Copyright
©2014 Baishideng Publishing Group Inc.
World J Cardiol. Jul 26, 2014; 6(7): 638-652
Published online Jul 26, 2014. doi: 10.4330/wjc.v6.i7.638
Published online Jul 26, 2014. doi: 10.4330/wjc.v6.i7.638
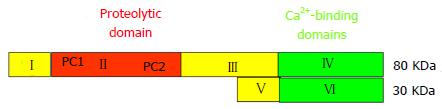
Figure 1 Domain structure of the catalytic 80-kDa and the regulatory 30-kDa subunits of the μ- und m-calpain dimers.
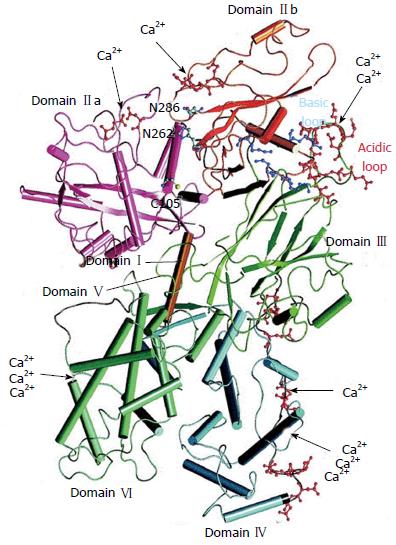
Figure 2 Crystallographic structure of human m-calpain by Suzuki et al[33].
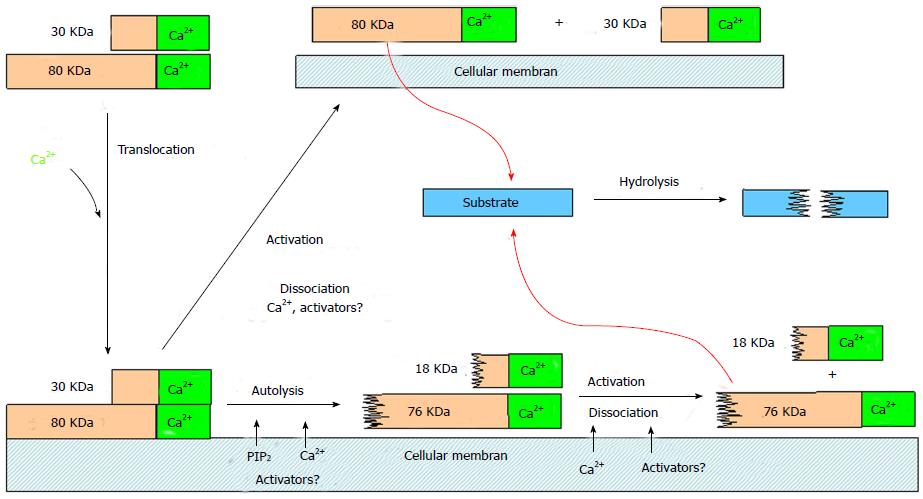
Figure 3 Mechanisms and consequences of calpain activation at biological membranes.
Modified from Suzuki et al[40].
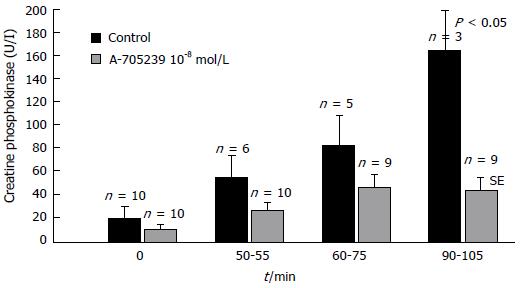
Figure 4 Release of creatine phosphokinase into the perfusion fluid of isolated rabbit hearts subjected to ischemia and reperfusion[180].
Control experiments without inhibitor are represented by black-coloured columns and inhibitor (A-705239 10-8 mol/L) treated hearts by grey-coloured columns. Data are expressed as means ± SE of n = 10 experiments each. Both groups differ significantly (P < 0.05) at the end of reperfusion.
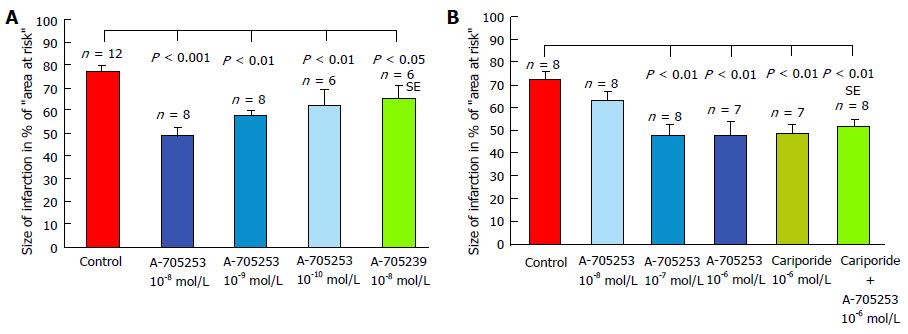
Figure 5 Development of myocardial infarction in isolated perfused rabbit hearts after occlusion of ramus interventricularis of left coronary artery for 60 min, followed by 120 min of reperfusion[209].
A: The inhibitors were added to the perfusion fluid before ischemia; B: With reperfusion. Infarct size is expressed in percentage of the area at risk (the transiently not perfused myocardium). Control experiments without inhibitor are represented by a red-coloured column and inhibitor treated hearts by blue-coloured columns. Data are presented as means ± SE. Infarct size is significantly reduced by calpain inhibition in all treated hearts compared to untreated controls.
- Citation: Neuhof C, Neuhof H. Calpain system and its involvement in myocardial ischemia and reperfusion injury. World J Cardiol 2014; 6(7): 638-652
- URL: https://www.wjgnet.com/1949-8462/full/v6/i7/638.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i7.638