-Eluting Metallic Stent: An Updated Meta-Analysis. JACC Cardiovasc Interv. 2017;10:308-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Sorrentino S, Giustino G, Mehran R, Kini AS, Sharma SK, Faggioni M, Farhan S, Vogel B, Indolfi C, Dangas GD. Everolimus-Eluting Bioresorbable Scaffolds Versus Everolimus-Eluting Metallic Stents. J Am Coll Cardiol. 2017;69:3055-3066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 10. | Tanaka A, Jabbour RJ, Mitomo S, Latib A, Colombo A. Hybrid Percutaneous Coronary Intervention With Bioresorbable Vascular Scaffolds in Combination With Drug-Eluting Stents or Drug-Coated Balloons for Complex Coronary Lesions. JACC Cardiovasc Interv. 2017;10:539-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | PCRonline. BVS/BRS trial updates and registries. Session comprising selected EuroPCR 2017 late-breaking trial submissions. [cited 1 June 2017]. Available from: URL: https://www.pcronline.com/Cases-resources-images/Resources/Course-videos-slides/2017/BVS-BRS-trial-updates-and-registries. |
| 12. | Kharlamov AN. Scaffold thrombosis: Exaggerated illusion, or when statistics rules. Int J Cardiol. 2016;209:206-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Iqbal J, Serruys PW, Silber S, Kelbaek H, Richardt G, Morel MA, Negoita M, Buszman PE, Windecker S. Comparison of zotarolimus- and everolimus-eluting coronary stents: final 5-year report of the RESOLUTE all-comers trial. Circ Cardiovasc Interv. 2015;8:e002230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 14. | Sedgwick P, Marston L. How to read a funnel plot in a meta-analysis. BMJ. 2015;351:h4718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 184] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 15. | Tenekecioglu E, Sotomi Y, Torii R, Bourantas C, Miyazaki Y, Collet C, Crake T, Su S, Onuma Y, Serruys PW. Strut protrusion and shape impact on endothelial shear stress: insights from pre-clinical study comparing Mirage and Absorb bioresorbable scaffolds. Int J Cardiovasc Imaging. 2017;33:1313-1322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Onuma Y, Serruys PW, Muramatsu T, Nakatani S, van Geuns RJ, de Bruyne B, Dudek D, Christiansen E, Smits PC, Chevalier B. Incidence and imaging outcomes of acute scaffold disruption and late structural discontinuity after implantation of the absorb Everolimus-Eluting fully bioresorbable vascular scaffold: optical coherence tomography assessment in the ABSORB cohort B Trial (A Clinical Evaluation of the Bioabsorbable Everolimus Eluting Coronary Stent System in the Treatment of Patients With De Novo Native Coronary Artery Lesions). JACC Cardiovasc Interv. 2014;7:1400-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 17. | Serruys PW, Katagiri Y, Sotomi Y, Zeng Y, Chevalier B, van der Schaaf RJ, Baumbach A, Smits P, van Mieghem NM, Bartorelli A. Arterial Remodeling After Bioresorbable Scaffolds and Metallic Stents. J Am Coll Cardiol. 2017;70:60-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 18. | Kharlamov AN. Cardiovascular burden and percutaneous interventions in Russian Federation: systematic epidemiological update. Cardiovasc Diagn Ther. 2017;7:60-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Muramatsu T, Onuma Y, Zhang YJ, Bourantas CV, Kharlamov A, Diletti R, Farooq V, Gogas BD, Garg S, García-García HM. Progress in treatment by percutaneous coronary intervention: the stent of the future. Rev Esp Cardiol (Engl Ed). 2013;66:483-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Kharlamov AN. Phenomenon of elongated struts: is optical coherence tomography accurate enough to analyze scaffold area? Int J Cardiol. 2013;168:4280-4284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Kharlamov AN. Can we adapt histological injury score for optical coherence tomography of coronaries? Int J Cardiol. 2013;168:4322-4324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Kharlamov AN. Why do we fail to achieve Glagovian atheroregression in lipid-lowering trials? Interv Cardiol. 2015;7:469-482. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Kharlamov AN. Bioresorbable Scaffolds for Atheroregression: Understanding of Transient Scaffolding. Curr Cardiol Rev. 2016;12:66-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Serruys PW, Onuma Y. Dmax for sizing, PSP-1, PSP-2, PSP-3 or OCT guidance: interventionalist’s jargon or indispensable implantation techniques for short- and long-term outcomes of Absorb BRS? EuroIntervention. 