Published online Oct 26, 2018. doi: 10.4330/wjc.v10.i10.153
Peer-review started: June 30, 2018
First decision: July 19, 2018
Revised: August 21, 2018
Accepted: August 30, 2018
Article in press: August 30, 2018
Published online: October 26, 2018
Processing time: 122 Days and 4.5 Hours
To assess the functionality of congenital coronary artery fistulas (CAFs) using adenosine stress 13N-ammonia positron emission tomography computed tomography (PET-CT).
Congenital CAFs were incidentally detected during coronary angiography (CAG) procedures in 11 adult patients (six males and five females) with a mean age of 64.3 years (range 41-81). Patients were collected from three institutes in the Netherlands. The characteristics of the fistulas (origin, pathway and termination), multiplicity of the origins and pathways of the fistulous vessels were assessed by CAG. Five patients underwent adenosine pharmacologic stress 13N-ammonia PET-CT to assess myocardial perfusion and the functional behavior of the fistula.
Eleven patients with 12 CAFs, 10 unilateral and one bilateral, originating from the left anterior descending coronary artery (n = 8), right coronary artery (n = 2) and circumflex (n = 2). All fistulas were of the vascular type, terminating into either the pulmonary artery (n = 11) or coronary sinus (n = 1). The CAG delineated the characteristics of the fistula (origin, pathway and termination). Multiplicity of the origins and pathways of the fistulous vessels were common in most fistulas (8/12, 67% and 9/12, 75%, respectively). Multiplicity was common among the different fistula components (23/36, 64%). Adenosine pharmacologic stress 13N-ammonia PET-CT revealed normal myocardial perfusion and ejection fraction in all but one patient, who showed a reduced ejection fraction.
PET-CT may be helpful for assessing the functional status of congenital CAFs in selected patients regarding clinical decision-making. Studies with a larger patient series are warranted.
Core tip: Congenital coronary artery fistulas are usually detected as a coincidental finding during non-invasive and invasive diagnostic modalities for the assessment of coronary artery disease. Positron emission tomography computed tomography (PET-CT) is not frequently applied for functional assessment. In the current study, five patients underwent adenosine 13N-ammonia PET-CT to assess myocardial perfusion and the functional behavior of the fistula. PET-CT revealed normal myocardial perfusion and ejection fraction in all but one patient, who showed a reduced ejection fraction. Combined with semi-quantitative results, patients with normal flow, revealed by PET-CT, could be treated medically, thereby avoiding the need for transcutaneous or surgical occlusion of the fistulas.
- Citation: Said SA, Agool A, Moons AH, Basalus MW, Wagenaar NR, Nijhuis RL, Schroeder-Tanka JM, Slart RH. Incidental congenital coronary artery vascular fistulas in adults: Evaluation with adenosine-13N-ammonia PET-CT. World J Cardiol 2018; 10(10): 153-164
- URL: https://www.wjgnet.com/1949-8462/full/v10/i10/153.htm
- DOI: https://dx.doi.org/10.4330/wjc.v10.i10.153
Congenital coronary artery fistulas (CAFs) are most often incidentally found during coronary angiography (CAG)[1], computed tomography coronary angiography (CTCA)[2] and transthoracic echocardiography (TTE)[3-5]. Coronary artery vascular and cameral fistulas are not only seen in humans, but are also occasionally observed in other mammals[6]. CAFs are classified as anomalies of termination[7]. Several diagnostic modalities are available for the morphological and functional detection of CAFs, including physical examination (presence of a continuous murmur), non-invasive methods such as echocardiography[8,9], myocardial perfusion imaging (MPI)[10,11], CTCA[3,9,12] and cardiovascular magnetic resonance imaging (MRI)[13], invasive techniques such as right heart catheterization, CAG and fractional flow reserve (FFR)[1,14-18], and incidental detection during positron emission tomography computed tomography (PET-CT)[19,20]. Exercise and pharmacological PET-CT is commonly applied for the assessment of myocardial perfusion and ischemia, and for the diagnosis and risk stratification of ischemic coronary artery disease (CAD)[21,22]. Adenosine 13N-ammonia PET-CT is useful for assessing coronary flow reserve and myocardial perfusion for risk stratification in patients with CAD[23], the results of which influence patient management strategies[24]. Myocardial ischemia can also be detected using single-photon emission computed tomography (SPECT) in patients with congenital CAFs[15]. It is unclear whether congenital fistulas are associated with reduced myocardial perfusion, which is sometimes associated with impaired left ventricular function. Indications for surgical intervention or percutaneous therapeutic embolization are based on the patient’s clinical presentation and on imaging findings. The aim of the present study was to evaluate the value of adenosine pharmacological stress 13N-ammonia PET-CT in a selected population of patients with incidentally identified congenital CAFs.
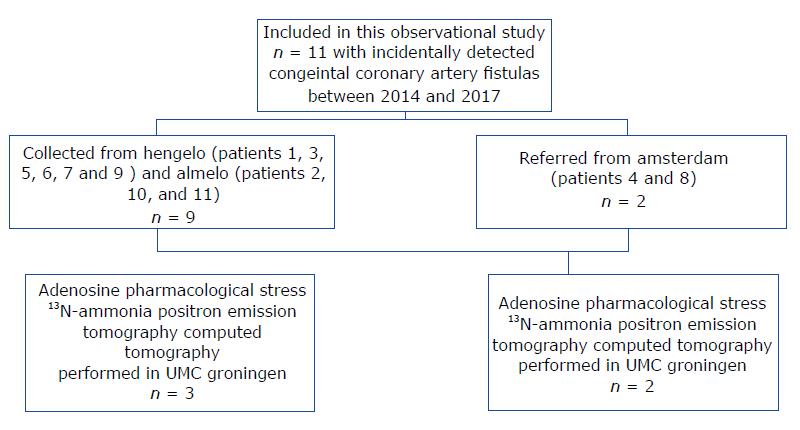
We collected 11 patients (six males and five females; mean age of 64.3 years, range 41-81 years) with CAFs which were incidentally detected during routine CAG between 2014 and 2017. The FFR was not determined due to a lack of facilities. The patients were collected from three centers in the Netherlands as follows: Amsterdam, patients 4 and 8; Hengelo, patients 1, 3, 5, 6, 7 and 9; and Almelo, patients 2, 10 and 11 (Figure 1). Patient data was obtained from the databases of three Dutch cardiac catheterization laboratories (Slotervaart Hospital, Amsterdam; Onze Lieve Vrouwe Gasthuis, Amsterdam; and Hospital Group Twente, Almelo-Hengelo).
All patients underwent electrocardiography (ECG) and TTE. Five patients were further analyzed by adenosine stress/rest 13N-ammonia PET-CT to quantify myocardial blood flow (MBF), myocardial perfusion and coronary flow reserve. Further studies to clarify the clinical presentation and fistula characteristics were performed at the clinician’s discretion. Stress and rest MPI SPECT (Symbia S, Siemens Medical Solutions, Erlangen, Germany) were performed in one patient (patient 7), CTCA and MRI were performed in two patients (patients 2 and 4), and shunt calculation by the oximetric method was performed in four patients (patients 3, 4, 7 and 8).
