Copyright
©2011 Baishideng Publishing Group Co.
World J Cardiol. Sep 26, 2011; 3(9): 281-302
Published online Sep 26, 2011. doi: 10.4330/wjc.v3.i9.281
Published online Sep 26, 2011. doi: 10.4330/wjc.v3.i9.281
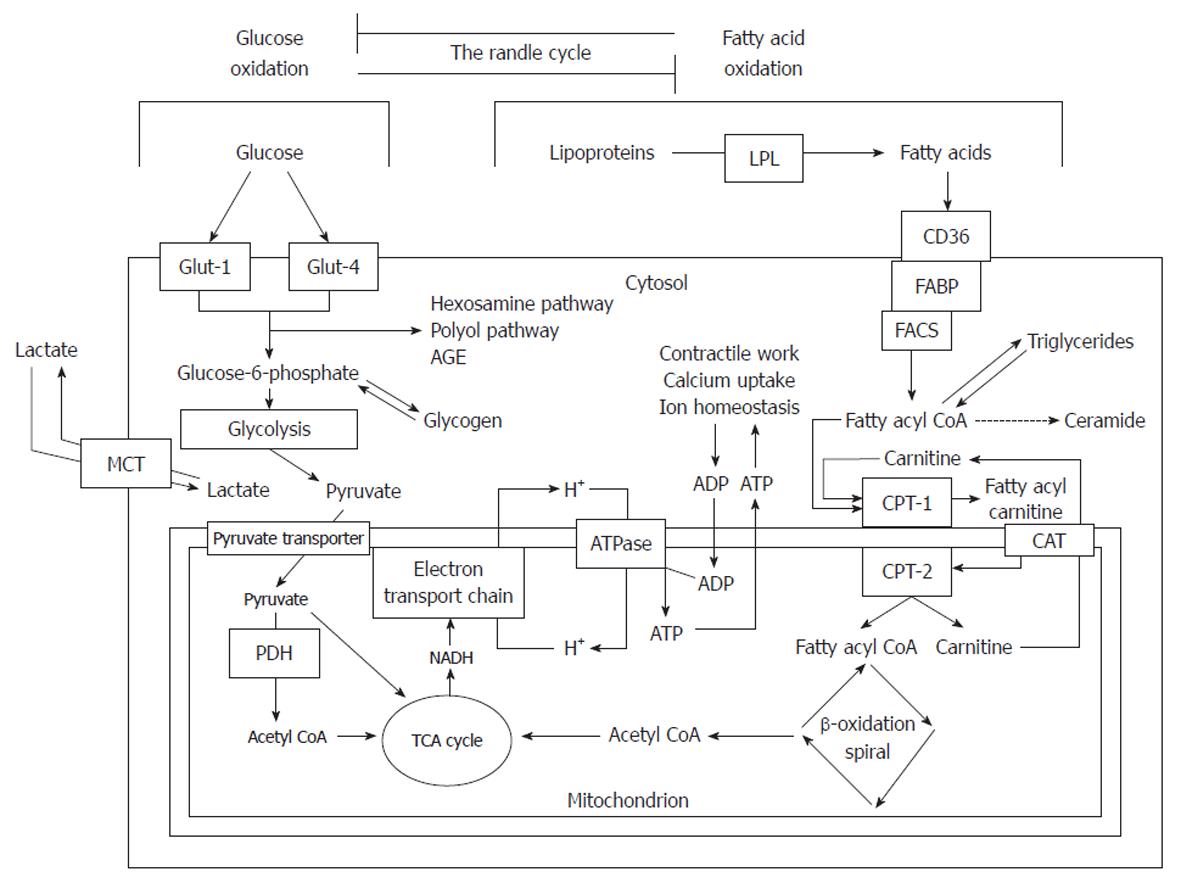
Figure 1 Summary of fatty acid and glucose metabolism.
Glucose is taken up by Glut-1 and Glut-4 transporters and is converted by glycolysis to pyruvate which enters the mitochondria to be oxidized, producing acetyl coenzyme A (CoA). Fatty acids are liberated from lipoproteins by lipoprotein lipase (LPL) and taken up by fatty acid translocase (CD36) and fatty acid binding protein (FABP). Long-chain fatty acyl-CoA synthetase (LCAS) converts the fatty acid to a CoA ester which is then taken up by the carnitine shuttle system to the mitochondria. The fatty acyl CoA undergoes β-oxidation, removing two carbons per turn of the cycle and generating acetyl CoA. Acetyl CoA, generated by either pathway, enters the tricarboxylic acid (TCA) cycle to generate reducing equivalents [reduced nicotinamide adenide dinucleotide (NADH)]. These pass electrons to the electron transport chain which creates an electrochemical proton gradient to drive adenosine triphosphate (ATP) synthesis. ATP synthesis is coupled to the systems which create the ATP demand. FACS: Fatty acyl-CoA synthase; CPT: Carnitine palmitoyltransferase; CAT: Carnitine acyl transferase; AGE: Advanced glycosylation end-product; PDH: Pyruvate dehydrogenase; MCT: Monocarboxylate transporter; PDH: Pyruvate dehydrogenase; ADP: Adenosine monophosphate. Modified from: Sharma et al[29].
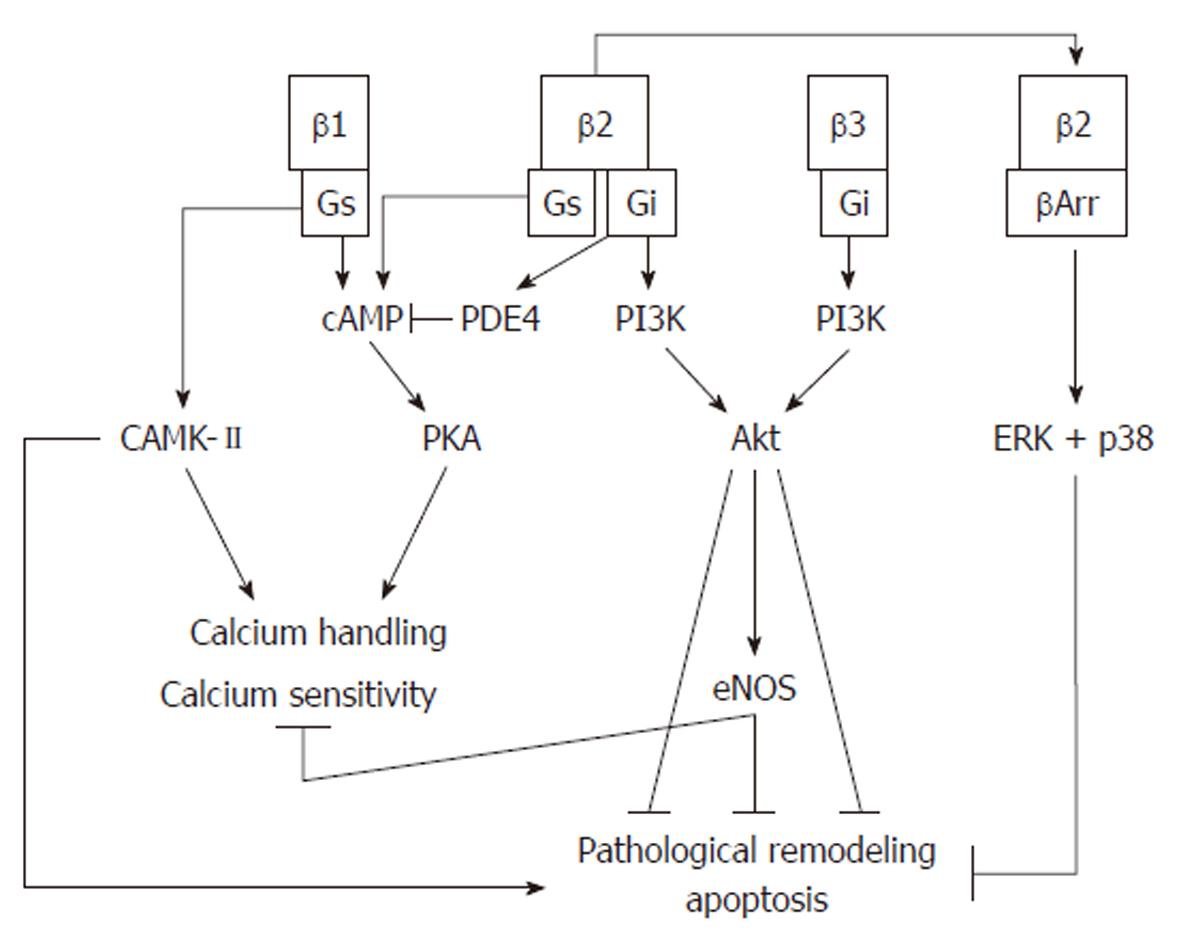
Figure 2 β-adrenergic signaling pathways.
β1-adrenergic receptors activate protein kinase A (PKA), which regulates calcium sensitivity and calcium handling. Prolonged activation of this receptor activates a harmful calmodulin-dependent protein kinase (CAMK)-II pathway which is pro-apoptotic and induces pathological remodeling. β2-adrenergic receptors also activate PKA, but prolonged activation causes a switch to Gi signaling which activates PDE4, inhibiting cAMP formation, and activates the cardioprotective phosphoinositol-3 kinase (PI3K)/protein kinase B (Akt) pathway. Desensitization of β2-adrenergic receptors by β-arrestin can recruit p38 and extracellular-signal-regulated kinase (ERK), which protect the cell from apoptosis. β3-adrenergic receptors produce a negative inotropic effect which is mediated by nitric oxide produced via the PI3K/Akt pathway.
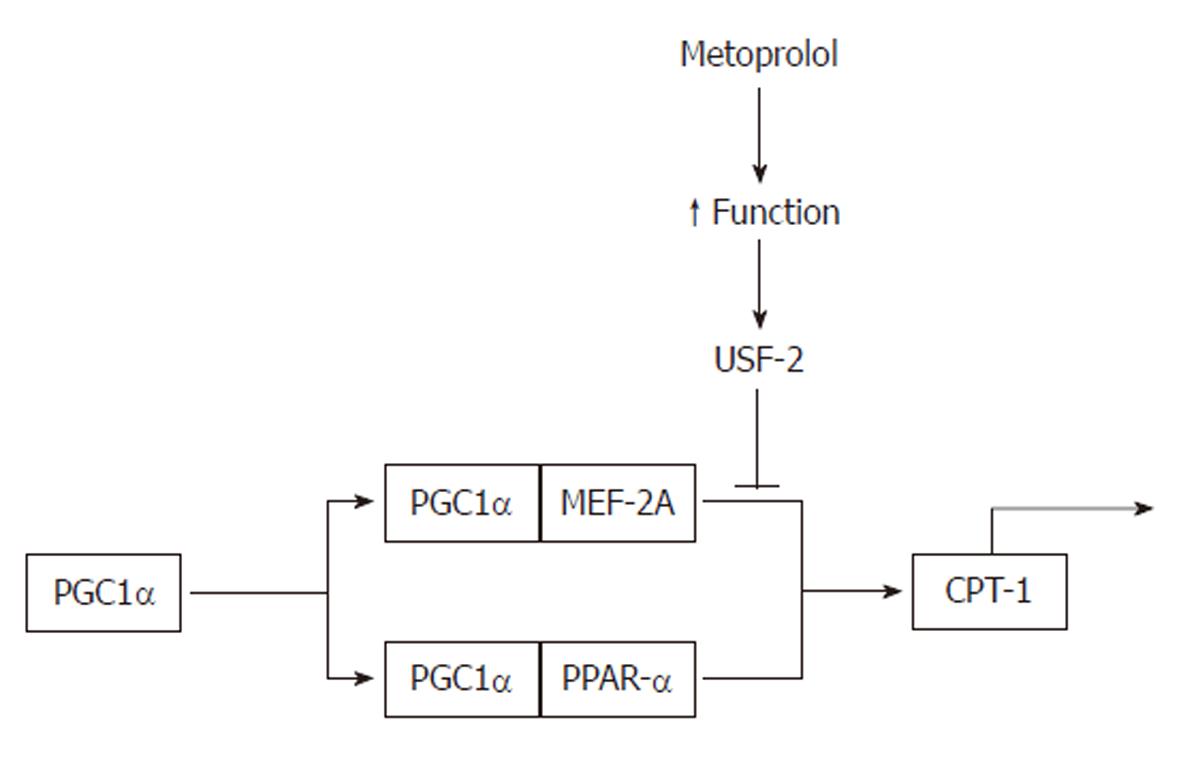
Figure 3 Repression of carnitine palmitoyltransferase-1 by metoprolol.
Improved contractile function in rat heart leads to stimulation of upstream stimulatory factor (USF)-2, which represses PGC1-α transcriptional complex and reduces the expression of carnitine palmitoyltransferase (CPT)-1. PPAR: Peroxisome proliferator-activated receptor.
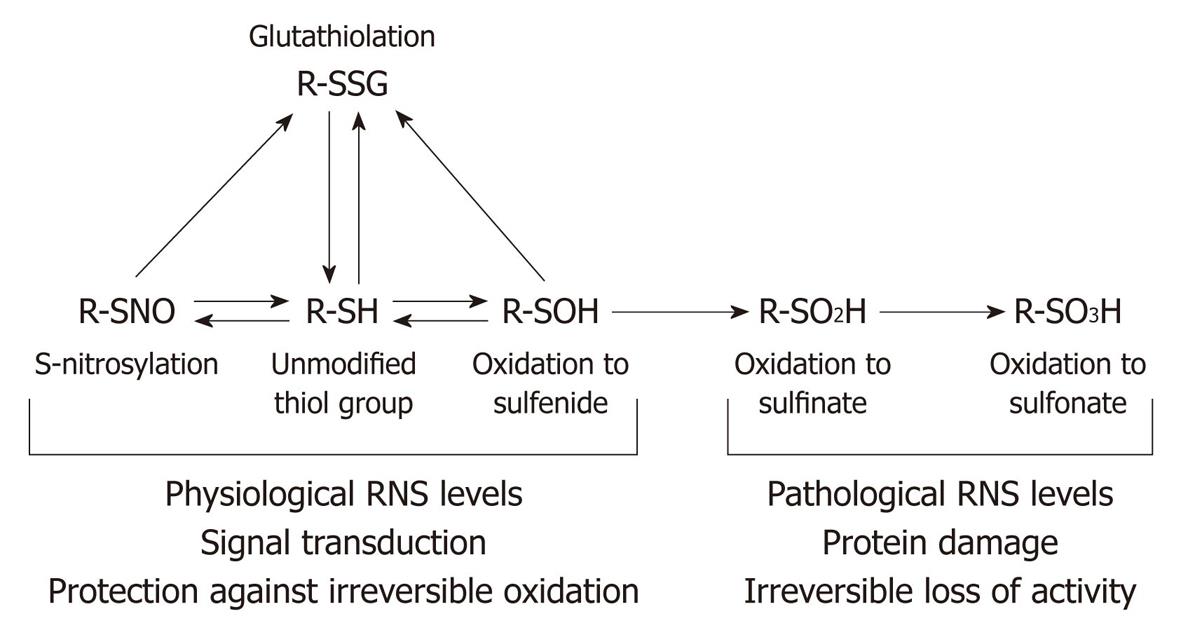
Figure 4 Nitric oxide and reactive nitrogen species-mediated modifications of thiol residues.
Thiol (SH) residues undergo a series of reversible modifications in response to changes in the redox potential or exposure to physiological levels of reactive nitrogen species or nitric oxide. Oxidation of the thiol to the corresponding sulfenic acidor the formation of a disulfide bond between the thiol and glutathione (glutathiolation) are reversible either by changes in the equilibrium, or enzymatic restoration of the thiol group by thiol transferases. Further oxidation of a glutathiolated residue is not possible, so glutathiolation confers protection against oxidative damage for as long as it persists. However, exposure of the thiol group or the sulfenic acid to pathological levels of reactive nitrogen or oxygen species results in the formation of sulfinate and then sulfonate; these are irreversible modifications which result in protein damage and loss of activity. RNS: Reactive nitrogen species. Modified from: Figure 2, Klattp et al[133].
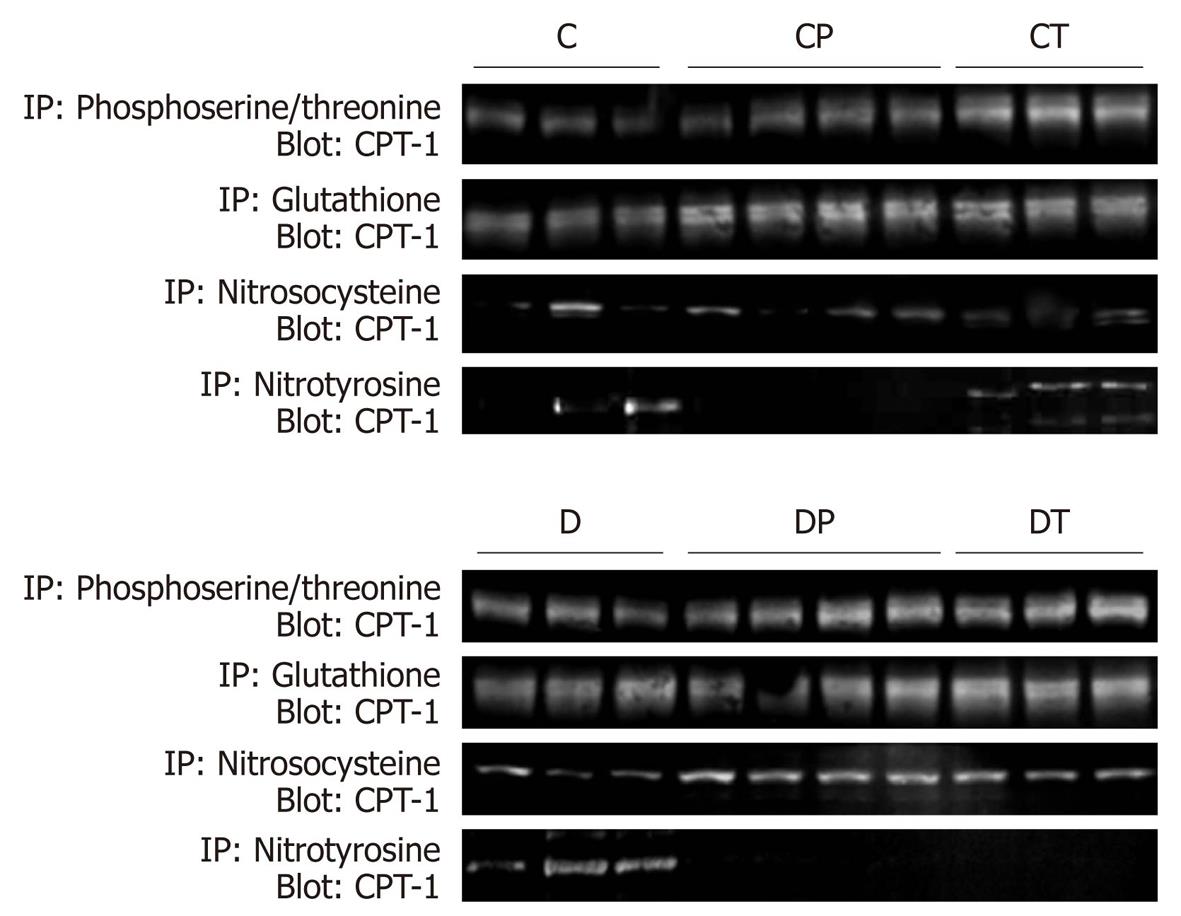
Figure 5 Covalent modifications of carnitine palmitoyltransferase-1 measured by immunoprecipitation.
Rat heart samples underwent immunoprecipitation with combined pan-specific anti-phosphoserine and anti-phosphothreonine antibodies, anti-glutathione antibodies or anti-nitrosocysteine antibodies, followed by immunoblotting with pan-specific carnitine palmitoyltransferase (CPT)-1 antibodies. Densitometric analyses of these data are presented in Figure 6. C: Control, n = 5; CP: Control perfused with metoprolol, n = 5; CT: Control treated with metoprolol, n = 5; D: Diabetic, n = 5; DP: Diabetic perfused with metoprolol, n = 5; DT: Diabetic treated with metoprolol, n = 5.
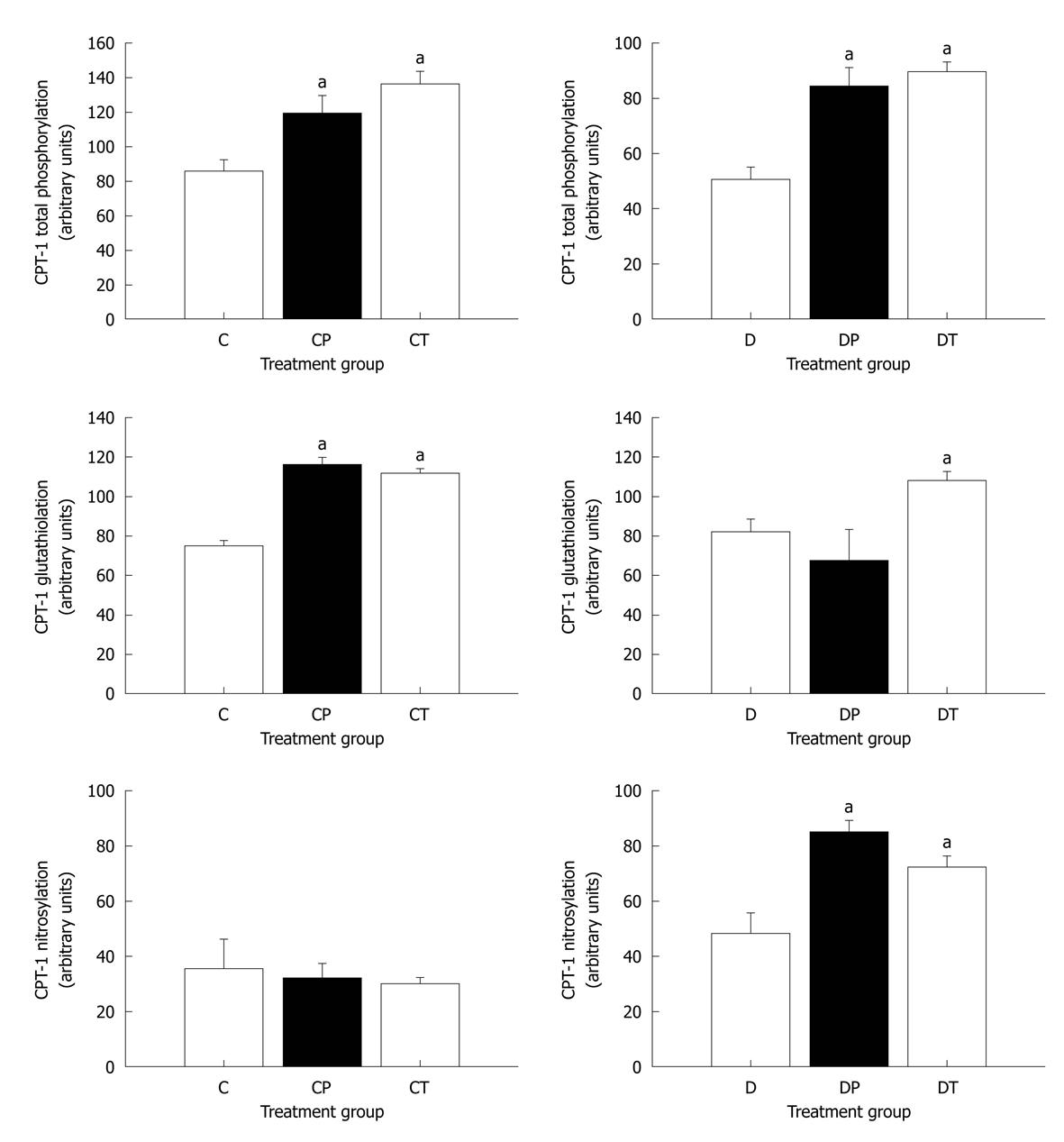
Figure 6 Densitometric analysis of carnitine palmitoyltransferase-1 covalent modifications.
Data represent mean ± SE. Data were analyzed using one-factor analysis of variance with the Neumann-Keuls post hoc test. aSignificantly different from C or D (P < 0.05). CPT: Carnitine palmitoyltransferase; C: Control, n = 5; CP: Control perfused with metoprolol, n = 5; CT: Control treated with metoprolol, n = 5; D: Diabetic, n = 5; DP: Diabetic perfused with metoprolol, n = 5; DT: Diabetic treated with metoprolol, n = 5.
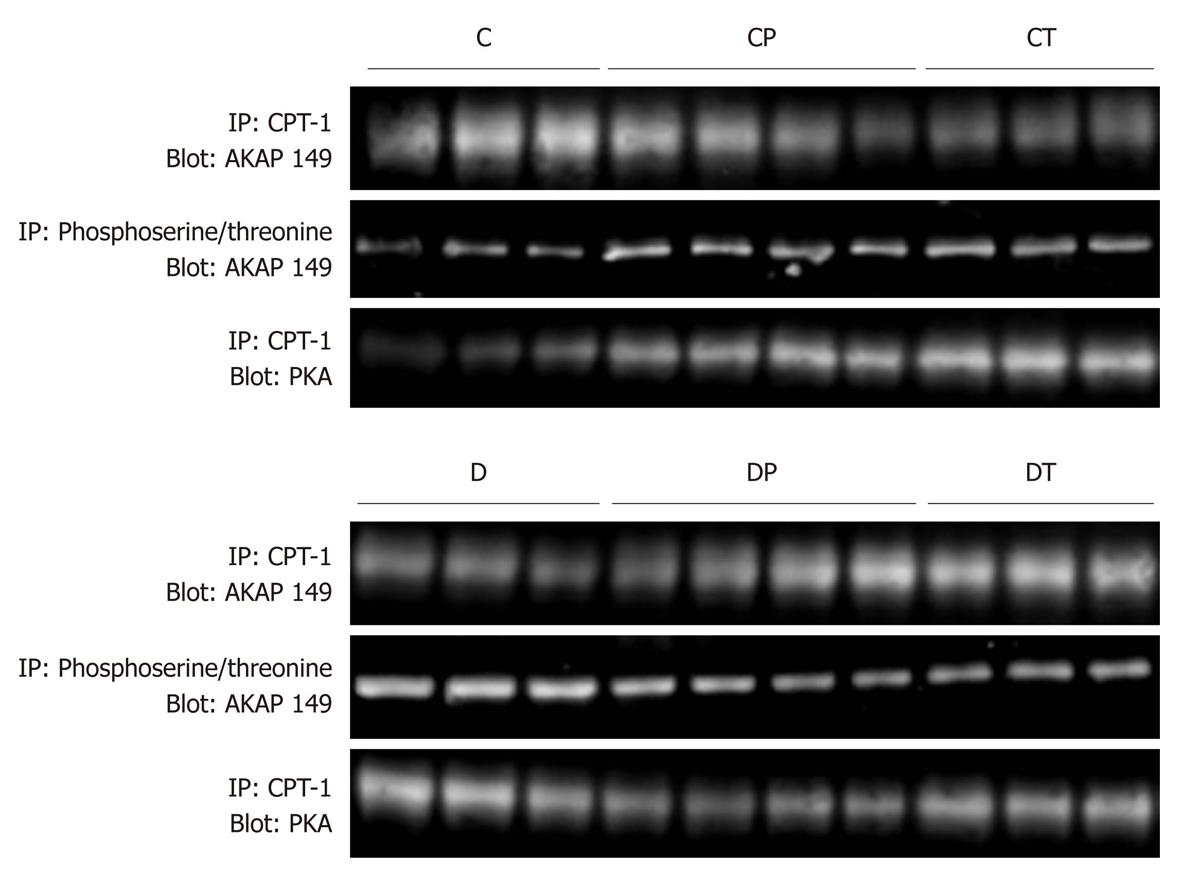
Figure 7 Co-immunoprecipitation of protein kinase A and A-kinase anchoring protein-149 with carnitine palmitoyltransferase-1, and phosphorylation state of A-kinase anchoring protein-149.
Densitometric analyses of these data are presented in Figure 8. AKAP: A-kinase anchoring protein; PKA: Protein kinase A; CPT: Carnitine palmitoyltransferase; C: Control, n = 5; CP: Control perfused with metoprolol, n = 5; CT: Control treated with metoprolol, n = 5; D: Diabetic, n = 5; DP: Diabetic perfused with metoprolol, n = 5; DT: Diabetic treated with metoprolol, n = 5.
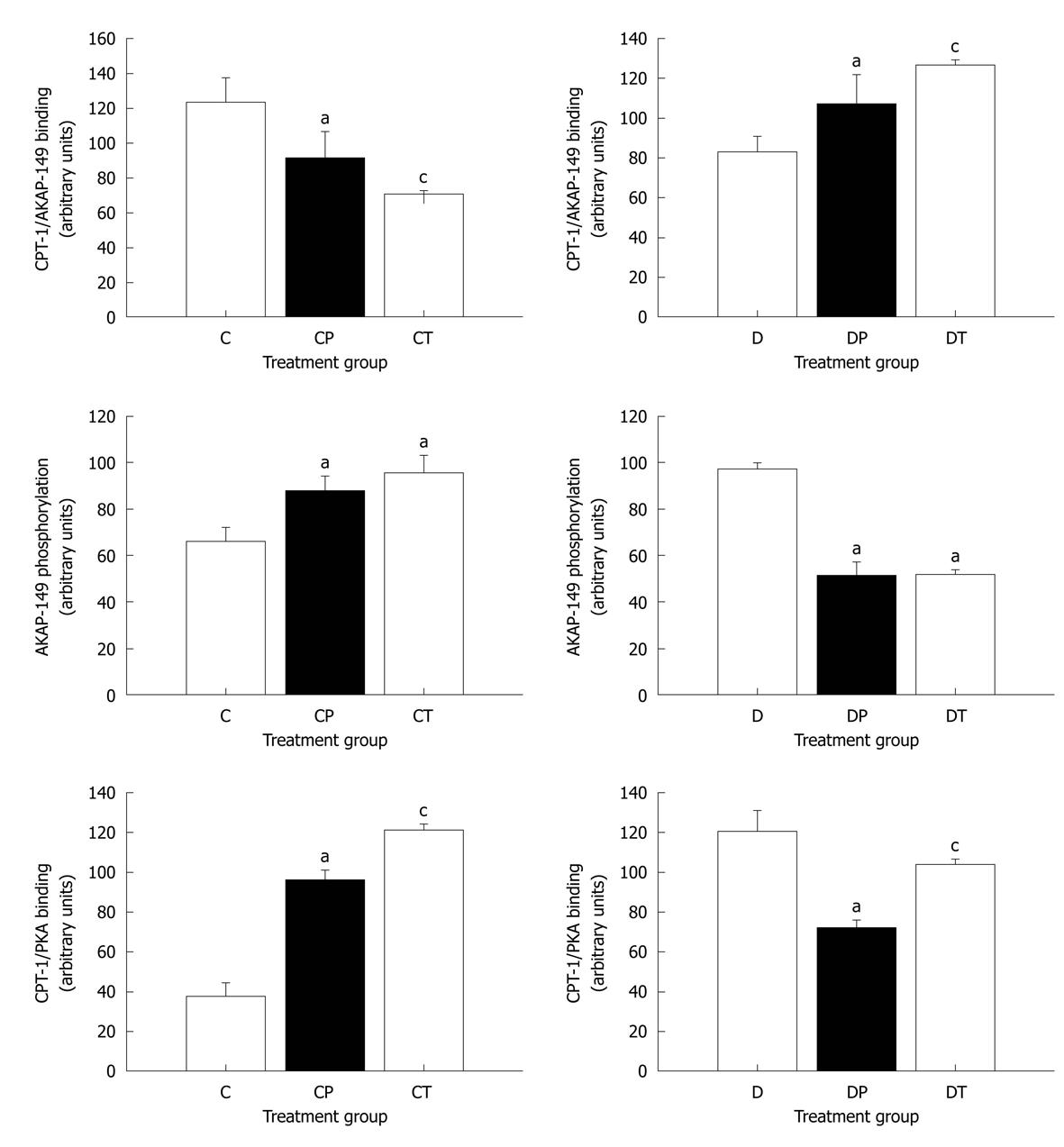
Figure 8 Densitometric analysis of the binding of protein kinase A and A-kinase anchoring protein-149 to carnitine palmitoyltransferase-1, and phosphorylation state of A-kinase anchoring protein-149.
Data represent mean ± SE. Data were analyzed using one-factor analysis of variance with the Neumann-Keuls post hoc test. AKAP: A-kinase anchoring protein; PKA: Protein kinase A; CPT: Carnitine palmitoyltransferase; C: Control; CP: Control perfused with metoprolol; CT: Control treated with metoprolol; D: Diabetic; DP: Diabetic perfused with metoprolol; DT: Diabetic treated with metoprolol. aSignificantly different from C or D; cSignificantly different from C and CP or D and DP (P < 0.05).
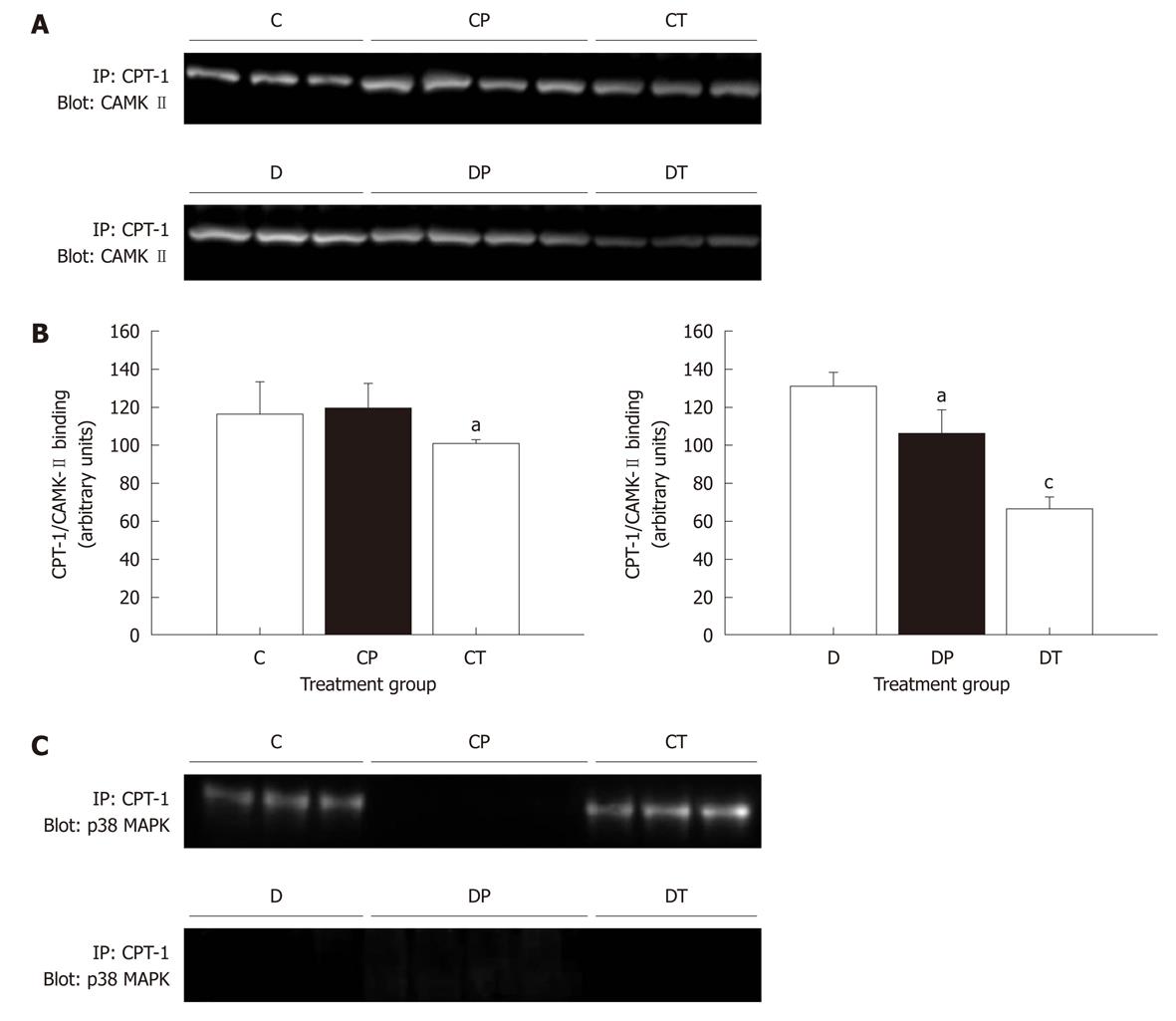
Figure 9 Binding of calmodulin-dependent protein kinase-II and p38 MPK carnitine palmitoyltransferase-1.
Data represent mean ± SE. Data were analyzed using one-factor analysis of variance with the Neumann-Keuls post hoc test. CAMK: Calmodulin-dependent protein kinase; CPT: Carnitine palmitoyltransferase; MAPK: Mitogen-activated protein kinase; C: Control; CP: Control perfused with metoprolol; CT: Control treated with metoprolol; D: Diabetic; DP: Diabetic perfused with metoprolol; DT: Diabetic treated with metoprolol. aSignificantly different from C or D; cSignificantly different from C and CP or D and DP (P < 0.05).
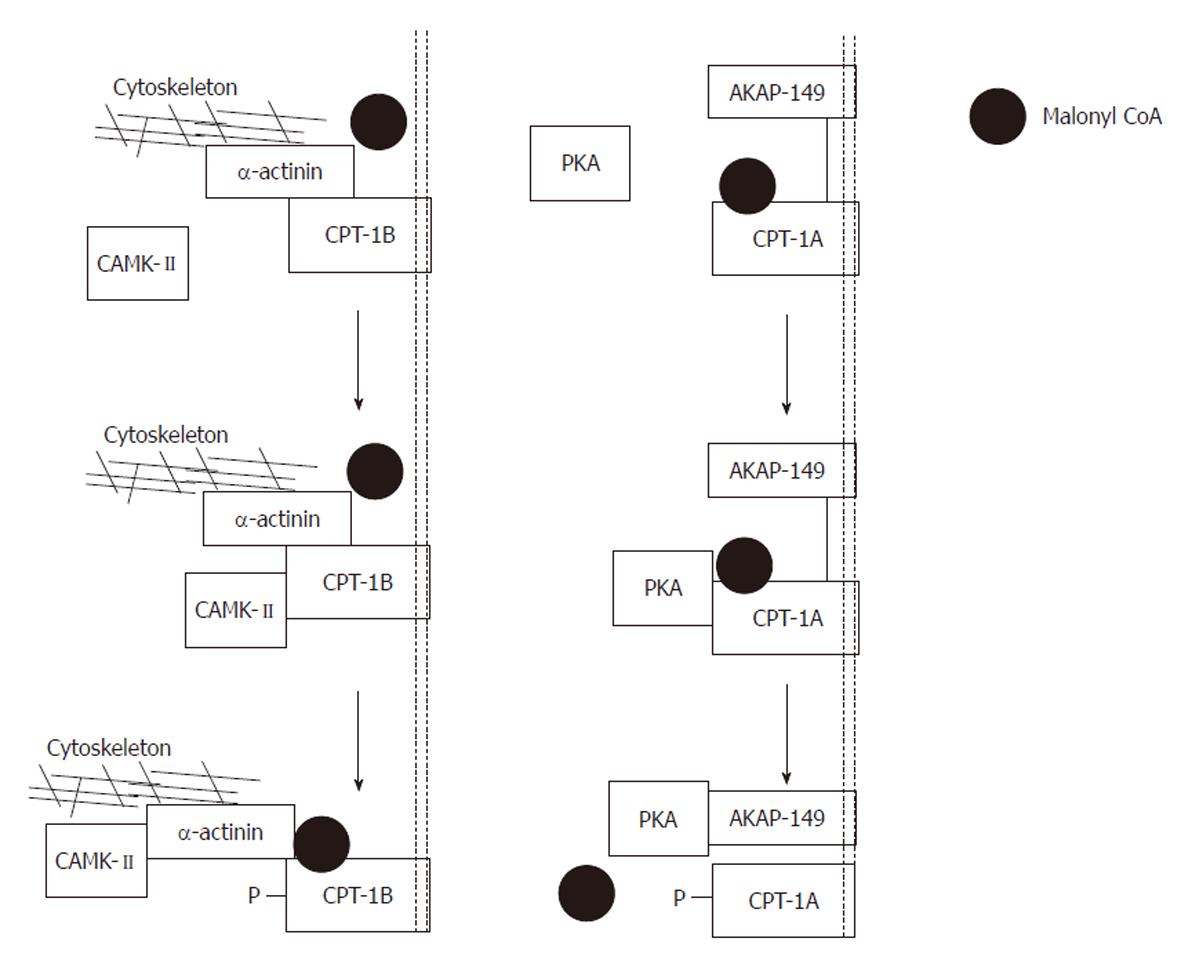
Figure 10 Proposed model of the actions of protein kinase A and calmodulin-dependent protein kinase-II.
Left panel: Exogenously applied protein kinase A (PKA) phosphorylates carnitine palmitoyltransferase (CPT)-1A and is then captured by its scaffolding protein. Phosphorylation produces a conformational change tightening the interaction between A-kinase anchoring protein (AKAP)-149 and CPT-1A. As a result, malonyl CoA is denied access to its binding site, and the sensitivity of CPT-1 to malonyl CoA is reduced. Note that CPT-1 and AKAP-149 are both anchored in the mitochondrial membrane; Right panel: Exogenously applied calmodulin-dependent protein kinase (CAMK)-II phosphorylates CPT-1B and is then captured by its scaffolding protein α-actinin. Phosphorylation produces a conformational change, loosening the interaction between α-actinin and CPT-1B. As a result, malonyl CoA has improved access to its binding site and the sensitivity of CPT-1 to malonyl CoA is increased. Note that CPT-1B is anchored to the mitochondrial membrane whereas α-actinin is anchored to the cytoskeleton. Modified from: Figure 4, supplementary information, Sharma et al[108].
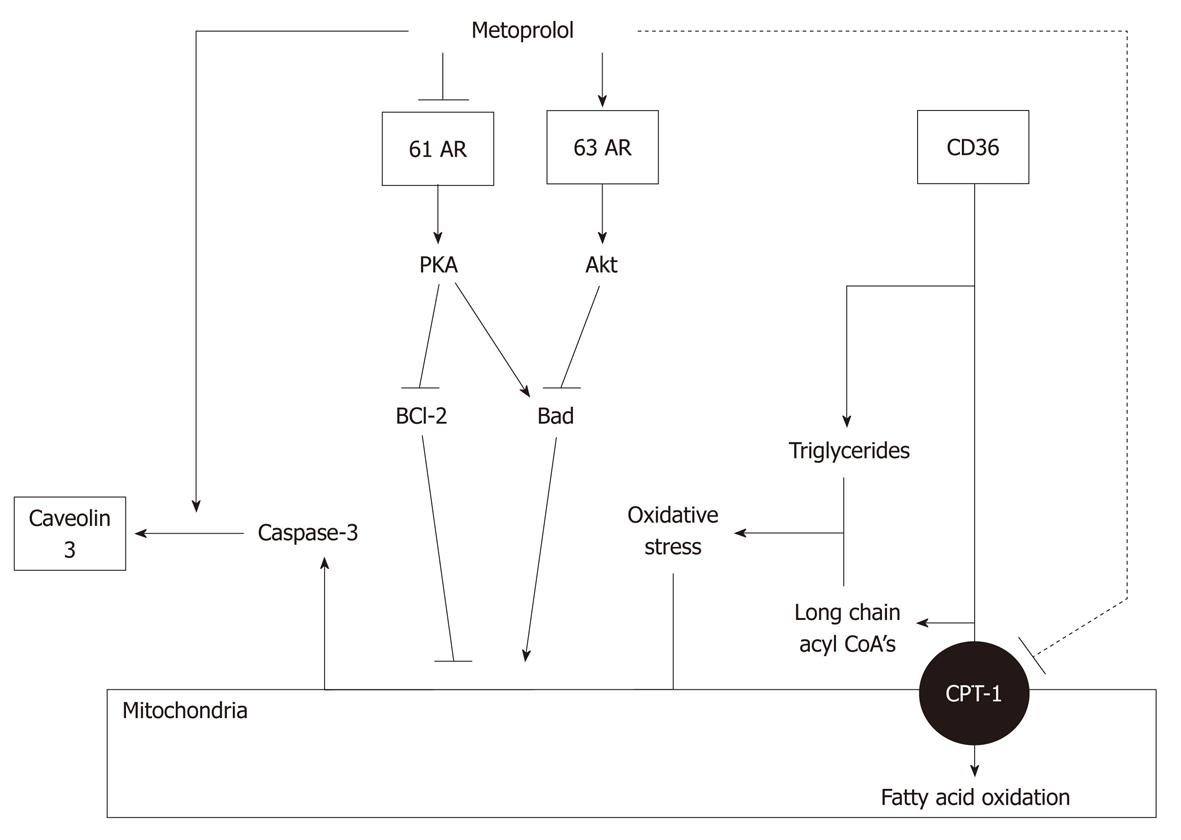
Figure 11 Mechanisms of action of metoprolol.
Metoprolol inhibits fatty acid oxidation by inhibiting carnitine palmitoyltransferase (CPT)-1, but has no effect on CD36. Triglyceride and long chain acyl CoA accumulation, and stimulation of oxidative stress, are therefore unaltered. Metoprolol also promotes β3 adrenoceptor signaling, leading to inhibition of Bad and stimulation of BCl-2, and inhibition of caspase-3 activation. Finally, metoprolol stimulates sequestration of caspase-3 by caveolins. The net effect is a prevention of caspase-3 activation. Modified from: Figure 7, Sharma et al[108].
- Citation: Sharma V, McNeill JH. Parallel effects of β-adrenoceptor blockade on cardiac function and fatty acid oxidation in the diabetic heart: Confronting the maze. World J Cardiol 2011; 3(9): 281-302
- URL: https://www.wjgnet.com/1949-8462/full/v3/i9/281.htm
- DOI: https://dx.doi.org/10.4330/wjc.v3.i9.281