2017;12:2047-2056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Onuma Y, Muramatsu T, Kharlamov A, Serruys PW. Freeing the vessel from metallic cage: what can we achieve with bioresorbable vascular scaffolds? Cardiovasc Interv Ther. 2012;27:141-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Sotomi Y, Onuma Y, Collet C, Tenekecioglu E, Virmani R, Kleiman NS, Serruys PW. Bioresorbable Scaffold: The Emerging Reality and Future Directions. Circ Res. 2017;120:1341-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 27. | Räber L, Brugaletta S, Yamaji K, O’Sullivan CJ, Otsuki S, Koppara T, Taniwaki M, Onuma Y, Freixa X, Eberli FR. Very Late Scaffold Thrombosis: Intracoronary Imaging and Histopathological and Spectroscopic Findings. J Am Coll Cardiol. 2015;66:1901-1914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 169] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 28. | Puricel S, Arroyo D, Corpataux N, Baeriswyl G, Lehmann S, Kallinikou Z, Muller O, Allard L, Stauffer JC, Togni M. Comparison of everolimus- and biolimus-eluting coronary stents with everolimus-eluting bioresorbable vascular scaffolds. J Am Coll Cardiol. 2015;65:791-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 178] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 29. | Wykrzykowska JJ, Kraak RP, Hofma SH, van der Schaaf RJ, Arkenbout EK, IJsselmuiden AJ, Elias J, van Dongen IM, Tijssen RYG, Koch KT. Bioresorbable Scaffolds versus Metallic Stents in Routine PCI. N Engl J Med. 2017;376:2319-2328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 343] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 30. | Byrne RA, Stefanini GG, Capodanno D, Onuma Y, Baumbach A, Escaned J, Haude M, James S, Joner M, Jüni P. Report of an ESC-EAPCI Task Force on the evaluation and use of bioresorbable scaffolds for percutaneous coronary intervention: executive summary. EuroIntervention. 2018;13:1574-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 31. | Serruys PW, Chevalier B, Sotomi Y, Cequier A, Carrié D, Piek JJ, Van Boven AJ, Dominici M, Dudek D, McClean D. Comparison of an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II): a 3 year, randomised, controlled, single-blind, multicentre clinical trial. Lancet. 2016;388:2479-2491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 420] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 32. | Kandzari DE, Mauri L, Koolen JJ, Massaro JM, Doros G, Garcia-Garcia HM, Bennett J, Roguin A, Gharib EG, Cutlip DE. Ultrathin, bioresorbable polymer sirolimus-eluting stents versus thin, durable polymer everolimus-eluting stents in patients undergoing coronary revascularisation (BIOFLOW V): a randomised trial. Lancet. 2017;390:1843-1852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 224] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 33. | Son D, Lee J, Lee DJ, Ghaffari R, Yun S, Kim SJ, Lee JE, Cho HR, Yoon S, Yang S. Bioresorbable Electronic Stent Integrated with Therapeutic Nanoparticles for Endovascular Diseases. ACS Nano. 2015;9:5937-5946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 34. | Kharlamov AN, Feinstein JA, Cramer JA, Boothroyd JA. WITHDRAWN: What exactly, regression of atherosclerosis or foreign body reaction with hyaline arteriosclerosis, drives transient scaffolding of coronary arteries? A pooled analysis of observational ABSORB studies with a serial multimodality imaging substudy of ABSORB cohort B1 trial. Int J Cardiol. 2017;pii:S0167-5273(16)32984-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 35. | Ali ZA, Serruys PW, Kimura T, Gao R, Ellis SG, Kereiakes DJ, Onuma Y, Simonton C, Zhang Z, Stone GW. 2-year outcomes with the Absorb bioresorbable scaffold for treatment of coronary artery disease: a systematic review and meta-analysis of seven randomised trials with an individual patient data substudy. Lancet. 2017;390:760-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 169] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 36. | Zhang XL, Zhu QQ, Kang LN, Li XL, Xu B. Mid- and Long-Term Outcome Comparisons of Everolimus-Eluting Bioresorbable Scaffolds Versus Everolimus-Eluting Metallic Stents: A Systematic Review and Meta-analysis. Ann Intern Med. 2017;167:642-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Xu B, Yang Y, Han Y, Huo Y, Wang L, Qi X, Li J, Chen Y, Kuo HC, Ying SW. Comparison of everolimus-eluting bioresorbable vascular scaffolds and metallic stents: three-year clinical outcomes from the ABSORB China randomised trial. EuroIntervention. 2017;pii:EIJ-D-17-00796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 38. | Elwany M, Latini RA, Di Palma G, Orrego PS, Cortese B. First experience of drug-coated balloons for treatment of bioresorbable vascular scaffold restenosis. Cardiovasc Revasc Med. 2017;18:482-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Ali ZA, Gao R, Kimura T, Onuma Y, Kereiakes DJ, Ellis SG, Chevalier B, Vu MT, Zhang Z, Simonton CA. Three-Year Outcomes With the Absorb Bioresorbable Scaffold: Individual-Patient-Data Meta-Analysis From the ABSORB Randomized Trials. Circulation. 