The ECG-gated PET images were acquired using a whole-body 64-slice PET-CT scanner (Biograph True Point; Siemens Medical Solutions). The PET data were acquired in 3D list mode. Patients were studied after an overnight fast, and all refrained from caffeine-containing beverages or theophylline-containing medications for 24 h before the study. Myocardial perfusion was assessed at rest and during vasodilator stress induced by adenosine, using 13N-ammonia as the perfusion radiotracer. Two CT-based transmission scans (120 kVp; 20–30 mA; helical scan mode with a pitch of 1.35) were obtained before and after the resting perfusion studies, performed with normal breathing to correct for photon attenuation of PET. The procedure involved the intravenous administration of 400 MBq of 13N-ammonia during rest and 400 MBq of 13N-ammonia during adenosine-induced pharmacological stress.
The PET and SPECT images were interpreted semi-quantitatively using the standard American Heart Association 5-point scoring system[25], and traditional metrics including summed difference score, summed stress score and summed rest score (SRS) were calculated. The SRS (in a fixed perfusion defect) was considered to be a measure of the extent and severity of a previous myocardial infarction (MI). Quantitative MBF values (mL/g per minute) at rest and under stress were computed for each sample on the polar map, as described previously[21], using a three-tissue compartment pharmacokinetic model for 13N-ammonia[26]. Myocardial perfusion reserve (MPR) was calculated as the ratio between stress MBF and rest MBF (making it a unitless variable). The resting MBF was corrected for the rate-pressure product. The global MPR and stress MBF were calculated for the whole left ventricular region (defined by the left ventricle long-axis plane), representing the parameters of interest for our analysis.
The ECG-gated images were analyzed using the QGS software package (Cedars-Sinai, Los Angeles, CA, United States)[27]. Short-axis images were processed, and ventricular edges and cavity volumes were calculated for each of the eight re-binned dynamic frames that were reconstructed for the average cardiac cycle. The algorithm for determining edges and calculating volume has been described previously.
The study was reviewed and approved by the local medical ethical committee of the eastern region, Enschede, the Netherlands (ID METC: K18-14, METC /18082.sai). As the patients’ personal information was protected, and therefore could not be identified and anonymized, it was exempt from consent by the local medical ethical committee.
Categorical data are expressed as numbers with percentages, and continuous data are expressed as means with a range.
This retrospective case series included 11 (Figure 1) patients with 12 congenital coronary vascular fistulas (CVFs). Of those patients, five underwent adenosine pharmacologic stress 13N-ammonia PET-CT to assess myocardial perfusion and functionality of the fistula.
Clinical presentations and features (Table 1) included limited posterior MI (patient 1), angina pectoris and chest pain (n = 6), dyspnea on exertion (n = 3) and asymptomatic abnormal resting ECG (n = 1). One patient (patient 9) presented with palpitation, transient ischemic attack, paroxysmal atrial fibrillation and non-sustained ventricular tachycardia. Previous MI was reported in two patients (patients 2 and 10). Patient 5 had exercise-induced non-sustained ventricular tachycardia. Physical examination was unremarkable in seven patients. Apical systolic murmur was heard in one patient, and systolic ejection murmur was heard in the second intercostal space in three patients.
| Case | Fistula origin and termination | Symptoms and clinical presentation | Previous history and risk factors | Physical findings | BMI | Intervention |
| 1 | RCA and LAD to PA | Chest pain | Tubular adenoma of sigmoid, celiac disease | Normal | 32.7 | None |
| NSTEMI/PMI | ||||||
| (CK 390 U/L) | ||||||
| 2 | LAD to PA | Chest pain | Old IMI | Normal | 25.9 | CABG and surgical closure of the fistula |
| NSTEMI | Asthma | |||||
| (CK 573 U/L) | ||||||
| 3 | LCx to PA | Chest pain | Blanco | Normal | 26.6 | Coiling of the fistula and PCI of LAD |
| Dyspnea on exertion | ||||||
| 4 | RCA to CS | Dyspnea on exertion | Diaphragmatic hernia, asthma and hypertension | Systolic ejection murmur grade 2/6 2 d ICS | 28.2 | None |
| 5 | LAD to PA | Non-sustained VT | DM, COPD, hypertension, hypothyroidism | Systolic ejection murmur grade 2/6 2 d ICS | 33.4 | PCI of OM branch and FFR of LAD. Coiling of the fistula |
| 6 | LAD to PA | Dyspnea on exertion | COPD | Apical mitral regurgitation murmur grade 2/6 | 25.5 | Mitral valve plasty |
| Severe mitral valve regurgitation | ||||||
| 7 | LAD to PA | Chest pain | Celiac disease | Normal | 24.2 | |
| 8 | LAD to PA | Angina pectoris | Hypercholesterolemia | Systolic ejection murmur grade 2/6 2 d ICS | 21.1 | PCI of LAD |
| Positive family history for CAD | ||||||
| 9 | LCx to PA | Palpitation | Ischemic CVA | Normal | 24.8 | None |
| PAF and non-sustained VT | ||||||
| Hypertension | ||||||
| 10 | LAD to PA | Angina pectoris | NSTEMI 2010 (CK 328 U/L). PCI of RCA 2012 | Normal | 24.2 | Coiling of the fistula |
| 11 | LAD to PA | Chest pain | Hypertension, hypercholesterolemia | Normal | 29.1 | None |
| Positive family history for CAD | ||||||
| Smoker |
In regard to the ECG, sinus rhythm was present in 10 patients and permanent atrial fibrillation was found in one patient. We also observed tall R-waves in precordial lead 2 (n = 1), signs of previous inferior MI (n = 1), non-specific repolarization abnormalities (n = 4), left bundle branch block (n = 1), left axis deviation (n = 1) and 1st degree atrioventricular delay (n = 1).
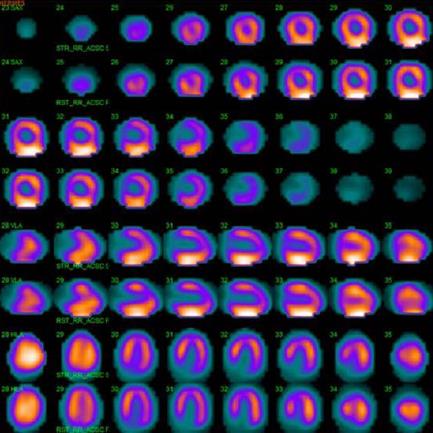
Echocardiography revealed normal findings in four patients, inferolateral hypokinesia in one patient, anteroseptal akinesia in one patient, mild aortic valvular stenosis with moderate aortic regurgitation (AR) in one patient, mild AR in one patient, mild mitral regurgitation (MR) in three patients, moderate to severe MR in one patient, and mild tricuspid regurgitation in three patients. Right ventricular systolic pressure was normal in all patients. Dilated coronary sinus (CS) was found in one patient (patient 4). One patient underwent 99mTc-sestamibi SPECT scintigraphy (patient 7), which revealed normal myocardial perfusion (Figure 2) and a normal left ventricular ejection fraction (LVEF) of 0.67. Two patients underwent CTCA (patient 2, patient 4), which confirmed the diagnosis of coronary artery vascular fistulas.
Cardiovascular MRI was performed in two patients (patients 2 and 4), which revealed moderate left ventricular hypertrophy and signs of healed MI in three flow territories (patient 2). Shunt calculation by oximetry was performed in four patients (patients 3, 4, 7 and 8) who showed left-to-right shunt ratios of 1.75:1, 1.3:1, 1.1:1 and 2.0:1, respectively, with a mean of 1.53:1 (range 1.1-2.0).
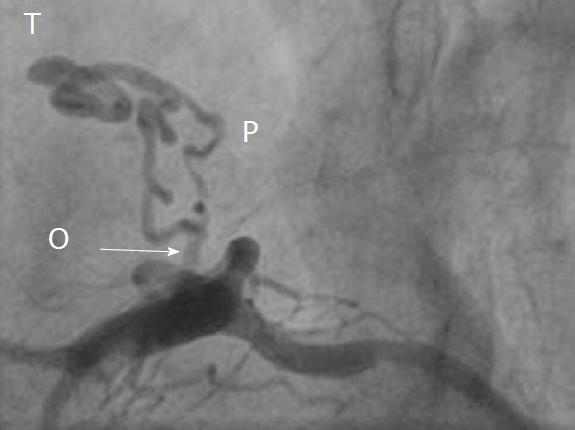
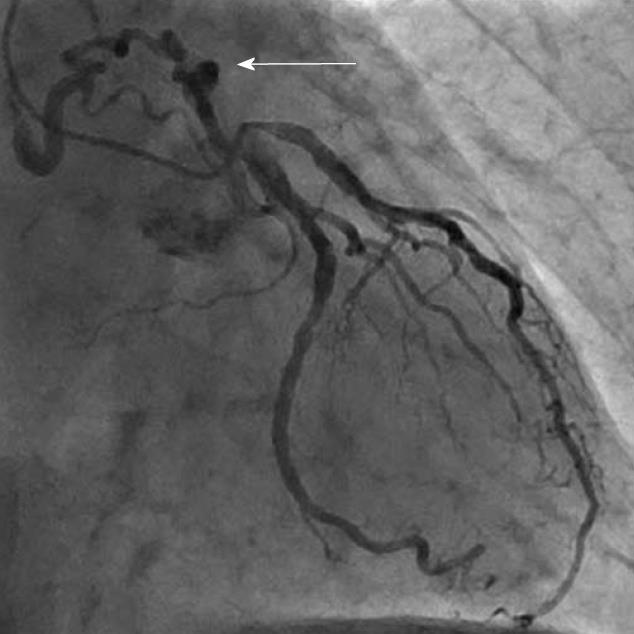
CAG was used to delineate the characteristics of the fistula (origin, pathway and termination; Figures 3-10 and Table 2). The multiplicity of the origin of the fistulous vessels and the pathway were plural in most fistulas (8/12, 67% and 9/12, 75%, respectively). The termination was equally distributed between single (6/12, 50%) and multiple (6/12, 50%) fistulous vessels. Multiplicity was common among the different fistula components (23/36, 64%). Tortuosity of the pathway was found in eight fistulas (8/12, 67%).
| Case | Fistula origin and termination | Yr of detection | Angiographic characteristics of fistula components | ||||||||
| LCx | RCA | LAD | |||||||||
| O | P | T | O | P | T | O | P | T | |||
| 1 | RCA and LAD to PA | 2014 | M | MT | M | S | MT | S | |||
| 2 | LAD to PA | 2015 | M | MT | M | ||||||
| 3 | LCx to PA | 2015 | S | ST | S | ||||||
| 4 | RCA to CS | 2015 | S | ST | S | ||||||
| 5 | LAD to PA | 2016 | S | ST | S | ||||||
| 6 | LAD to PA | 2016 | M | M | M | ||||||
| 7 | LAD to PA | 2016 | M | MT | M | ||||||
| 8 | LAD to PA | 2015 | M | MT | S | ||||||
| 9 | LCx to PA | 2016 | M | MT | M | ||||||
| 10 | LAD to PA | 2017 | M | M | M | ||||||
| 11 | LAD to PA | 2017 | M | MT | S | ||||||
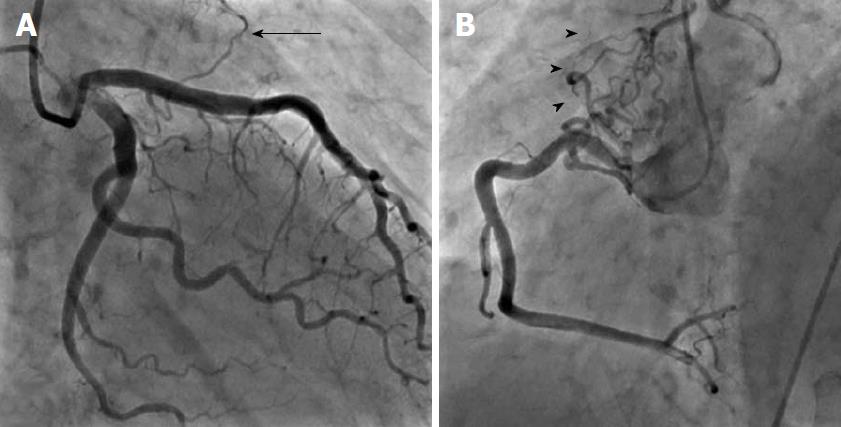
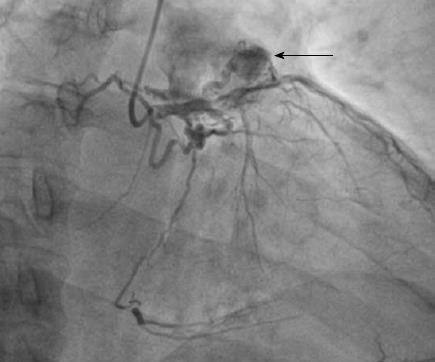
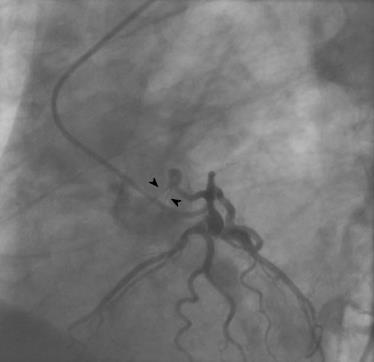
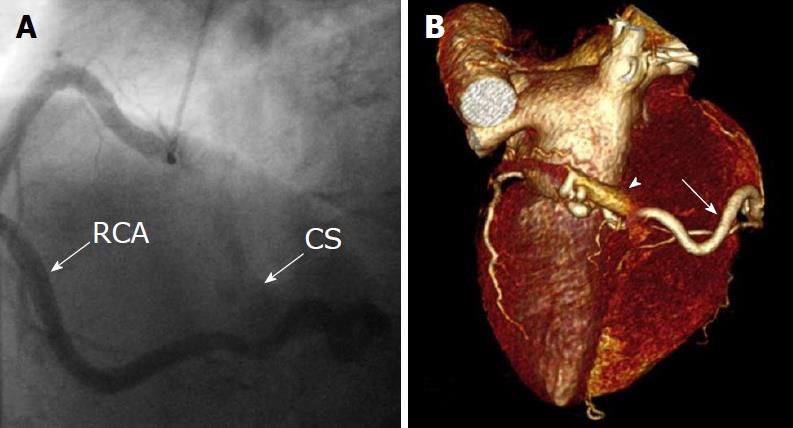
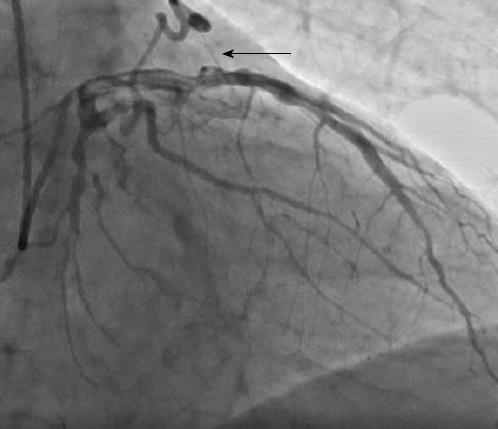
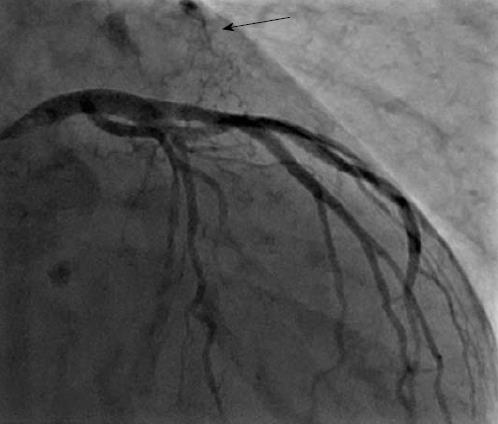
There were 10 unilateral (Figure 4) and one bilateral (Figure 3A and B) fistulas. All fistulas were of the coronary vascular type, terminating into the pulmonary artery (PA, n = 11; Figure 5) or the coronary sinus (n = 1; Figure 6), and originating from the left anterior descending coronary artery (LAD; n = 8; Figure 7), right coronary artery (RCA; n = 2; Figures 3B and 6) or left circumflex coronary artery (LCx; n = 2; Figures 5 and 10). The characteristics of the fistula (origin, pathway and termination) showed multiple origins of fistulous vessels and pathways in most fistulas (8/12, 67% and 9/12, 75%, respectively; Figure 9). Multiplicity was common among the different fistula components (22/36, 61%; Figures 3B, 4 and 10). In contrast, single (7/12, 58%) termination of the fistulous vessels was more common than multiple (5/12, 42%) termination of fistulous vessels.
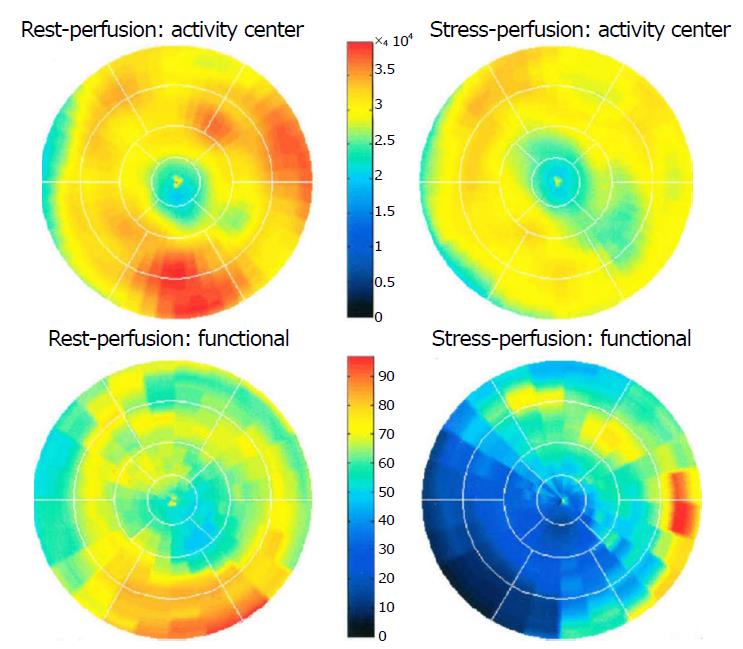
Dilated RCA was found in one patient (patient 4; Figure 6A), while large and small aneurysmal formation was present in two patients (patients 2 and 6, respectively; Figures 4 and 8). The FFR was not measured. The ECG-gated imaging of adenosine stress/rest 13N-ammonia PET-CT (Table 3) demonstrated homogenous distribution of perfusion in two patients, who showed no perfusion defects (patients 3 and 11). One patient showed diffuse, reversible reductions in perfusion in the apical and antero-septal regions, and partly in the basal anterior segment (patient 4) (Figure 11). In another patient, perfusion of the LAD area was slightly lower than the inferior segment, but it was equal to that of the lateral wall (patient 7). Normal perfusion with reduced LVEF (rest 33% and stress 39%) was probably underestimated in one patient (patient 8). In the five patients, the mean global stress/rest ratio for MPR was 2.9 (range 2.33-3.90). The mean regional stress/rest ratio was 3.0 for the LAD (range 2.35-4.50), 2.9 for the RCA (range 2.49-3.60) and 2.8 for the LCx (range 2.36-3.20). Blood flow through the LAD was slightly higher than flow through the RCA and LCx.
| Case | CAF | Stress/rest perfusion segments | LVEF (%) | Semi-quantitative findings | CAD | Prior procedure | Management of CAF | ||||
| LCx | RCA | LAD | Global | Rest | Stress | ||||||
| 3 | LCx-PA | 2.74 | 2.49 | 2.56 | 2.59 | 52 | 57 | No perfusion defects | 1-VD | PCI-LAD | PTE |
| 4 | RCA-CS | 2.65 | 2.7 | 2.71 | 2.69 | 65 | 65 | Diffuse reversible reduction of perfusion in the apical, antero-septal and partially in basal anterior segment | None | None | CMM |
| 7 | LAD-PA | 2.36 | 2.55 | 2.35 | 2.33 | 60 | 65 | Perfusion of LAD area is less than inferior segment | None | None | CMM |
| 8 | LAD-PA | 3.02 | 2.91 | 2.94 | 2.95 | 33 | 39 | Normal perfusion with reduced LVEF | 1-VD | PCI-LAD | CMM |
| 11 | LAD-PA | 3.2 | 3.6 | 4.5 | 3.9 | 65 | 65 | No perfusion defects | None | None | CMM |
Absolute flow quantification in one patient (patient 3) yielded normal myocardial perfusion with high flow in the LCx, which was the fistula-related vessel, when compared to RCA and LAD flow, confirming successful percutaneous transcatheter embolization (PTE) closure of the fistulous vessel. On the other hand, semi-quantitative analysis revealed normal perfusion in two patients and a diffuse reduction in perfusion in the other two patients. When combined with the semi-quantitative results, findings of normal flow by PET-CT indicate that conservative medical management (CMM) can be used as a management option for fistulous vessels, thereby avoiding the need for occlusion of the fistula.
The interventions included leaving a small fistula without percutaneous or surgical intervention; surgical ligation (SL) of the fistula in combination with CABG (patient 2); percutaneous closure of the fistulous tract (LCx to PA) and percutaneous coronary intervention (PCI) of the LAD due to chest pain and dyspnea upon exertion (patient 3); PCI of the OM branch of the LCx and coiling of the fistula (LAD to PA) in a patient (patient 5) with non-sustained ventricular tachycardia; and transcutaneous obliteration of the fistula secondary to angina pectoris (patient 10).
Treatment and management of the fistula included CMM in seven patients, PTE in three patients and SL combined with coronary artery bypass grafting (CABG) in one patient. Of the patients who underwent adenosine stress/rest 13N-ammonia PET-CT, PTE was performed in only one patient (patient 3). This patient showed high flow in the LCx, which was the fistula-related vessel, and PTE was successfully performed with angiographic documentation. In the other four patients (patients 4, 7, 8 and 11), PET-CT showed no flow restrictions, indicating CMM and avoiding the need for fistula closure.
Five patients had known CAD (one vessel disease in four patients and three vessel diseases in one patient). Of these patients, three underwent PCI procedures, one patient was managed with optimal medical treatment, and one patient underwent CABG in combination with fistula ligation.
In this study, we describe congenital CAFs that were incidentally found in 11 adult patients during routine CAG. To investigate the functional characteristics of these fistulas, PET-CT was performed at rest and after adenosine pharmacological stress in five subjects. To date, the role of PET-CT has not been extensively studied in adult patients with congenital CAFs.
Congenital CAFs are usually asymptomatic in many adult patients and are usually incidentally detected during routine CAG for suspected CAD. Over the past century, congenital CAFs associated with continuous cardiac murmur have often been confused with patent ductus arteriosus Botalli[28,29]. More recently, congenital CAFs have been misdiagnosed as a right atrial myxoma[30].
Coronary artery vascular and cameral fistulas are increasingly found during echocardiography[3,31,32], CTCA[2,33] and diagnostic CAG procedures[1]. One study reported the diagnosis of a small coronary-pulmonary fistula (CPF) by echocardiography in asymptomatic dizygotic twin infant brothers[31], suggesting a possible genetic cause of CAFs. Invasive CAG is considered the gold standard for the detection and diagnosis of congenital CAFs; however, non-invasive imaging techniques such as contrast echocardiography[34], 2D and color Doppler echocardiography, cardiovascular MRI and CTCA provide valuable complementary data for the visualization of fistula components (origin, pathway and termination sites) that are not visible on the CAG[20,35-37].
The value of FFR has been emphasized by several authors. Ouyang et al[38] reported deferral of treatment of the fistula-related artery and tandem intermediate stenosis in a patient with a huge CAF originating from the LAD and draining into the PA based on the findings of FFR. In the case of an adult subject assessed by both FFR and intravascular ultrasound, PCI was performed for significant stenosis of the LAD, which was the fistula-related artery (LAD-PA), but the congenital fistula remained untouched due to the absence of myocardial ischemia[18]. In congenital CAFs, the steal phenomenon has been demonstrated by FFR[17]. Few reports have been published regarding the functional assessment of congenital CAFs (left main PA) in which FFR was used to guide clinical decision-making for intervention. In one of these reports, normal FFR was observed for a patient with concomitant moderate coronary artery stenosis, which led to the deferral of surgical or transcutaneous interventions[39]. Furthermore, Hollenbeck et al[40] demonstrated ischemic changes due to the combination of a moderate LAD stenosis and CVF, which was relieved by PCI. Oh et al[41] assessed the hemodynamic significance of congenital multiple micro-fistulas and coronary cameral fistulas by FFR, which ruled out ischemia. In 2014, Sasi et al[42] assessed CAF using coronary flow reserve (CFR) and FFR. The LCx FFR and CFR were 0.97 and 2.33, respectively, and the LAD FFR of 0.86 was associated with a CFR of 1.56. This patient was treated medically. In 2016, Ito et al[43] successfully performed FFR-guided PCI of an intermediate LAD stenosis associated with CAFs, and the fistula was treated conservatively. Bi-directional flow in the fistula may occur in the left ventricle[44] and the right ventricle[45]; however, none of our patients demonstrated bi-directional fistulous flow.
In the 1990s, myocardial perfusion scintigraphy SPECT using Tl-201 was performed in some patients to determine fistula-related ischemia. A report by Gupta et al[46] in 1991, followed by Glynn et al[47] in 1994, reported the first case studies documenting the use of thallium-201 perfusion imaging for physiological assessment of CAF (LAD-PA) and as the cause of an exercise-induced reversible thallium-201 perfusion defect in an adult patient[46,47]. The pharmacological stress MPI SPECT technique may indicate segmental perfusion defects in patients with congenital CPF in the absence of CAD[11]. In a patient series evaluated by Lee et al[11], 35% of patients with congenital CPF without CAD developed perfusion defects, but the findings were only clinically relevant in 12%.
Adenosine stress-induced 13N-ammonia PET-CT uptake in the myocardium is related to perfusion and may offer better assessment of myocardial ischemia. Adenosine stress 13N-ammonia PET-CT has several advantages over SPECT, such as higher spatial resolution and sensitivity, absolute quantification, better counting efficiency and improved attenuation correction[11,24]. In 2011, a report described the application of adenosine 13N-ammonia PET-CT in congenital CAFs for the first time[19].
Adenosine 13N-ammonia PET-CT scanning is routinely applied to assess the functional status and quantify flow in patients with CAD[26,48], which could also be used in subjects with congenital coronary artery anomalies of origin (i.e., single coronary artery[49]) and termination (i.e., CAFs[19]).
Quantification of myocardial perfusion facilitates the high-performance detection and localization of perfusion abnormalities. In the same patient with CAF, PET-CT was reported to show greater areas of ischemic change than 99mTc-sestamibi scintigraphy[50]. In a comparative study by Kong et al[20], adenosine stress 13N-ammonia PET-CT imaging was shown to have higher diagnostic sensitivity (91% vs 65%) and specificity (89% vs 82%) compared with SPECT using Tc-99m sestamibi, providing better assessment of myocardial perfusion. Thus, MPI may guide decisions regarding which patients will benefit from invasive treatment. Adenosine 13N-ammonia PET-CT may prove to be valuable in clinical decision-making for whether to close the fistulous communication based on the presence or absence of distribution abnormalities[51]. Successful SL of the fistula and reconstruction of the CS was performed in an adult male with a congenital RCA-CS fistula, which was associated with aneurysmal dilatation of the CS and stenosis of the CS ostium[3]. In the patient series reported by Zhang et al[52], SL of isolated congenital CAFs was related to lower morbidity and mortality associated with residual shunt in 8/47 (17%) patients, two of whom required PTE. Based on the findings of adenosine 13N-ammonia PET-CT in our five patients, transcutaneous or surgical intervention was avoided in four subjects. In the current series, treatment and management of the fistula included CMM in seven patients, PTE in three patients and SL in one patient.
In conclusion, adenosine 13N-ammonia PET-CT is a useful technique that provides additional information for diagnosis and decision-making related to the management of incidentally detected congenital CAFs. The technique may also be useful for determining the functional status of the fistula and may add some clues for clinical decision-making in adult patients with congenital CAFs. Further prospective studies with a large number of patients are warranted.
Firstly, the study was retrospective in nature, with patients collected from different cardiac catheterization laboratories in the Netherlands. Secondly, this study contained a small sample size, involving a limited number of patients collected from three centers which used different diagnostic modalities. There is increasing need for a prospective international registry.
Congenital coronary artery fistulas (CAFs) are uncommon coronary artery vascular anomalies which are often incidentally found during coronary angiography (CAG) performed for suspected atherosclerotic coronary artery disease (CAD). Moreover, most asymptomatic patients are diagnosed with CAFs during evaluation for cardiac murmur. Nowadays, congenital CAFs are increasingly detected due to the widespread use of non-invasive techniques such as echocardiography and computed tomography coronary angiography (CTCA). Functional assessment and determination of the clinical significance of CAFs is of great importance for therapeutic decision-making. The choice of treatment strategy depends on the size and location of the fistula, the magnitude of left-to-right shunt and the characteristics of the fistulous tract. Many diagnostic modalities are currently used to evaluate the functional characteristics of the fistula, including non-invasive methods such as Doppler echocardiography, CTCA and radionuclide angiography, and invasive methods such as right heart catheterization and fractional flow reserve (FFR), among others. In the current study, the role of positron emission tomography computed tomography (PET-CT) is described in an observational setting. We aimed to determine the impact of the fistula on the clinical status of the patient, in addition to whether PET-CT can be used to assess the functional status of the fistula. This information was used to determine the best therapeutic strategy, which included monitoring, conservative medical management (CMM), transcutaneous catheter embolization or surgical ligation (SL). In general, echocardiography represents the first diagnostic imaging approach, but it may be limited by an inappropriate acoustic window. Modern echocardiography equipment has greater sensitivity, which explains why congenital CAFs are frequently diagnosed by this method. Echocardiography can be used to diagnose and evaluate the hemodynamic significance, anatomy and physiopathology of CAFs. CTCA is a widely used technique that can be used for morphological and functional analyses, as well as for perfusion studies of CAFs. CTCA allows for comprehensive cardiac evaluation, providing morphological and functional data on coronary circulation and myocardial perfusion status, as well as anatomical images. Electrocardiography (ECG)-gated cardiovascular computed tomography may play an important role in the evaluation of the origin, pathway, termination and morphology of the fistula in relation to the adjacent anatomical structures, as well as cardiac morphology and contractility. Cardiovascular magnetic resonance does not use ionic radiation and plays a crucial role in determining myocardial wall viability, characterizing the myocardial tissue and its morphology, as well as providing detailed data related to cardiac function, estimated blood flow within the fistula and the anatomical characteristics. Myocardial perfusion imaging (MPI) is used to identify abnormalities in cardiac and pulmonary circulation, providing the Qp: Qs ratio is required to diagnose and quantify left-to-right shunt, and to assess myocardial perfusion defects that occur as a result of segmental hypoperfusion caused by the fistula-bearing vessel. Myocardial perfusion single-photon emission computed tomography (SPECT) is used to detect myocardial ischemia and to stratify the risk of experiencing a cardiac event in patients with CAFs. The hemodynamic significance of this modality remains unclear. The closure technique for congenital CAFs will be chosen after thorough diagnostic imaging and functional investigation has been performed to assess the hemodynamic and functional significance of the fistula, its anatomical morphology, and its impact on the clinical status of the patient.
The aim of this study was to review and present the current data on non-invasive and invasive diagnostic methods used to evaluate the anatomical morphology and functional significance of CAFs. Medical imaging is important for assessing the location and size of CAFs.
We assessed the hemodynamic impact of CAFs using 13N-ammonia PET-CT imaging under pharmacological adenosine-induced stress and at rest. Future research in a larger group of symptomatic and asymptomatic patients with a greater magnitude of left-to-right shunt is warranted.
This was an observational study of 11 subjects with congenital CAFs that had been incidentally found during CAG performed for suspected atherosclerotic CAD. In all patients, physical examination, ECG, echocardiography, chest X-ray and laboratory investigation were performed. The patients were collected from three non-academic hospitals in the West and East regions of the Netherlands. FFR was not measured due to a lack of interventional cardiology facilities in these non-interventional hospitals. Different fistula characteristics were delineated using coronary angiographic imaging techniques. Five subjects underwent pharmacological adenosine stress/rest 13N-ammonia PET-CT to assess the hemodynamic impact of the fistula. PET-CT was performed in a different academic center. PET-CT is considered a superior diagnostic modality as it provides data on the metabolic status of the myocardial tissue.
The patients involved in this study had a variety of clinical presentations, including limited posterior non-ST-elevation myocardial infarction (MI), angina pectoris and chest pain (n = 6), dyspnea upon exertion (n = 3), and an asymptomatic presentation with abnormal resting electrocardiogram (n = 1). One patient presented with palpitation, ischemic cerebrovascular accident, paroxysmal atrial fibrillation and non-sustained ventricular tachycardia. Another patient presented with exercise-induced non-sustained ventricular tachycardia. Previous MI was reported in two patients. The physical examination was unremarkable in seven patients. Apical systolic murmur was heard in one patient, and systolic ejection murmur was heard in the second intercostal space in three other patients. Although continuous cardiac murmur is usually present in patients with CAF, no continuous murmur was heard in the current group of patients. In this case series, the body mass index of subjects ranged between 21.1 to 33.4 kg/m2, with four patients classified as normal weight, five as overweight and two as obese. The electrocardiogram demonstrated sinus rhythm in 10 patients and permanent atrial fibrillation in one patient. Echocardiography revealed dilated coronary sinus in one patient. None of the patients showed pulmonary hypertension, with normal results for right ventricular systolic pressure. There were 10 single-sided and one double-sided fistulas. All fistulas were of the coronary vascular type, terminating into the pulmonary artery (n = 11) or coronary sinus (n = 1), and originating from the LAD (n = 8), right coronary artery (RCA, n = 2) or left circumflex coronary artery (LCx, n = 2). In regard to the characteristics of the fistula (origin, pathway and termination), the origin and pathway of the fistulous vessels was plural in most fistulas (8/12, 67% and 9/12, 75%, respectively). Multiplicity was common among the different fistula components (22/36, 61%). In contrast, single (7/12, 58%) termination of the fistulous vessels was more common than multiple (5/12, 42%) termination of fistulous vessels. The termination was equally distributed between single (6/12, 50%) and multiple (6/12, 50%) fistulous vessels. Multiplicity was common among the different fistula components (23/36, 64%). Tortuosity of the pathway was found in eight fistulas (8/12, 67%). A dilated RCA was found in one patient, and large and small aneurysmal formation was present in two patients. The presence of tortuosity and multiplicity of the fistulous tract meant that percutaneous intervention would be very challenging. In patient 2, who had symptomatic significant CAD, SL of the fistula was performed in combination with coronary artery bypass grafting. The characteristics of the fistula components in this patient were multiple origin and termination with multiple-tortuous pathways, which meant the percutaneous approach could not be used. In four patients (patients 4, 7, 8 and 11), PET-CT showed no flow restrictions. Thus, CMM could be implemented, avoiding the need for fistula closure either transcutaneous or surgically. The adenosine stress/rest 13N-ammonia PET-CT performed in five subjects demonstrated homogenous distribution of perfusion in two patients, and no perfusion defects in two patients. One patient showed diffuse, reversible reduction in perfusion in the apical and antero-septal regions, and also partly in the basal anterior segment. In another patient, perfusion of the left anterior descending coronary artery (LAD) area was slightly lower than the inferior segment, but it was equal to the lateral wall. Normal perfusion with reduced left ventricular ejection fraction (rest 33%, stress 39%) was probably underestimated in one patient. In these five patients, the mean global stress/rest ratio was 2.9 (range 2.33-3.90). The mean regional stress/rest ratio was 3.0 for the LAD (range 2.35-4.50), 2.9 for the RCA (range 2.49-3.60) and 2.8 for the LCx (range 2.36-3.20). Blood flow through the LAD was slightly higher than through the RCA and LCx. Absolute flow quantification revealed normal myocardial perfusion with high flow in the LCx, which was the fistula-related vessel, compared to the flow of the RCA and the LAD, indicating successful PTE closure procedure of the fistulous vessel. On the other hand, semi-quantitative analysis revealed normal perfusion in two patients and a reduction in diffuse perfusion in the other two patients.
The hemodynamic characteristics of incidentally found CAFs are of great importance to guide decision-making for whether to treat patients or perform periodic monitoring. Pharmacological adenosine stress/rest 13N-ammonia PET-CT in patients with incidentally found congenital CAFs provided adequate and clear information regarding the hemodynamic burden of the fistula in this small patient population. For better diagnosis of incidentally found congenital CAFs, pharmacological adenosine stress/rest 13N-ammonia PET-CT should be performed as part of the diagnostic imaging work-up. However, this needs to be confirmed in a large, prospective, international study or registry. In the current study, pharmacological adenosine stress/rest 13N-ammonia PET-CT was performed in a limited number of adult patients with incidentally found congenital CAFs. This test is achievable in patients with congenital CAFs. That pharmacological adenosine stress-rest 13N-ammonia PET-CT in incidentally found congenital CAFs is currently, in this patient population, can provide adequate and clear answer regarding the hemodynamic burden of the fistula and guiding the clinical decision making. For patients with CAFs, multiple imaging modalities are required to assess the anatomical morphology, hemodynamic significance and behavior of the fistula in order to assist in the choice of therapeutic strategy. Angiographic characterization of the individual fistula components (origin, pathway and termination) may help guide the selection of closure technique, either percutaneously or surgically.
Further studies on a larger number of patients with congenital CAFs (small or large, symptomatic or asymptomatic, treated or untreated) are required to determine the prospective incidence and other characteristics, such as history and long-term outcomes. In 2018-2019, we are planning to initiate an international registry on CAFs (Euro-CAF Survey) to address the diagnostic and therapeutic issues.
The authors would like to thank the members and staff of the catheterization unit of the Department of Cardiology and the Department of Nuclear Medicine, as well as Mr. Jeroen Geerdink, Hospital System Integrator, for his assistance during the preparation of the manuscript.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: The Netherlands
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D, D, D
Grade E (Poor): 0
P- Reviewer: Altarabsheh SE, Barik R, Korosoglou G, Petix NR, Uehara Y, Vermeersch P S- Editor: Ji FF L- Editor: A E- Editor: Wu YXJ
| 1. | Karazisi C, Eriksson P, Dellborg M. Coronary Artery Fistulas: Case Series and Literature Review. Cardiology. 2017;136:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Ghaffari S, Akbarzadeh F, Pourafkari L. Aneurysmal coronary arteriovenous fistula closing with covered stent deployment: a case report and review of literature. Cardiol J. 2011;18:556-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Pu L, Li R, Yang Y, Liu G, Wang Y. Right coronary artery coronary sinus fistula with coronary sinus ostium stenosis. Echocardiography. 2017;34:1102-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Bittencourt MS, Seltman M, Achenbach S, Rost C, Ropers D. Right coronary artery fistula to the coronary sinus and right atrium associated with giant right coronary enlargement detected by transthoracic echocardiography. Eur J Echocardiogr. 2011;12:E22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Green T, Crilley J. Endocarditis and coronary artery fistula: a case report. Eur Heart J. 2018;1-4. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Scansen BA. Coronary Artery Anomalies in Animals. Vet Sci. 2017;4:pii: E20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Villa AD, Sammut E, Nair A, Rajani R, Bonamini R, Chiribiri A. Coronary artery anomalies overview: The normal and the abnormal. World J Radiol. 2016;8:537-555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 187] [Cited by in RCA: 205] [Article Influence: 22.8] [Reference Citation Analysis (19)] |
| 8. | Magro V, Cacciapuoti F, Cacciapuoti F, Lama D. An unexpected finding in a diabetic patient studied with transthoracic echocardiography. MOJ Gerontol Ger. 2017;2:00041. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Zhang P, Cai G, Chen J, Wang Y, Duan S. Echocardiography and 64-multislice computed tomography angiography in diagnosing coronary artery fistula. J Formos Med Assoc. 2010;109:907-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Iskandrian AS, Kimbiris D, Bemis CE, Segal BL. Coronary artery to pulmonary artery fistulas. Am Heart J. 1978;96:605-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Lee SK, Jung JI, O JH, Kim HW, Youn HJ. Coronary-to-pulmonary artery fistula in adults: Evaluation with thallium-201 myocardial perfusion SPECT. PLoS One. 2017;12:e0189269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Jani L, Mester A, Hodas R, Kovacs I, Benedek T, Bajka B, Benedek I. Computed tomography assessment of coronary fistulas. J Interdisc Med. 2017;2:155-159. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Detorakis EE, Foukarakis E, Karavolias G, Dermitzakis A. Cardiovascular magnetic resonance and computed tomography in the evaluation of aneurysmal coronary-cameral fistula. J Radiol Case Rep. 2015;9:10-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Siddiqi MS, Sharma AK, Sharma J. Giant left circumflex artery aneurysm with coronary artery fistula. Open Access J Surg. 2016;1:555-572. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Challoumas D, Pericleous A, Dimitrakaki IA, Danelatos C, Dimitrakakis G. Coronary arteriovenous fistulae: a review. Int J Angiol. 2014;23:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 16. | Sunil Roy TN, Sajeev CG, Francis J, Krishnan MN, Venugopal K. Three major coronary artery-to-left ventricular fistula: an unusual cause of a diastolic murmur at the apex. J Am Soc Echocardiogr. 2006;19:1402.e1-1402.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Härle T, Kronberg K, Elsässer A. Coronary artery fistula with myocardial infarction due to steal syndrome. Clin Res Cardiol. 2012;101:313-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Ouyang F, Chen M, Yi T, Wu M, Peng H, Huang H, Huang H, Zhou S. Successful percutaneous coronary intervention for multivessel stenosis complicated by a huge coronary artery fistula with the combined physiology and intracoronary anatomy techniques. Int J Cardiol. 2015;192:70-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Said SA, Nijhuis RL, Op den Akker JW, Kimman GP, Van Houwelingen KG, Gerrits D, Huisman AB, Slart RH, Nicastia DM, Koomen EM. Diagnostic and therapeutic approach of congenital solitary coronary artery fistulas in adults: Dutch case series and review of literature. Neth Heart J. 2011;19:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Kong EJ, Cho IH, Chun KA, Won KC, Lee HW, Park JS, Shin DG, Kim YJ, Shim BS. Comparison of clinical usefulness between N-13 ammonia PET/CT and Tc-99m sestamibi SPECT in coronary artery disease. Nucl Med Mol Imaging. 2008;5:354-361. |
| 21. | Knuuti J. Integrated positron emission tomography/computed tomography (PET/CT) in coronary disease. Heart. 2009;95:1457-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Beanlands RS, Chow BJ, Dick A, Friedrich MG, Gulenchyn KY, Kiess M, Leong-Poi H, Miller RM, Nichol G, Freeman M. CCS/CAR/CANM/CNCS/CanSCMR joint position statement on advanced noninvasive cardiac imaging using positron emission tomography, magnetic resonance imaging and multidetector computed tomographic angiography in the diagnosis and evaluation of ischemic heart disease--executive summary. Can J Cardiol. 2007;23:107-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Herzog BA, Husmann L, Valenta I, Gaemperli O, Siegrist PT, Tay FM, Burkhard N, Wyss CA, Kaufmann PA. Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Am Coll Cardiol. 2009;54:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 489] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 24. | Siegrist PT, Husmann L, Knabenhans M, Gaemperli O, Valenta I, Hoefflinghaus T, Scheffel H, Stolzmann P, Alkadhi H, Kaufmann PA. (13)N-ammonia myocardial perfusion imaging with a PET/CT scanner: impact on clinical decision making and cost-effectiveness. Eur J Nucl Med Mol Imaging. 2008;35:889-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Dilsizian V, Bacharach SL, Beanlands RS, Bergmann SR, Delbeke D, Gropler RJ, Knuuti J, Schelbert HR, Travin MI. PET myocardial perfusion and metabolism clinical imaging. J Nucl Cardiol. 2009;16:651. [RCA] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 26. | Tio RA, Dabeshlim A, Siebelink HM, de Sutter J, Hillege HL, Zeebregts CJ, Dierckx RA, van Veldhuisen DJ, Zijlstra F, Slart RH. Comparison between the prognostic value of left ventricular function and myocardial perfusion reserve in patients with ischemic heart disease. J Nucl Med. 2009;50:214-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 156] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 27. | Germano G, Kiat H, Kavanagh PB, Moriel M, Mazzanti M, Su HT, Van Train KF, Berman DS. Automatic quantification of ejection fraction from gated myocardial perfusion SPECT. J Nucl Med. 1995;36:2138-2147. [PubMed] |
| 28. | Bogers AJ, Quaegebeur JM, Huysmans HA. Early and late results of surgical treatment of congenital coronary artery fistula. Thorax. 1987;42:369-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | BIORCK G, CRAFOORD C. Arteriovenous aneurysm on the pulmonary artery simulating patent ductus arteriosus botalli. Thorax. 1947;2:65-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 30. | Wen B, Yang J, Jiao Z, Fu G, Zhao W. Right coronary artery fistula misdiagnosed as right atrial cardiac myxoma: A case report. Oncol Lett. 2016;11:3715-3718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Caputo S, Sorice N, Sansone R, Simeone D, Caruso V, Russo R, Cicchella N, Izzo A, Saviano C, Casani A. Echocardiographic diagnosis of coronary artery fistula in both dizygotic twin brothers: environmental mechanism? J Cardiovasc Med (Hagerstown). 2017;18:378-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Ganji JL, Click RL, Najib MQ, Schaff HV, Chaliki HP. Coronary arteriovenous fistula in a patient with aortic valve regurgitation. Echocardiography. 2013;30:E85-E86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 33. | Pursnani A, Jacobs JE, Saremi F, Levisman J, Makaryus AN, Capuñay C, Rogers IS, Wald C, Azmoon S, Stathopoulos IA. Coronary CTA assessment of coronary anomalies. J Cardiovasc Comput Tomogr. 2012;6:48-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Hong SM, Yoon SJ, Rim SJ. Contrast echo-a simple diagnostic tool for a coronary artery fistula. Korean Circ J. 2012;42:205-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Latson LA. Coronary artery fistulas: how to manage them. Catheter Cardiovasc Interv. 2007;70:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 132] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 36. | Cademartiri F, Malagò R, La Grutta L, Alberghina F, Palumbo A, Maffei E, Brambilla V, Pugliese F, Runza G, Midiri M. Coronary variants and anomalies: methodology of visualisation with 64-slice CT and prevalence in 202 consecutive patients. Radiol Med. 2007;112:1117-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Black IW, Loo CK, Allan RM. Multiple coronary artery-left ventricular fistulae: clinical, angiographic, and pathologic findings. Cathet Cardiovasc Diagn. 1991;23:133-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Ouyang F, Wu M, Peng H, Zhang M, Huang H, Chen M, Huang H, Zhou S. Fractional flow reserve, an effective preoperative guideline to a patient with a huge coronary artery fistula and tandem stenosis. Int J Cardiol. 2015;199:333-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 39. | Yew KL, Ooi PS, Law CS. Functional assessment of sequential coronary artery fistula and coronary artery stenosis with fractional flow reserve and stress adenosine myocardial perfusion imaging. J Saudi Heart Assoc. 2015;27:283-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Hollenbeck RD, Salloum JG. Sequential moderate coronary artery fistula and moderate coronary artery stenosis causing ischemia demonstrated by fractional flow reserve and relieved following percutaneous coronary intervention. Catheter Cardiovasc Interv. 2014;83:443-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Oh JH, Lee HW, Cha KS. Hemodynamic significance of coronary cameral fistula assessed by fractional flow reserve. Korean Circ J. 2012;42:845-848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Sasi V, Nemes A, Forster T, Ungi I. Functional assessment of a left coronary-pulmonary artery fistula by coronary flow reserve. Postepy Kardiol Interwencyjnej. 2014;10:141-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 43. | Ito T, Murai S, Fujita H, Tani T, Ohte N. Fractional flow reserve-guided percutaneous coronary intervention for an intermediate stenosis complicated by a coronary-to-pulmonary artery fistula. Heart Vessels. 2016;31:816-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | Chen JP, Rodie J. Bi-directional flow in coronary-to-left ventricular fistula. Int J Cardiol. 2009;133:e41-e42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 45. | Ono M, Otake S, Fukushima N, Sawa Y, Ichikawa H, Kagisaki K, Matsuda H. Huge right ventricle-right coronary artery fistula compromising right ventricular function in a patient with pulmonary atresia and intact ventricular septum: a case report. J Thorac Cardiovasc Surg. 2001;122:1030-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 46. | Gupta NC, Beauvais J. Physiologic assessment of coronary artery fistula. Clin Nucl Med. 1991;16:40-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 47. | Glynn TP Jr, Fleming RG, Haist JL, Hunteman RK. Coronary arteriovenous fistula as a cause for reversible thallium-201 perfusion defect. J Nucl Med. 1994;35:1808-1810. [PubMed] |
| 48. | Hasbak P, Kjær A. 82 Rubidium PET to replace myocardial scintigraphy. Ugeskr Laeger. 2011;173:567-572. [PubMed] |
| 49. | Said SA, de Voogt WG, Bulut S, Han J, Polak P, Nijhuis RL, Op den Akker JW, Slootweg A. Coronary artery disease in congenital single coronary artery in adults: A Dutch case series. World J Cardiol. 2014;6:196-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 50. | Said SA, Nijhuis RL, Akker JW, Takechi M, Slart RH, Bos JS, Hoorntje CR, Houwelingen KG, Bakker-de Boo M, Braam RL. Unilateral and multilateral congenital coronary-pulmonary fistulas in adults: clinical presentation, diagnostic modalities, and management with a brief review of the literature. Clin Cardiol. 2014;37:536-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 51. | Said SA, Oortman RM, Hofstra JH, Verhorst PM, Slart RH, de Haan MW, Eerens F, Crijns HJ. Coronary artery-bronchial artery fistulas: report of two Dutch cases with a review of the literature. Neth Heart J. 2014;22:139-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 52. | Zhang W, Hu R, Zhang L, Zhu H, Zhang H. Outcomes of surgical repair of pediatric coronary artery fistulas. J Thorac Cardiovasc Surg. 2016;152:1123-1130.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |