Revised: December 26, 2009
Accepted: December 28, 2009
Published online: December 31, 2009
Atrial fibrillation (AF) management requires knowledge of its pattern of presentation, underlying conditions, and decisions about restoration and maintenance of sinus rhythm, control of the ventricular rate, and anti-thrombotic therapy. Maintenance of sinus rhythm is a desirable goal in AF patients because the prevention of recurrence may improve cardiac function, relieve symptoms and reduce the likelihood of adverse events. Anti-arrhythmic drug therapy is the first-line treatment for patients with paroxysmal and persistent AF based on current guidelines. However, currently used drugs have limited efficacy and cause cardiac and extracardiac toxicity. Thus, there is a continued need to develop new drugs, device and ablative approaches to rhythm management. Additionally, simpler and safer stroke prevention regimens are needed for AF patients on life-long anticoagulation, including occlusion of the left atrial appendage. The results of the Randomized Evaluation of Long-Term Anticoagulant Therapy study are encouraging in these settings. Knowledge on the pathophysiology of AF is rapidly expanding and identification of focally localized triggers has led to the development of new treatment options for this arrhythmia. Conversely, the clinical decision whether to restore and maintain sinus rhythm or simply control the ventricular rate has remained a matter of intense debate. In the minority of patients in whom AF cannot be adequately managed by pharmacological therapy, the most appropriate type of non-pharmacological therapy must be selected on an individualized basis. Curative treatment of AF with catheter ablation is now a legitimate option for a large number of patients. The evolution of hybrid therapy, in which two or more different strategies are employed in the same patient, may be an effective approach to management of AF. In any case, planning a treatment regimen for AF should include evaluation of the risks inherent in the use of various drugs as well as more invasive strategies.
- Citation: Forleo GB, Santini L, Romeo F. Present concepts in management of atrial fibrillation: From drug therapy to ablation. World J Cardiol 2009; 1(1): 11-22
- URL: https://www.wjgnet.com/1949-8462/full/v1/i1/11.htm
- DOI: https://dx.doi.org/10.4330/wjc.v1.i1.11
Atrial fibrillation (AF) is the most common cardiac rhythm disturbance seen in clinical practice[1], accounting for approximately one-third of hospitalizations for this condition. AF may occur in isolation or in association with structural heart disease, contributing substantially to cardiac morbidity and mortality. The estimated prevalence of AF is 0.4%-1% in the general population, increasing with age[2,3], and it is associated with an increased long-term risk of stroke, heart failure, and all-cause mortality, especially in women[4,5]. Although there are clear guidelines for the acute management of symptomatic AF[6,7], the best long-term approach for patients with a first or recurrent AF is still debated with regard to quality of life, risk of re-hospitalizations, and possible disabling complications, such as thromboembolic stroke, major bleeding, and death. Management of patients with AF requires knowledge of its pattern of presentation (paroxysmal, persistent, or permanent), underlying conditions, and decisions about restoration and maintenance of sinus rhythm, control of the ventricular rate, and anti-thrombotic therapy. The goal of treatment is to reduce symptoms and risk of thromboembolic events and to avoid tachycardia-induced unfavorable myocardial remodeling. As epidemiological studies shed light on the importance of AF, treatment progressed from the occasional use of cardiac glycosides, such as digoxin to control ventricular rate, to the use of powerful anti-arrhythmic drugs which has been the mainstay of AF treatment for decades. Regardless of the strategy initially chosen, attention must also be directed to anti-thrombotic therapy for prevention of thromboembolism.
AF is associated with substantial morbidity and mortality, mostly due to the consequences of thromboembolism. Currently, acetylsalicylic acid (a platelet inhibitor) and vitamin K antagonists, including Warfarin, are the only approved anti-thrombotic agents for stroke prevention in patients with AF. Although there is modest benefit from anti-platelets agents, randomized trials have shown that it is consistently and substantially less effective than vitamin K antagonists[8]. Patients with AF who are at low risk for stroke or who have contraindications to Warfarin should take aspirin 81 to 325 mg daily[6]. In high-risk patients with non-valvular AF, anticoagulation with Warfarin is recommended to reduce the risk of stroke and thromboembolic events[9].
It is crucial to estimate the risk of stroke before deciding the anticoagulation therapy for individual AF patient. The threshold risk that warrants anticoagulation is still controversial. To stratify the risk of ischemic stroke in AF patients and to identify patients who benefit most and least from anticoagulation, several clinical schemes have been proposed[10]. An easy score to estimate the risk of stroke in such patients is the CHADS2 risk score. It is based on a point system in which 2 points are assigned for a history of stroke or TIA and 1 point each is assigned for age over 75 years, a history of hypertension, diabetes, or recent heart failure. According to guidelines[6], aspirin is recommended in low-risk patients with a CHADS2 score of 0. In high-risk patients with a CHADS2 score ≥ 2, only oral anticoagulant therapy is recommended. In intermediate risk patients with a CHADS2 score of 1, physicians can choose between aspirin and warfarin depending on the individual patient.
For primary and secondary prevention in most AF patients under age of 75 years, an INR of 2.5 (target range, 2.0-3.0) is recommended. A target INR of 2.0 (target range, 1.6-2.5) seems reasonable for primary prevention in patients older than 75 years who are considered at high risk of bleeding.
While the available vitamin K antagonists are highly effective for the prevention and/or treatment of most thrombotic diseases, the significant inter- and intra-patient variability in dose-response, the narrow therapeutic index, and the numerous drug and dietary interactions associated with these agents have led clinicians, and investigators to search for alternative agents.
Three new orally administered anticoagulants (apixaban, dabigatran and rivaroxaban) are in the late phase of development and several others are still in the (or moving through) early phase of investigation. Direct thrombin inhibitors are new oral agents with predictable efficacy, rapid onset of action and no need of laboratory monitoring. According to the results of the Randomized Evaluation of Long-Term Anticoagulant Therapy trial[11], Dabigatran etexilate, an oral thrombin inhibitor, showed similar efficacy to Warfarin in reducing stroke and embolism (primary outcome) at the dose of 110 mg, but lowered the rate of major hemorrhage by 20%. In contrast, higher doses (150 mg) of Dabigratan were more effective than Warfarin in reducing the primary outcomes, but had similar rates of major hemorrhage. Therefore, the two effective doses, with different benefit risk profiles, make it possible to tailor the therapy to individual patient.
Although the only adverse effect of Dabigratan was dyspepsia, many aspects of the therapy are still controversial. First, while the Dabigratan doses were blinded, patients with at least one risk factor for stroke received Warfarin (open-label). Compared with Warfarin (0.53% per year), the rate of myocardial infarction was higher in - Dabigratan-treated group (0.72% per year at dose of 110 mg and 0.74% at 150 mg). Finally, the price of Dabigratan is 10 times higher than the Warfarin.
Also questionable is the the addition of clopidogrel to aspirin in patients considered unsuitable for warfarin therapy. The results of the Atrial Fibrillation Clopidogrel Trial With Irbesartan for Prevention of Vascular Events[12], which is the largest trial ever performed in patients with AF who cannot take warfarin, have clearly shown that the combination of clopidogrel (75 mg/d) and aspirin (75-100 mg/d) reduced major vascular events, particularly stroke, compared with placebo, although at the expense of an increase in major bleeding. Whereas no conclusive data are available at present, this combination-treatment alternative is not recommended yet in guidelines.
It has been documented that the left atrial appendage (LAA) is the main source of left atrial thrombus, especially in non-rheumatic AF. Several surgical and percutaneous endovascular techniques have been explored to occlude the LAA. As an alternative of the surgical closure, percutaneous exclusion of the LAA is a new approach used to prevent strokes in high-risk patients with AF and contraindication to long-term oral anticoagulant therapy[13]. Devices have recently been developed that will exclude the LAA from the circulation and potentially replace the standard Warfarin therapy for patients with AF. Two percutaneous approaches to LAA obliteration have been studied to date. The PLAATO device has been tested in patients with a contraindication to anticoagulant and at least one additional risk factor for stroke[14]. This device is no longer being evaluated or supported in the United States. The Watchman device has been recently investigated in the Embolic Protection in Patients with Atrial Fibrillation trial[15]. The study enrolled 707 patients randomly assigned in a 2:1 ratio to percutaneous closure of the LAA using the Watchman device plus short-term Warfarin or to conventional Warfarin therapy. After 1065 patient/year of follow-up, the rate of the primary composite end point (stroke, cardiovascular death and systemic embolism) was 32% lower in the Watchman group than in the conventional therapy group, a result that met the prespecified criterion for non-inferiority. However, 12.3% of patients had serious procedural complications, including pericardial effusion, acute ischemic stroke, device embolization and post-implantation sepsis.
In conclusion, although clinical application of these devices could provide a new therapeutic option, the concerns about procedural safety and the need for long-term follow-up should be addressed before this potentially important technology is put into a wide use.
The long-term management of this arrhythmia includes two generally acceptable strategies: (1) one strategy attempts restoration and/or maintenance of sinus rhythm with pharmacological and non-pharmacological anti-arrhythmic approaches; (2) in the second approach, the ventricular rate is controlled with no commitment to restore or maintain sinus rhythm. Regardless of whether the rate- or rhythm-control strategy is pursued, attention must also be paid to the anti-thrombotic therapy for prevention of thromboembolism.
An attempt to restore sinus rhythm is a reasonable approach to a first episode of AF. After this episode, the arrhythmia-free period is unpredictable, and it may not be necessary to prescribe either long-term anti-arrhythmic or anti-coagulant drugs for all patients after the first episode. Intravenous administration of anti-arrhythmic drugs (AADs) class Ic or III represents the first choice to obtain cardioversion of a new onset AF and they are widely used particularly for the emergency cases. Cardioversion might even be performed initially without the use of antiarrhythmic drugs. This approach may result in the maintenance of sinus rhythm for a year or more in about 25% patients. If arrhythmia recurs and if symptoms persist despite AAD, repeated cardioversion with the addition of anti-arrhythmic drugs should be considered.
A few randomized trials comparing outcomes of rhythm- vs rate-control strategies have been published. In particular, the AF Follow-up Investigation of Rhythm Management (AFFIRM), Rate Control versus Electrical Cardioversion for AF (RACE), and Strategies for Treatment of AF (STAF) trials compared a strategy of rate control and a rhythm control approach using AADs[16-18]. In addition, the Atrial Fibrillation and Congestive Heart Failure trial[19] compared these strategies in patients with congestive HF. The analysis of these trials demonstrated no difference in mortality or stroke rate between patients assigned to one strategy or the other. These results are generally interpreted as that either rate control or rhythm control is a suitable strategy in AF patients.
However, it would be incorrect to extrapolate that it is not worthwhile to restore sinus rhythm for a multitude of reasons. First, these trials did not compare the sinus rhythm and AF. Indeed, in one study (RACE), only 39% of patients in the rhythm-control group had sinus rhythm at the end of follow-up. Consequently, a significant limitation of these studies is the non-efficacy of rhythm-control strategy with AAD. Many patients in the rate control arm were spontaneously in sinus rhythm by the end of the study period from 10% in STAF and RACE to 35% in AFFIRM. Therefore, the results of these studies may reflect the ineffectiveness of the rhythm control methods used. When the data from these trials are analyzed according to the patient’s actual rhythm, the benefit of sinus rhythm over AF becomes apparent[20]. This benefit might have been reduced by AAD, which increased the risk of death. The reduced mortality with sinus rhythm has also been demonstrated in virtually every study that has monitored this end point.
Another methodological concern is that in the rhythm control group, continuous anticoagulation was encouraged but could be stopped at the physician’s discretion whereas in the rate-control group, continuous anticoagulation was mandated by the protocol. Importantly, most strokes were diagnosed after discontinuation of anticoagulation or at sub-therapeutic intensity (International Normalized Ratio below 2.0). In addition, while recurrent AF was detected in only one-third of those in the rhythm-control groups who developed stroke, and at the time of ischemic stroke, patients in the rate-control groups typically had AF. We strongly believe that adequate anticoagulation with Warfarin would have substantially lowered in the rhythm-control groups.
Finally, it is also important to acknowledge that the patients enrolled in these trials do not represent the full spectrum of AF patients. In particular, the patients with severe symptoms of AF who would benefit most from sinus rhythm were largely excluded from the AFFIRM trial. Clearly, in such patients the goal is still to maintain the sinus rhythm, for which, search for better drugs and techniques should continue.
A more cost-effective approach is to control the ventricular rate without attaining the sinus rhythm. Drugs that prolong the AV node refractory period are generally effective for rate control. The efficacy of pharmacological interventions designed to achieve rate control in AF patients is about 80% in clinical trials[21]. However, the adverse effects of the drugs such as bradycardia and heart block may occur, especially in the elderly. Radiofrequency ablation of the atrioventricular junction with pacemaker implantation (the “ablate and pace” strategy) can improve symptoms and LV function in some patients, but the growing concern about the negative effects of long-term right ventricular pacing makes this a drawback rather than a primary treatment strategy.
Randomized studies suggest combining β-blockers or calcium channel blockers with digoxin to achieve a better rate control at rest and during exercise[22,23]. The oldest drug, digoxin, still is used frequently. The advantage of digoxin is that it has positive inotropic effects, making it highly suitable for patients with left ventricular dysfunction. The disadvantage is that it acts by increasing vagotonus and thus has limited or virtually no rate-controlling properties during exercise. Digoxin, used intravenously or orally, should be reserved only for heart failure patients. β-blocking agents may be the drugs of choice in patients with systolic dysfunction and/or coronary artery disease. Importantly, the negative inotropic action of β-blockers can cause deterioration in patients with (decompensated) systolic heart failure. However, if carefully titrated, β-blockers may even improve left ventricular function and survival in patients with poor left ventricular function. Intravenous β-blockers, verapamil, or diltiazem may be used to immediately slow a fast ventricular rate associated with AF. Non-pharmacological therapies should be administered to patients with symptomatic AF in whom a rapid ventricular rate cannot be slowed by drug therapy.
The aims to control the heart rate in AF patients treated with drugs are to minimize symptoms and prevent excessive tachycardia. However, the optimal level of heart rate for AF patients remains unclear. Rate control in RACE and AFFIRM trial defined a resting heart rate < 80 or < 100 beats/min, respectively. However, a sub-study of the AFFIRM[24] showed that the rate-control approach was successfully achieved in two-thirds of the patients. Additionally, to obtain adequate rate control, atrioventricular node ablation and pacemaker implantation was performed in 5.3% patients, and 17.3% patients had a pacemaker implanted for symptomatic bradycardia.
Unfortunately, these studies give no data on the influence of the level of rate control on mortality and morbidity. It is essential to achieve good rate control to minimize symptoms and the risk of tachycardia-mediated cardiomyopathy, and a 24-h heart rate that mimics normal sinus rhythm is a reasonable end point. As it still remains unknown whether strict rate control is associated with an improved prognosis, a long-term prospective, randomized trial would be useful.
Management of AF includes treatment of underlying causes and precipitating factors. Immediate cardioversion should be performed in patients with AF and acute myocardial infarction, chest pain, hypotension, severe heart failure, or syncope. Elective direct-current cardioversion has a higher success rate and a lower incidence of cardiac adverse effects than medical cardioversion in converting AF to sinus rhythm. Unless transesophageal echocardiography shows no thrombus in the LAA before cardioversion, oral Warfarin should be given for 3 wk before elective cardioversion, and continue for at least 4 wk after maintenance of sinus rhythm. Direct-current cardioversion involves delivery of an electrical shock synchronized with the intrinsic activity of the heart by sensing the R wave of the ECG to ensure that electrical stimulation does not occur during the vulnerable phase of the cardiac cycle.
Different techniques are used to perform an electrical cardioversion, each with specific indications, advantages and limitations[25] (Table 1). The method most frequently used to restore sinus rhythm is external direct current cardioversion, which was found to be a safe and effective technique, since biphasic waveform defibrillators are widely available[26,27]. However, this technique requires high energies and needs general anaesthesia or deep sedation. An alternative method to obtain restoration of sinus rhythm is esophageal cardioversion that could obviate these limitations of the external one, which uses lower energy and avoids general anaesthesia and is extremely well tolerated by patients and can be easily performed in an outpatient setting[28,29]. Recently the two techniques have been compared[30] and the results showed that AF might be cardioverted safely and effectively by either a transthoracic or a transesophageal approach. The sedation of moderate depth using midazolam renders cardioversion by either approach acceptable. As transesophageal cardioversion shows no clear advantage, transthoracic cardioversion using a conscious sedation by midazolam should remain the approach of first choice.
| Advantages | Disadvantages | Efficacy (%) | |
| External cardioversion | Safety Effectiveness Feasibility Outpatient regimen (not in all the centers) | General anaesthesia (physical presence of anaesthesiologist) Need of high energies | 94 |
| Oesophageal cardioversion | Efficacy with lower energies Outpatient regimen No general anesthesia First choice in obese and COPD patients Safety in patients with pacemaker or ICD Atrial pacing back up | High cost of the catheter Contraindicated in patients with oesophageal diseases | 95 |
| Internal cardioversion | Very high effectiveness Use of very low energy No general anaesthesia | Need of an electrophysiology laboratory Invasive approach Pain perception | 93 |
Another technique performed during the last two decades is the internal cardioversion[31], but its advantage is limited to a small percentage of unsuccessful external cardioversions or in those patients who are more difficult to defibrillate such as overweight or obese patients, patients with chronic obstructive pulmonary disease or those with implanted devices which may be injured by high energy shocks.
Anti-arrhythmic drug therapy is the first line of treatment for patients with paroxysmal and persistent AF based on current guidelines. However, the currently available anti-arrhythmic agents have poor efficacy and are associated with significant side effects, both cardiac and non-cardiac. Consequently,the limited efficacy and proarrhythmic risks of AAD for AF have led to the development of nonpharmacologic therapeutic approaches.
Patients who do not receive AAD have a 1-year AF recurrence rate of about 75%. With anti-arrhythmic drugs, sinus rhythm may be maintained in 50%-65% of cases. The choice of anti-arrhythmic agents should be guided by the presence or absence of structural heart disease, tolerability, ease of administration and side effect profile. The optimal pharmacological means to restore and maintain sinus rhythm in AF patients remains controversial.
Several drugs including amiodarone, propafenone, flecainide, and sotalol have been shown to be effective in the prevention of AF recurrences. These agents often do not totally abolish the arrhythmia, but increase the length of the arrhythmia-free interval. The best available agent for rhythm control is amiodarone[32,33]. In the Canadian Trial of Atrial Fibrillation, amiodarone was compared with propafenone and sotalol for suppression of AF. Amiodarone was associated with a 35% rate of AF recurrence at 16 mo compared with a 63% rate of recurrence in the other drugs. Amiodarone has been approved by the Food and Drug Administration for the treatment of ventricular arrhythmias but not for the AF management. Even so, it is widely prescribed and it is an excellent choice for patients with structural heart disease or congestive heart failure as most other anti-arrhythmic medications are contraindicated in heart failure patients. Amiodarone is less proarrhythmic than other agents but can adversely affect lungs, thyroid, and other organs[34]. After 5 years, 30% of patients on amiodarone are expected to discontinue the therapy because of side effects[35]. Dronedarone, a new derivative of amiodarone, lacks the iodine component that is largely responsible for the multiple organ toxicities. Recent randomized trials[36,37] showed that dronedarone was significantly more effective than placebo in maintaining sinus rhythm and in reducing the ventricular rate during recurrence of arrhythmia. In the ATHENA trial which randomized 4628 moderate- to high-risk AF patients, dronedarone resulted in a significant reduction (hazard ratio 0.76) in the primary endpoint of cardiovascular hospitalizations or death. However, the efficacy of dronedarone to suppress AF seems not as strong as that of amiodarone. Moreover, the potential adverse effects of dronedarone in patients with symptomatic heart failure and severe left ventricular systolic dysfunction remains an unresolved concern[38].
Discontinuation rates for AAD are consistently high in most trials. The careful use of these medications as demonstrated in AFFIRM can minimize this risk but can not eliminate it entirely[39]. A careful history taking and physical examination are mandatory in order to evaluate any potentially negative effect that the therapy for the arrhythmia may have on the underlying heart disease. However, in some patients the efficacy is lower than desired, and the prediction of anti-arrhythmic vs arrhythmogenic effects of AAD in a particular case is nearly impossible.
Rapidly developing experimental work has provided new insights into AF pathophysiology that will lead to new mechanism-based therapies. Oxidative stress and inflammation may be involved in the genesis of AF. Agents that modulate non-ionic current targets (termed ‘upstream’ therapies) targeting inflammation, oxidative injury, atrial myocyte metabolism, extracellular matrix remodeling, and fibrosis, may help modify the substrate for AF maintenance and have theoretical advantages as novel therapeutic strategies[40]. Angiotensin II type 1 receptor antagonists, immunosuppressive agents, statins and omega-3 polyunsaturated fatty acids have shown potential anti-arrhythmic effects related to the treatment of underlying heart disease in some but not in all studies[41-43]. These agents could be explored to prevent or delay atrial remodeling in AF patients, even in the absence of routine indications for such therapy, but the potential value of these novel therapeutic options is still under active investigations.
For many years, a pharmacological approach was the only therapeutic modality available for managing AF. Because anti-arrhythmic therapy has several limitations, including unacceptable rates of AF recurrence and other proarrhythmic sequelae, non-pharmacological approaches have become increasingly important therapeutic alternatives. Recent observations on the mechanisms of AF have resulted in the development of different non-pharmacological treatment to eliminate the triggers and to modify the electrophysiological substrate for the prevention and treatment of the disorder.
Role of cardiac rhythm management devices in AF patients: Pacemakers play an important role in the non-pharmacological management of AF. Atrial or dual-chamber pacing has been proven to prevent or delay the progression to permanent AF in patients with sinus node dysfunction as compared with ventricular pacing[44,45]. However, its utility as a treatment for paroxysmal AF in patients without conventional indications for pacing has not been proved.
There may be additional benefits associated with the use of particular sites of pacing, specific pacing algorithms designed to target potential triggers of AF, and pace-termination of atrial tachycardia. Anti-tachycardia pacing algorithms incorporated in implantable cardioverter-defibrillators and pacemakers are currently under investigation and may offer a valuable alternative to anti-arrhythmic drug therapy in elderly patients with left ventricular dysfunction at high risk of proarrhythmia or worsening heart failure.
Low energy internal defibrillation which was assumed to be safe, has prompted the development of implantable devices for terminating AF. These devices can be patient-activated or programmed to deliver automatically therapies include pacing and/or shocks, once atrial tachyarrhythmias are detected. Studies have shown that despite shock discomfort, quality of life was improved in patients with atrial defibrillators and the need for repeated hospitalizations was reduced. But due to the bad feedback from physicians regarding the shock discomfort, industry did not further develop such systems. Moreover, the cost of these devices remains a concern for the treatment of a non-lethal arrhythmia.
Advantages and limitations of atrial defibrillators and approaches to reduce shock related discomfort may be an important concern in some patients and would need further reviews.
Newer implantable pacemakers not only play a role in the management of AF for the available algorithms and therapies, but also offer an important diagnostic tool. The data storage capabilities permit detection of multiple episodes ofAF, including asymptomatic ones. The evaluation of the AF Burden may be useful to assess the thromboembolic risk profile of the patients and to optimize the antiarrhythmic drug therapy.
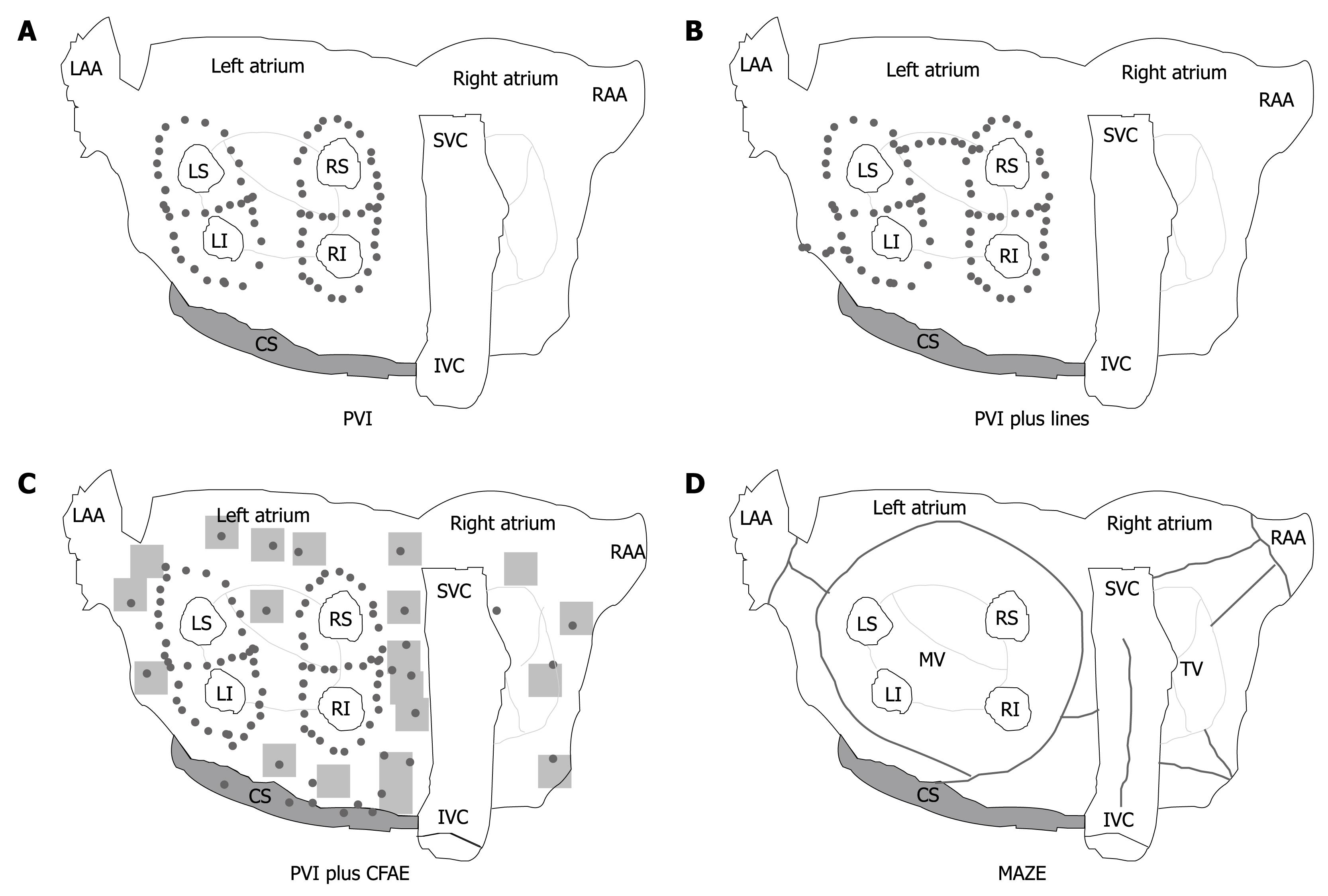
Surgical approach: With the surgical technique introduced by Cox and colleagues[46] to create conduction barriers at the critical area in order to reduce the critical mass within both atria, the possibility of a surgical cure of AF was raised. There are different surgical ablative techniques that can effectively modify the atrial substrate[47-49]; by making a series of atrial incisions and cryolesions, this procedure results in the interruption of the multiple reentry circuits necessary for the propagation of AF (Figure 1D).
These procedures require thoracotomy and cardiopulmonary bypass, however, and they are associated with morbidity as well as the risk of serious complications. Most surgical procedures are performed in conjunction with other cardiac operations (concomitant MAZE) particularly mitral-valve surgery. A success rate of around 95% over 15 years of follow-up was reported in patients undergoing mitral valve surgery[50,51]. Other studies gave a success rate of around 70%. The surgical procedures using alternate energy sources, thoracoscopic and catheter-based epicardial techniques may become more acceptable alternatives for a wider population of AF patients.
Catheter ablation: Over the past decade, catheter-based AF ablation (CA) has been proposed as a definitive cure in a broad spectrum of patients, from patients with paroxysmal AF to those with long-lasting persistent AF. With continuing advances in this field, more patients will be offered this treatment option. A number of different ablation strategies have been used, including pulmonary vein isolation, targeting of fractionated electrograms, autonomic ganglionated plexi ablation, compartmentalizing the atria with linear lesions and various combinations and modifications of these lesion sets (Figure 1A-C). The optimal ablation strategy for both paroxysmal and long-lasting persistent AF is unknown.
Randomized, controlled trials (Table 2) comparing radiofrequency energy (RF) ablation with anti-arrhythmic medications in the treatment of AF have been published[52-57]. Most studies included patients with paroxysmal or persistent AF who had failed at least one or two anti-arrhythmic medications or who were intolerant of anti-arrhythmic medications. These studies demonstrated the superiority of catheter ablation over anti-arrhythmic drugs in AF patients with regard to maintenance of sinus rhythm and improvement of symptoms, exercise capacity, and quality of life. Thus, the primary selection criterion for catheter ablation should be the presence of symptomatic AF refractory or intolerant to at least one class 1 or 3 antiarrhythmic medication. The current guidelines recommend catheter ablation in this setting. However, one trial assessed the efficacy of ablation in patients with permanent AF[58], whereas another study randomized patients as first-line therapy[52] suggesting that catheter ablation can be considered early in the management of the patients. A recent systematic review showed that in patients with paroxysmal and persistent AF and structurally normal hearts, ablation therapy results in a 65% reduction in the RR of AF recurrence compared with standard antiarrhythmic therapy[59].
| Study | n | Follow-up(mo) | Patients free of AF | No. of ablation procedures | Major complications(ablation arm) (%) | Type of AF | ||
| Ablation strategy (%) | AAD strategy (%) | P | ||||||
| Wazni et al[48] (2005) | 70 | 12 | 88 | 37 | < 0.001 | 1 | 6.3 | Paroxysmal persistent |
| Oral et al[50] (2006) | 146 | 12 | 74 | 58 | 0.05 | 1.4 | 0 | Permanent |
| Stabile et al[49] (2006) | 137 | 12 | 56 | 9 | < 0.001 | 1 | 4.4 | Paroxysmal persistent |
| Pappone et al[51] (2006) | 198 | 12 | 86 | 22 | < 0.001 | 1 | 2.0 | Paroxysmal |
| Jaïs et al[52] (2008) | 112 | 12 | 88 | 24 | < 0.001 | 1.8 | 1.9 | Paroxysmal |
| Forleo et al[53] (2009) | 70 | 12 | 80 | 57 | 0.001 | 1 | 2.9 | Paroxysmal persistent |
Of note, a recent study that compared the cost of ablation as first-line treatment of symptomatic AF vs anti-arrhythmic drug therapy, demonstrated that CA was cost neutral 2 years after the initial procedure. Accumulating evidences from clinical studies have documented long-term improvement in quality of life, functional capacity, and left ventricular function in patients with impaired systolic function who undergo CA for AF[60-63]. A major advantage of CA is that patients with low ejection fraction are at an increased risk of AAD adverse effects,while its disadvantages include a higher procedural risk in these patients. Nevertheless, many aspects of the therapy are still controversial, from ablation techniques to procedural endpoints, patient management, definition of success and long-term results. The definition of a successful intervention for the management of AF remains a challenge. It is uncertain whether elimination of AF or transformation into an asymptomatic form of AF unrecognized by the patient or the physician represents the cure of the disease. The distinction has great significance from the point of view of preventing thromboembolic episodes in patients with risk factors for stroke associated with AF.
Controversies exist with regard to the procedural safety of AF ablation. Reports from highly sophisticated centers claim very low complication rates. However, recent surveys showed that this procedure is associated with approximately 5% rate of major complications[64,65]. Pulmonary vein stenosis, pericardial effusion, embolic cerebral and peripheral vascular complications constitute the most frequent complications. Continuing advances in this field might reduce the rate of major complications, and the increasing number of pulmonary veins (PV) ablation procedures has allowed electrophysiologists to become aware of the peculiarities and potential dangers of these procedures. Useful tools are required in order to develop the ablation strategy and to avoid more complex procedures with longer durations and higher periprocedural risks. Phased-array intracardiac echocardiography has been shown to be helpful in minimizing complications associated with ablation procedures, allowing real-time monitoring of both PV ostium and RF delivery[66].
Techniques and endpoints for AF ablation: It is likely that in humans AF is caused by different mechanisms. Recent observations have focused attention on the PV as a source of ectopic activity determining AF[67]. However, a predisposing atrial substrate of sufficient mass capable of maintaining re-entrant circuits is necessary and other anatomical structures are critical in this regard.
Since its original description in 1998, the technique of CA of AF has undergone several modifications[68]. Isolating or encircling all accessible PV is identified as the cornerstone of any ablation approach, and most of the trials did use PV isolation as an endpoint for radiofrequency ablation. This approach, called “empirical PV isolation”, targets all of the PVs without regard to the initiation of ectopic beats (Figure 1A). However, PVI as a stand-alone strategy is insufficient to eliminate recurrent AF in most patients with persistent/permanent AF. In these patients, there is considerable evidence that ablation of residual triggers or drivers of AF outside the PV is required[69].
Various adjunctive atrial modifications such as wider ablation around the veins, linear lines, or ablation of complex fractionated atrial electrograms (CFAE) have been proposed. Substrate modification can be achieved by additional linear lesions (Figure 1B) in the LA, but the optimal lesion set to be deployed has yet to be elucidated. The most common sites of linear lesions are the LA roof between the superior aspects of the left and right upper PV isolation lesions, and the mitral isthmus between the mitral valve and the left inferior PV.
Elimination of CFAE is another possible approach (Figure 1C)[70]. However, in a large proportion of patients, Oral et al[71] showed that ablation of CFAE is not sufficient to eliminate the driving mechanisms of AF, suggesting the routine isolation of all PVs.
It is uncertain whether all patients need further substrate modifications for AF treatment. Studies have shown that the combined approach of PVI and CFAE ablation in persistent/permanent AF yielded mixed results[69,72]. Therefore, the role of this strategy should be proven in larger series. Tools allowing the differentiation between active and passive CFAE sites are crucial for the understanding and treatment of persistent AF.
The ablation procedure is often guided by 3D electroanatomical mapping systems. Currently, the Ensite NavX (St Jude Medical, Minnetonka, MN, USA) and Carto (Biosense Webster Inc, Diamond Bar, CA, USA) systems are increasingly used during CA of AF because they facilitate the difficult interventional ablation procedure while providing accurate visualization of the atrial anatomy and provide a guide for atrial substate modifications. The 3-dimensional mapping systems also shorten the fluoroscopic time and assist in identifying the critical substrate during the ablation, preventing gap formation and guiding post-ablation atrial tachycardia or flutter ablation. Additionally, image integration improves the safety and long-term success rate.
Most ablation procedures are being performed with close- or open-irrigation RF catheters[73], which are capable only for focal ablations. Achieving PVI with this technique,however, remains lengthy, technically challenging and requires a high degree of skill. Balloon and coil platforms, using different energy sources, are being tested as potential alternatives for focal RF catheters, with the hope of providing a safer, faster and more effective technology[74-76]. Cryo-balloon has emerged as a promising tool allowing PV isolation in a safe and effective manner. Results from early pre-clinical and clinical studies showed that the use of cryoablation is associated with a very low rate of complications including thrombogenicity, PV stenosis and esophageal injury[77]. It has been suggested that cryothermal balloon ablation for paroxysmal AF results in a clinical success rate comparable to studies using radiofrequency ablation. However, the clinical success of cryoballoon PVI in paroxysmal AF has not been achieved in patients with persistent AF[78], likely because of the need for additional atrial substrate modification in this subgroup. Extensive substrate modifications using focal cryoablation catheters is technically feasible but plagued with the need for prolonged application time and the inability to create “dragging” ablation lesions due to cryocatheter adherence to tissues, significantly limited their use.
Despite these differences in technique, there remain remarkable consistencies in the AF outcome data between centers, with overall single-procedure efficacy of > 70% in achieving long-term arrhythmia control for patients with paroxysmal AF but significantly lower success rates in achieving a similar outcome for patients with persistent or permanent AF.
Recent clinical trials that compared strategies of rhythm control with rate control in patients with AF lacked information about the best appropriate endpoints for determining “successful” rate or rhythm control in individual patients. Various endpoints have been used for judging the success of rhythm control strategies, including time to first recurrence of AF, any AF recurrence, AF burden, and a reduction in symptoms.
Time to first recurrence of AF has been frequently utilized, however, it has a poor value in the clinical care of patients. Of note, suppression of AF in a patient at high risk of stroke does not obviate the need for concomitant Warfarin treatment. A reasonable endpoint for rhythm control is a marked reduction in the frequency and duration of symptomatic AF episodes. For this reason we strongly believe that asymptomatic patients should not be treated with a rhythm control strategy. In addition, it is not essential to eliminate all episodes of AF when evaluating the success of the therapy, most patients can live comfortably with occasional episodes of AF, which is an entirely acceptable endpoint. Unfortunately, a few patients are bothered by even infrequent brief AF episodes. For such patients, it is a difficult task to find a strategy that eliminates nearly all AF recurrences.
Continuous monitoring of patients is another approach that can be used to measure the success of anti-arrhythmic therapy. Although continuous loop recording with regular transtelephonic data transmission throughout a uniform period of follow-up would be the “gold standard” for assessment of cardiac rhythm, it is impractical, inconvenient, and expensive.
AF remains the most common and most challenging arrhythmia. Current treatment guidelines state that rhythm and rate control strategies should consider equivalent therapeutic approaches, but recognize that no “one size fits all”. Physicians have to determine strategies most appropriate for particular clinical conditions. More attention should be paid to the severity of symptoms (symptomatic burden) but less to the frequency and duration of AF, and treatment should be delivered accordingly. Currently, the limited efficacy and proarrhythmic risks of anti-arrhythmic drugs highlights the importance for safer and more effective treatment options for AF. Several new nonpharmacologic treatment modalities have been developed; however, they are not applicable to all patients with AF; therefore, drug therapy will remain an important option.
In the minority of patients in whom AF cannot be adequately managed by pharmacological therapy, the most appropriate type of nonpharmacological therapy must be selected on an individualized basis.
Ablative techniques that offer the potential of a complete cure from AF are gaining popularity in the treatment of highly symptomatic patients with AF who are refractory to drug therapy. Nevertheless, many aspects of the therapy are still controversial. Even though the results of published studies favor ablation therapy, large, well-designed, multi-center clinical trials are needed to confirm the efficacy and safety of this approach.
Peer reviewers: Jonathan S Steinberg, MD, Chief, Division of Cardiology, Al-Sabah Endowed Director, Arrhythmia Institute, St. Luke’s and Roosevelt Hospitals, Professor of Medicine, Columbia University College of Physicians & Surgeons, 1111 Amsterdam Ave, New York, NY 10025, United States; Mehmet Ozaydin, Associate Professor, Sevket Demirel Kalp Merkezi, 32100, Isparta, Turkey; Klaus Kettering, MD, Department of Cardiology, University of Mainz, Langenbeckstrasse 1, D-55131 Mainz, Germany
S- Editor Cheng JX L- Editor Ma JY E- Editor Zheng XM
| 1. | Kannel WB, Abbott RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation: the Framingham study. N Engl J Med. 1982;306:1018-1022. |
| 2. | Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370-2375. |
| 3. | Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med. 1995;155:469-473. |
| 4. | Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113:359-364. |
| 5. | Kannel WB, Benjamin EJ. Status of the epidemiology of atrial fibrillation. Med Clin North Am. 2008;92:17-40, ix. |
| 6. | Fuster V, Rydén LE, Asinger RW, Cannom DS, Crijns HJ, Frye RL, Halperin JL, Kay GN, Klein WW, Lévy S. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to develop guidelines for the management of patients with atrial fibrillation) developed in collaboration with the North American Society of Pacing and Electrophysiology. Eur Heart J. 2001;22:1852-1923. |
| 7. | Calkins H, Brugada J, Packer DL, Cappato R, Chen SA, Crijns HJ, Damiano RJ Jr, Davies DW, Haines DE, Haissaguerre M. HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2007;4:816-861. |
| 8. | Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med. 1999;131:492-501. |
| 10. | Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864-2870. |
| 11. | Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151. |
| 12. | Connolly SJ, Pogue J, Hart RG, Hohnloser SH, Pfeffer M, Chrolavicius S, Yusuf S. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med. 2009;360:2066-2078. |
| 13. | Halperin JL, Gomberg-Maitland M. Obliteration of the left atrial appendage for prevention of thromboembolism. J Am Coll Cardiol. 2003;42:1259-1261. |
| 14. | Ostermayer SH, Reisman M, Kramer PH, Matthews RV, Gray WA, Block PC, Omran H, Bartorelli AL, Della Bella P, Di Mario C. Percutaneous left atrial appendage transcatheter occlusion (PLAATO system) to prevent stroke in high-risk patients with non-rheumatic atrial fibrillation: results from the international multi-center feasibility trials. J Am Coll Cardiol. 2005;46:9-14. |
| 15. | Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, Mullin CM, Sick P. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374:534-542. |
| 16. | Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825-1833. |
| 17. | Carlsson J, Miketic S, Windeler J, Cuneo A, Haun S, Micus S, Walter S, Tebbe U. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol. 2003;41:1690-1696. |
| 18. | Hagens VE, Ranchor AV, Van Sonderen E, Bosker HA, Kamp O, Tijssen JG, Kingma JH, Crijns HJ, Van Gelder IC. Effect of rate or rhythm control on quality of life in persistent atrial fibrillation. Results from the Rate Control Versus Electrical Cardioversion (RACE) Study. J Am Coll Cardiol. 2004;43:241-247. |
| 19. | Roy D, Talajic M, Nattel S, Wyse DG, Dorian P, Lee KL, Bourassa MG, Arnold JM, Buxton AE, Camm AJ. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667-2677. |
| 20. | Corley SD, Epstein AE, DiMarco JP, Domanski MJ, Geller N, Greene HL, Josephson RA, Kellen JC, Klein RC, Krahn AD. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109:1509-1513. |
| 21. | Weerasooriya R, Davis M, Powell A, Szili-Torok T, Shah C, Whalley D, Kanagaratnam L, Heddle W, Leitch J, Perks A. The Australian Intervention Randomized Control of Rate in Atrial Fibrillation Trial (AIRCRAFT). J Am Coll Cardiol. 2003;41:1697-1702. |
| 22. | Farshi R, Kistner D, Sarma JS, Longmate JA, Singh BN. Ventricular rate control in chronic atrial fibrillation during daily activity and programmed exercise: a crossover open-label study of five drug regimens. J Am Coll Cardiol. 1999;33:304-310. |
| 23. | Tamariz LJ, Bass EB. Pharmacological rate control of atrial fibrillation. Cardiol Clin. 2004;22:35-45. |
| 24. | Olshansky B, Rosenfeld LE, Warner AL, Solomon AJ, O'Neill G, Sharma A, Platia E, Feld GK, Akiyama T, Brodsky MA. The Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study: approaches to control rate in atrial fibrillation. J Am Coll Cardiol. 2004;43:1201-1208. |
| 25. | Santini L, Forleo GB, Topa A, Romeo F, Santini M. Electrical cardioversion of atrial fibrillation: different methods for a safe and effective technique. Expert Rev Cardiovasc Ther. 2005;3:601-610. |
| 26. | Mittal S, Ayati S, Stein KM, Schwartzman D, Cavlovich D, Tchou PJ, Markowitz SM, Slotwiner DJ, Scheiner MA, Lerman BB. Transthoracic cardioversion of atrial fibrillation: comparison of rectilinear biphasic versus damped sine wave monophasic shocks. Circulation. 2000;101:1282-1287. |
| 27. | Page RL, Kerber RE, Russell JK, Trouton T, Waktare J, Gallik D, Olgin JE, Ricard P, Dalzell GW, Reddy R. Biphasic versus monophasic shock waveform for conversion of atrial fibrillation: the results of an international randomized, double-blind multicenter trial. J Am Coll Cardiol. 2002;39:1956-1963. |
| 28. | Santini L, Forleo GB, Santini M, Romeo F. Oesophageal electrical cardioversion of atrial fibrillation. Minerva Cardioangiol. 2004;52:73-80. |
| 29. | Santini L, Magris B, Topa A, Gallagher MM, Forleo GB, Papavasileiou LP, Borzi M, Romeo F, Santini M. Outpatient oesophageal-precordial electrical cardioversion of atrial fibrillation: an effective and safe technique to restore sinus rhythm. J Cardiovasc Med (Hagerstown). 2007;8:488-493. |
| 30. | Santini L, Gallagher MM, Papavasileiou LP, Romano V, Topa A, Di Battista L, Aracri M, Romeo F. Transthoracic versus transesophageal cardioversion of atrial fibrillation under light sedation: a prospective randomized trial. Pacing Clin Electrophysiol. 2007;30:1469-1475. |
| 31. | Santini M, Pandozi C, Altamura G, Gentilucci G, Villani M, Scianaro MC, Castro A, Ammirati F, Magris B. Single shock endocavitary low energy intracardiac cardioversion of chronic atrial fibrillation. J Interv Card Electrophysiol. 1999;3:45-51. |
| 32. | Roy D, Talajic M, Dorian P, Connolly S, Eisenberg MJ, Green M, Kus T, Lambert J, Dubuc M, Gagné P. Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of Atrial Fibrillation Investigators. N Engl J Med. 2000;342:913-920. |
| 33. | Singh BN, Singh SN, Reda DJ, Tang XC, Lopez B, Harris CL, Fletcher RD, Sharma SC, Atwood JE, Jacobson AK. Amiodarone versus sotalol for atrial fibrillation. N Engl J Med. 2005;352:1861-1872. |
| 34. | Zimetbaum P. Amiodarone for atrial fibrillation. N Engl J Med. 2007;356:935-941. |
| 35. | Chun SH, Sager PT, Stevenson WG, Nademanee K, Middlekauff HR, Singh BN. Long-term efficacy of amiodarone for the maintenance of normal sinus rhythm in patients with refractory atrial fibrillation or flutter. Am J Cardiol. 1995;76:47-50. |
| 36. | Singh BN, Connolly SJ, Crijns HJ, Roy D, Kowey PR, Capucci A, Radzik D, Aliot EM, Hohnloser SH. Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med. 2007;357:987-999. |
| 37. | Hohnloser SH, Crijns HJ, van Eickels M, Gaudin C, Page RL, Torp-Pedersen C, Connolly SJ. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med. 2009;360:668-678. |
| 38. | Køber L, Torp-Pedersen C, McMurray JJ, Gøtzsche O, Lévy S, Crijns H, Amlie J, Carlsen J. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med. 2008;358:2678-2687. |
| 39. | AFFIRM First Antiarrhythmic Drug Substudy Investigators. Maintenance of sinus rhythm in patients with atrial fibrillation: an AFFIRM substudy of the first antiarrhythmic drug. J Am Coll Cardiol. 2003;42:20-29. |
| 40. | Casaclang-Verzosa G, Gersh BJ, Tsang TS. Structural and functional remodeling of the left atrium: clinical and therapeutic implications for atrial fibrillation. J Am Coll Cardiol. 2008;51:1-11. |
| 41. | Goette A, Bukowska A, Lendeckel U. Non-ion channel blockers as anti-arrhythmic drugs (reversal of structural remodeling). Curr Opin Pharmacol. 2007;7:219-224. |
| 42. | Savelieva I, Camm J. Statins and polyunsaturated fatty acids for treatment of atrial fibrillation. Nat Clin Pract Cardiovasc Med. 2008;5:30-41. |
| 43. | Salehian O, Healey J, Stambler B, Alnemer K, Almerri K, Grover J, Bata I, Mann J, Matthew J, Pogue J. Impact of ramipril on the incidence of atrial fibrillation: results of the Heart Outcomes Prevention Evaluation study. Am Heart J. 2007;154:448-453. |
| 44. | Lamas GA, Lee KL, Sweeney MO, Silverman R, Leon A, Yee R, Marinchak RA, Flaker G, Schron E, Orav EJ. Ventricular pacing or dual-chamber pacing for sinus-node dysfunction. N Engl J Med. 2002;346:1854-1862. |
| 45. | Andersen HR, Nielsen JC, Thomsen PE, Thuesen L, Mortensen PT, Vesterlund T, Pedersen AK. Long-term follow-up of patients from a randomised trial of atrial versus ventricular pacing for sick-sinus syndrome. Lancet. 1997;350:1210-1216. |
| 46. | Cox JL, Schuessler RB, D'Agostino HJ Jr, Stone CM, Chang BC, Cain ME, Corr PB, Boineau JP. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg. 1991;101:569-583. |
| 47. | Kim KB, Huh JH, Kang CH, Ahn H, Sohn DW. Modifications of the Cox-Maze III procedure. Ann Thorac Surg. 2001;71:816-822. |
| 48. | Cox JL, Boineau JP, Schuessler RB, Jaquiss RD, Lappas DG. Modification of the maze procedure for atrial flutter and atrial fibrillation. I. Rationale and surgical results. J Thorac Cardiovasc Surg. 1995;110:473-484. |
| 49. | Kosakai Y, Kawaguchi AT, Isobe F, Sasako Y, Nakano K, Eishi K, Tanaka N, Kito Y, Kawashima Y. Cox maze procedure for chronic atrial fibrillation associated with mitral valve disease. J Thorac Cardiovasc Surg. 1994;108:1049-1054; discussion 1054-1055. |
| 50. | Damiano RJ Jr, Gaynor SL, Bailey M, Prasad S, Cox JL, Boineau JP, Schuessler RP. The long-term outcome of patients with coronary disease and atrial fibrillation undergoing the Cox maze procedure. J Thorac Cardiovasc Surg. 2003;126:2016-2021. |
| 51. | Gillinov AM, McCarthy PM. Advances in the surgical treatment of atrial fibrillation. Cardiol Clin. 2004;22:147-157. |
| 52. | Wazni OM, Marrouche NF, Martin DO, Verma A, Bhargava M, Saliba W, Bash D, Schweikert R, Brachmann J, Gunther J. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA. 2005;293:2634-2640. |
| 53. | Stabile G, Bertaglia E, Senatore G, De Simone A, Zoppo F, Donnici G, Turco P, Pascotto P, Fazzari M, Vitale DF. Catheter ablation treatment in patients with drug-refractory atrial fibrillation: a prospective, multi-centre, randomized, controlled study (Catheter Ablation For The Cure Of Atrial Fibrillation Study). Eur Heart J. 2006;27:216-221. |
| 54. | Oral H, Pappone C, Chugh A, Good E, Bogun F, Pelosi F Jr, Bates ER, Lehmann MH, Vicedomini G, Augello G. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Engl J Med. 2006;354:934-941. |
| 55. | Pappone C, Augello G, Sala S, Gugliotta F, Vicedomini G, Gulletta S, Paglino G, Mazzone P, Sora N, Greiss I. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study. J Am Coll Cardiol. 2006;48:2340-2347. |
| 56. | Jaïs P, Cauchemez B, Macle L, Daoud E, Khairy P, Subbiah R, Hocini M, Extramiana F, Sacher F, Bordachar P. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation. 2008;118:2498-2505. |
| 57. | Forleo GB, Mantica M, De Luca L, Leo R, Santini L, Panigada S, De Sanctis V, Pappalardo A, Laurenzi F, Avella A. Catheter ablation of atrial fibrillation in patients with diabetes mellitus type 2: results from a randomized study comparing pulmonary vein isolation versus antiarrhythmic drug therapy. J Cardiovasc Electrophysiol. 2009;20:22-28. |
| 58. | Oral H, Pappone C, Chugh A, Good E, Bogun F, Pelosi F Jr, Bates ER, Lehmann MH, Vicedomini G, Augello G. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Engl J Med. 2006;354:934-941. |
| 59. | Nair GM, Nery PB, Diwakaramenon S, Healey JS, Connolly SJ, Morillo CA. A systematic review of randomized trials comparing radiofrequency ablation with antiarrhythmic medications in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2009;20:138-144. |
| 60. | Chen MS, Marrouche NF, Khaykin Y, Gillinov AM, Wazni O, Martin DO, Rossillo A, Verma A, Cummings J, Erciyes D. Pulmonary vein isolation for the treatment of atrial fibrillation in patients with impaired systolic function. J Am Coll Cardiol. 2004;43:1004-1009. |
| 61. | Hsu LF, Jaïs P, Sanders P, Garrigue S, Hocini M, Sacher F, Takahashi Y, Rotter M, Pasquié JL, Scavée C. Catheter ablation for atrial fibrillation in congestive heart failure. N Engl J Med. 2004;351:2373-2383. |
| 62. | Tondo C, Mantica M, Russo G, Avella A, De Luca L, Pappalardo A, Fagundes RL, Picchio E, Laurenzi F, Piazza V. Pulmonary vein vestibule ablation for the control of atrial fibrillation in patients with impaired left ventricular function. Pacing Clin Electrophysiol. 2006;29:962-970. |
| 63. | Khan MN, Jaïs P, Cummings J, Di Biase L, Sanders P, Martin DO, Kautzner J, Hao S, Themistoclakis S, Fanelli R. Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N Engl J Med. 2008;359:1778-1785. |
| 64. | Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Packer D, Skanes A. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. 2005;111:1100-1105. |
| 65. | Bertaglia E, Zoppo F, Tondo C, Colella A, Mantovan R, Senatore G, Bottoni N, Carreras G, Corò L, Turco P. Early complications of pulmonary vein catheter ablation for atrial fibrillation: a multicenter prospective registry on procedural safety. Heart Rhythm. 2007;4:1265-1271. |
| 66. | Marrouche NF, Martin DO, Wazni O, Gillinov AM, Klein A, Bhargava M, Saad E, Bash D, Yamada H, Jaber W. Phased-array intracardiac echocardiography monitoring during pulmonary vein isolation in patients with atrial fibrillation: impact on outcome and complications. Circulation. 2003;107:2710-2716. |
| 67. | Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659-666. |
| 68. | Natale A, Raviele A, Arentz T, Calkins H, Chen SA, Haïssaguerre M, Hindricks G, Ho Y, Kuck KH, Marchlinski F. Venice Chart international consensus document on atrial fibrillation ablation. J Cardiovasc Electrophysiol. 2007;18:560-580. |
| 69. | Elayi CS, Verma A, Di Biase L, Ching CK, Patel D, Barrett C, Martin D, Rong B, Fahmy TS, Khaykin Y. Ablation for longstanding permanent atrial fibrillation: results from a randomized study comparing three different strategies. Heart Rhythm. 2008;5:1658-1564. |
| 70. | Nademanee K, McKenzie J, Kosar E, Schwab M, Sunsaneewitayakul B, Vasavakul T, Khunnawat C, Ngarmukos T. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004;43:2044-2053. |
| 71. | Oral H, Chugh A, Good E, Wimmer A, Dey S, Gadeela N, Sankaran S, Crawford T, Sarrazin JF, Kuhne M. Radiofrequency catheter ablation of chronic atrial fibrillation guided by complex electrograms. Circulation. 2007;115:2606-2612. |
| 72. | Oral H, Chugh A, Yoshida K, Sarrazin JF, Kuhne M, Crawford T, Chalfoun N, Wells D, Boonyapisit W, Veerareddy S. A randomized assessment of the incremental role of ablation of complex fractionated atrial electrograms after antral pulmonary vein isolation for long-lasting persistent atrial fibrillation. J Am Coll Cardiol. 2009;53:782-789. |
| 73. | Kanj MH, Wazni O, Fahmy T, Thal S, Patel D, Elay C, Di Biase L, Arruda M, Saliba W, Schweikert RA. Pulmonary vein antral isolation using an open irrigation ablation catheter for the treatment of atrial fibrillation: a randomized pilot study. J Am Coll Cardiol. 2007;49:1634-1641. |
| 74. | Natale A, Pisano E, Shewchik J, Bash D, Fanelli R, Potenza D, Santarelli P, Schweikert R, White R, Saliba W. First human experience with pulmonary vein isolation using a through-the-balloon circumferential ultrasound ablation system for recurrent atrial fibrillation. Circulation. 2000;102:1879-1882. |
| 75. | Nakagawa H, Antz M, Wong T, Schmidt B, Ernst S, Ouyang F, Vogtmann T, Wu R, Yokoyama K, Lockwood D. Initial experience using a forward directed, high-intensity focused ultrasound balloon catheter for pulmonary vein antrum isolation in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18:136-144. |
| 76. | Mansour M, Forleo GB, Pappalardo A, Heist EK, Avella A, Laurenzi F, De Girolamo P, Bencardino G, Dello Russo A, Mantica M. Initial experience with the Mesh catheter for pulmonary vein isolation in patients with paroxysmal atrial fibrillation. Heart Rhythm. 2008;5:1510-1516. |
| 77. | Sarabanda AV, Bunch TJ, Johnson SB, Mahapatra S, Milton MA, Leite LR, Bruce GK, Packer DL. Efficacy and safety of circumferential pulmonary vein isolation using a novel cryothermal balloon ablation system. J Am Coll Cardiol. 2005;46:1902-1912. |
| 78. | Neumann T, Vogt J, Schumacher B, Dorszewski A, Kuniss M, Neuser H, Kurzidim K, Berkowitsch A, Koller M, Heintze J. Circumferential pulmonary vein isolation with the cryoballoon technique results from a prospective 3-center study. J Am Coll Cardiol. 2008;52:273-278. |