2018;137:464-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 146] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 40. | Stone GW, Abizaid A, Onuma Y, Seth A, Gao R, Ormiston J, Kimura T, Chevalier B, Ben-Yehuda O, Dressler O. Effect of Technique on Outcomes Following Bioresorbable Vascular Scaffold Implantation: Analysis From the ABSORB Trials. J Am Coll Cardiol. 2017;70:2863-2874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 132] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 41. | Liu JJ, Bell CM, Matelski JJ, Detsky AS, Cram P. Payments by US pharmaceutical and medical device manufacturers to US medical journal editors: retrospective observational study. BMJ. 2017;359:j4619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 42. | Chevalier B, Cequier A, Dudek D, Haude M, Carrie D, Sabaté M, Windecker S, Reith S, de Sousa Almeida M, Campo G. Four-year follow-up of the randomised comparison between an everolimus-eluting bioresorbable scaffold and an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II Trial). EuroIntervention. 2018;13:1561-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 43. | Kereiakes DJ, Ellis SG, Metzger C, Caputo RP, Rizik DG, Teirstein PS, Litt MR, Kini A, Kabour A, Marx SO. 3-Year Clinical Outcomes With Everolimus-Eluting Bioresorbable Coronary Scaffolds: The ABSORB III Trial. J Am Coll Cardiol. 2017;70:2852-2862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 198] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 44. | Bangalore S, Bezerra HG, Rizik DG, Armstrong EJ, Samuels B, Naidu SS, Grines CL, Foster MT, Choi JW, Bertolet BD. The State of the Absorb Bioresorbable Scaffold: Consensus From an Expert Panel. JACC Cardiovasc Interv. 2017;10:2349-2359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 45. | Kang SH, Gogas BD, Jeon KH, Park JS, Lee W, Yoon CH, Suh JW, Hwang SS, Youn TJ, Chae IH. Long-term safety of bioresorbable scaffolds: insights from a network meta-analysis including 91 trials. EuroIntervention. 2018;13:1904-1913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Boon B. Leonardo da Vinci on atherosclerosis and the function of the sinuses of Valsalva. Neth Heart J. 2009;17:496-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Williams KJ, Feig JE, Fisher EA. Rapid regression of atherosclerosis: insights from the clinical and experimental literature. Nat Clin Pract Cardiovasc Med. 2008;5:91-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 153] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 48. | Friedman M, Byers SO, Rosenman RH. Resolution of aortic atherosclerotic infiltration in the rabbit by phosphatide infusion. Proc Soc Exp Biol Med. 1957;95:586-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | Brown BG, Zhao XQ, Sacco DE, Albers JJ. Lipid lowering and plaque regression. New insights into prevention of plaque disruption and clinical events in coronary disease. Circulation. 1993;87:1781-1791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 488] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 50. | Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316:1371-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2687] [Cited by in RCA: 2405] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 51. | Callister TQ, Raggi P, Cooil B, Lippolis NJ, Russo DJ. Effect of HMG-CoA reductase inhibitors on coronary artery disease as assessed by electron-beam computed tomography. N Engl J Med. 1998;339:1972-1978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 492] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 52. | Wada H, Mattson PC, Iwata H. Stent or Scaffold Thrombosis: Past, Current and Future Perspectives. EMJ. 2017;5:55-61. |
| 53. | Ormiston JA, Serruys PW, Onuma Y, van Geuns RJ, de Bruyne B, Dudek D, Thuesen L, Smits PC, Chevalier B, McClean D. First serial assessment at 6 months and 2 years of the second generation of absorb everolimus-eluting bioresorbable vascular scaffold: a multi-imaging modality study. Circ Cardiovasc Interv. 2012;5:620-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 167] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 54. | Brugaletta S, Garcia-Garcia HM, Garg S, Gomez-Lara J, Diletti R, Onuma Y, van Geuns RJ, McClean D, Dudek D, Thuesen L. Temporal changes of coronary artery plaque located behind the struts of the everolimus eluting bioresorbable vascular scaffold. Int J Cardiovasc Imaging. 2011;27:859-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 55. | Serruys PW, Onuma Y, Garcia-Garcia HM, Muramatsu T, van Geuns RJ, de Bruyne B, Dudek D, Thuesen L, Smits PC, Chevalier B. Dynamics of vessel wall changes following the implantation of the absorb everolimus-eluting bioresorbable vascular scaffold: a multi-imaging modality study at 6, 12, 24 and 36 months. EuroIntervention. 