Copyright
©The Author(s) 2016.
World J Cardiol. Feb 26, 2016; 8(2): 163-179
Published online Feb 26, 2016. doi: 10.4330/wjc.v8.i2.163
Published online Feb 26, 2016. doi: 10.4330/wjc.v8.i2.163
Figure 1 Schematic of factors involved in cardiomyocyte differentiation in embryology, embryonic stem cell/induced-pluripotent stem cells and cardiac fibroblast reprogramming.
Factors that influence the progression through the five steps in cardiomyocyte differentiation and maturation: mesoderm differentiation, mesoderm specification, cardiac specification, cardiomyocyte differentiation and cardiomyocyte maturation in: A: Embryonic cardiomyocyte differentiation; B: Cardiomyocyte differentiation from ESC and iPSC using exogenous (growth) factors; C: In direct reprogramming of cardiac fibroblasts into cardiomyocytes. Transcription factors associated with each of the seven cell types during cardiomyocyte differentiation are presented in the boxes below. ESC: Embryonic stem cell; iPSC: Induced-pluripotent stem cells.
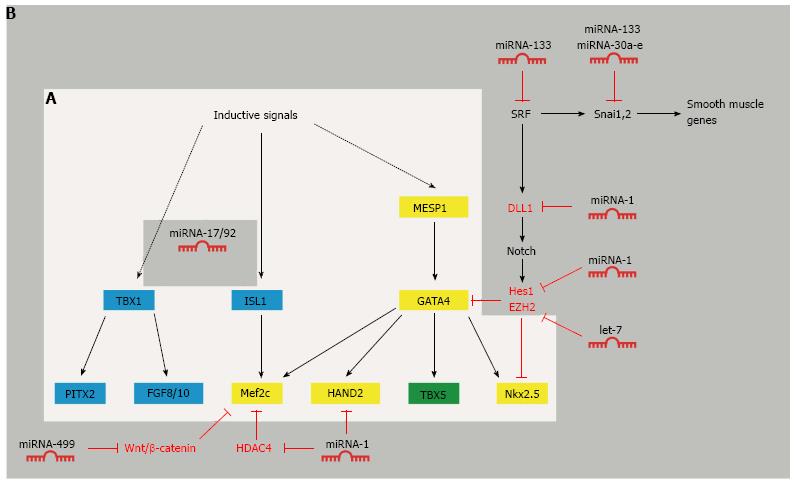
Figure 2 The complex web of transcription factors in cardiac specification and their regulation by microRNAs.
A: Crosstalk between transcription factors involved in the formation of the first and second heart field (light grey box). MESP1, GATA4, Mef2c, HAND2 and Nkx2.5 are central transcription factors in the first and second heart field (yellow). TBX5 is only expressed in the first heart field (green). ISL1 and TBX1 are expressed in the second heart field (blue); B: MicroRNA-mediated regulation of cardiac transcription factors during cardiomyocyte differentiation (dark grey box).
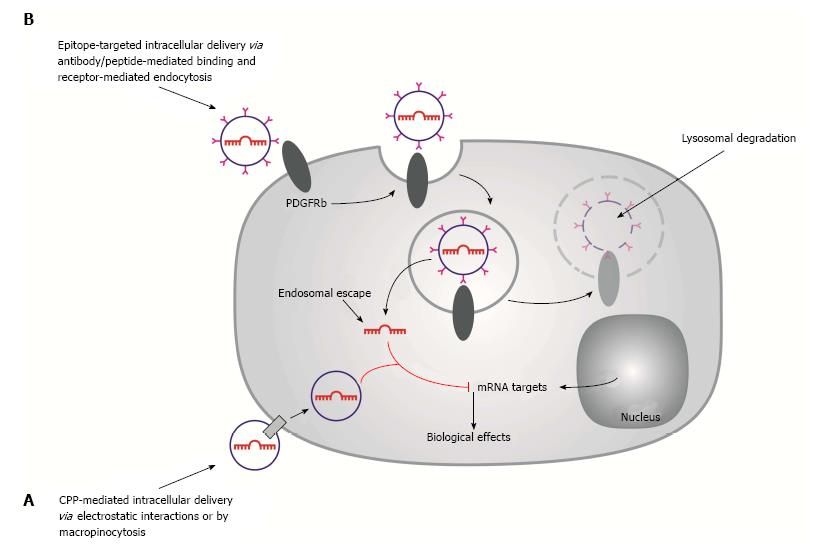
Figure 3 Schematic of passive and active targeted drug delivery systems for microRNA delivery.
A: Passive targeting by cell-penetrating peptide-coated nanoparticles are internalized by receptor-mediated endocytosis; B: Active targeting by PDGFRb-targeted liposomes. Liposomes interact with cell surface receptors (PDGFRb) and internalized via receptor-mediated endocytosis. The endocytotic vesicles fuse to form early endosomes which ultimately become part of the lysosomes, where proteins and nucleic acids are degraded by acid hydrolases. To achieve target gene silencing, microRNAs need to be released from the liposome and escape from the endosomes into the cytoplasm, where the microRNA directs the cleavage of target mRNAs.
- Citation: Kamps JA, Krenning G. Micromanaging cardiac regeneration: Targeted delivery of microRNAs for cardiac repair and regeneration. World J Cardiol 2016; 8(2): 163-179
- URL: https://www.wjgnet.com/1949-8462/full/v8/i2/163.htm
- DOI: https://dx.doi.org/10.4330/wjc.v8.i2.163