2014;9:1271-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 195] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 56. | Serruys PW, Ormiston J, van Geuns RJ, de Bruyne B, Dudek D, Christiansen E, Chevalier B, Smits P, McClean D, Koolen J. A Polylactide Bioresorbable Scaffold Eluting Everolimus for Treatment of Coronary Stenosis: 5-Year Follow-Up. J Am Coll Cardiol. 2016;67:766-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 137] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 57. | Onuma Y, Dudek D, Thuesen L, Webster M, Nieman K, Garcia-Garcia HM, Ormiston JA, Serruys PW. Five-year clinical and functional multislice computed tomography angiographic results after coronary implantation of the fully resorbable polymeric everolimus-eluting scaffold in patients with de novo coronary artery disease: the ABSORB cohort A trial. JACC Cardiovasc Interv. 2013;6:999-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 181] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 58. | Karanasos A, Simsek C, Serruys P, Ligthart J, Witberg K, van Geuns RJ, Sianos G, Zijlstra F, Regar E. Five-year optical coherence tomography follow-up of an everolimus-eluting bioresorbable vascular scaffold: changing the paradigm of coronary stenting? Circulation. 2012;126:e89-e91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 59. | Onuma Y, Serruys PW, Perkins LE, Okamura T, Gonzalo N, García-García HM, Regar E, Kamberi M, Powers JC, Rapoza R. Intracoronary optical coherence tomography and histology at 1 month and 2, 3, and 4 years after implantation of everolimus-eluting bioresorbable vascular scaffolds in a porcine coronary artery model: an attempt to decipher the human optical coherence tomography images in the ABSORB trial. Circulation. 2010;122:2288-2300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 259] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 60. | Kharlamov AN, Krams R, Fayad Z, Matter C, Gabinsky JL. Understanding of the transient scaffolding: new frontiers in vascular interventional medicine for atheroregression. Treatment Strategies: Interventional Cardiology. 2013;3. |
| 61. | Otsuka F, Pacheco E, Perkins LE, Sakakura K, Yahagi K, Ladich E, Kolodgie FD, Rapoza R, Joner M, Virman R. Detailed morphologic characterization of the strut composition following Absorb scaffold placement in a porcine coronary artery model through 48 months. J Am Coll Cardiol. 2014;64:B179-B179. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 62. | Otsuka F, Pacheco E, Perkins LE, Lane JP, Wang Q, Kamberi M, Frie M, Wang J, Sakakura K, Yahagi K. Long-term safety of an everolimus-eluting bioresorbable vascular scaffold and the cobalt-chromium XIENCE V stent in a porcine coronary artery model. Circ Cardiovasc Interv. 2014;7:330-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 148] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 63. | Kraak RP, de Boer HH, Elias J, Ambarus CA, van der Wal AC, de Winter RJ, Wykrzykowska JJ. Coronary Artery Vessel Healing Pattern, Short and Long Term, After Implantation of the Everolimus-Eluting Bioresorbable Vascular Scaffold. J Am Heart Assoc. 2015;4:pii: e002551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 64. | Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4004] [Cited by in RCA: 3381] [Article Influence: 198.9] [Reference Citation Analysis (0)] |
| 65. | Kourtzelis I, Rafail S, DeAngelis RA, Foukas PG, Ricklin D, Lambris JD. Inhibition of biomaterial-induced complement activation attenuates the inflammatory host response to implantation. FASEB J. 2013;27:2768-2776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 66. | Hobbs FD, Banach M, Mikhailidis DP, Malhotra A, Capewell S. Is statin-modified reduction in lipids the most important preventive therapy for cardiovascular disease? A pro/con debate. BMC Med. 2016;14:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 67. | Calvert PA, Obaid DR, O’Sullivan M, Shapiro LM, McNab D, Densem CG, Schofield PM, Braganza D, Clarke SC, Ray KK. Association between IVUS findings and adverse outcomes in patients with coronary artery disease: the VIVA (VH-IVUS in Vulnerable Atherosclerosis) Study. JACC Cardiovasc Imaging. 2011;4:894-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 392] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 68. | Steinvil A, Rogers T, Torguson R, Waksman R. Overview of the 2016 U.S. Food and Drug Administration Circulatory System Devices Advisory Panel Meeting on the Absorb Bioresorbable Vascular Scaffold System. JACC Cardiovasc Interv. 2016;9:1757-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 69. | Zeng Y, Tateishi H, Cavalcante R, Tenekecioglu E, Suwannasom P, Sotomi Y, Collet C, Nie S, Jonker H, Dijkstra J. Serial Assessment of Tissue Precursors and Progression of Coronary Calcification Analyzed by Fusion of IVUS and OCT: 5-Year Follow-Up of Scaffolded and Nonscaffolded Arteries. JACC Cardiovasc Imaging. 2017;10:1151-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |