Copyright
©2011 Baishideng Publishing Group Co.
World J Cardiol. Jun 26, 2011; 3(6): 186-200
Published online Jun 26, 2011. doi: 10.4330/wjc.v3.i6.186
Published online Jun 26, 2011. doi: 10.4330/wjc.v3.i6.186
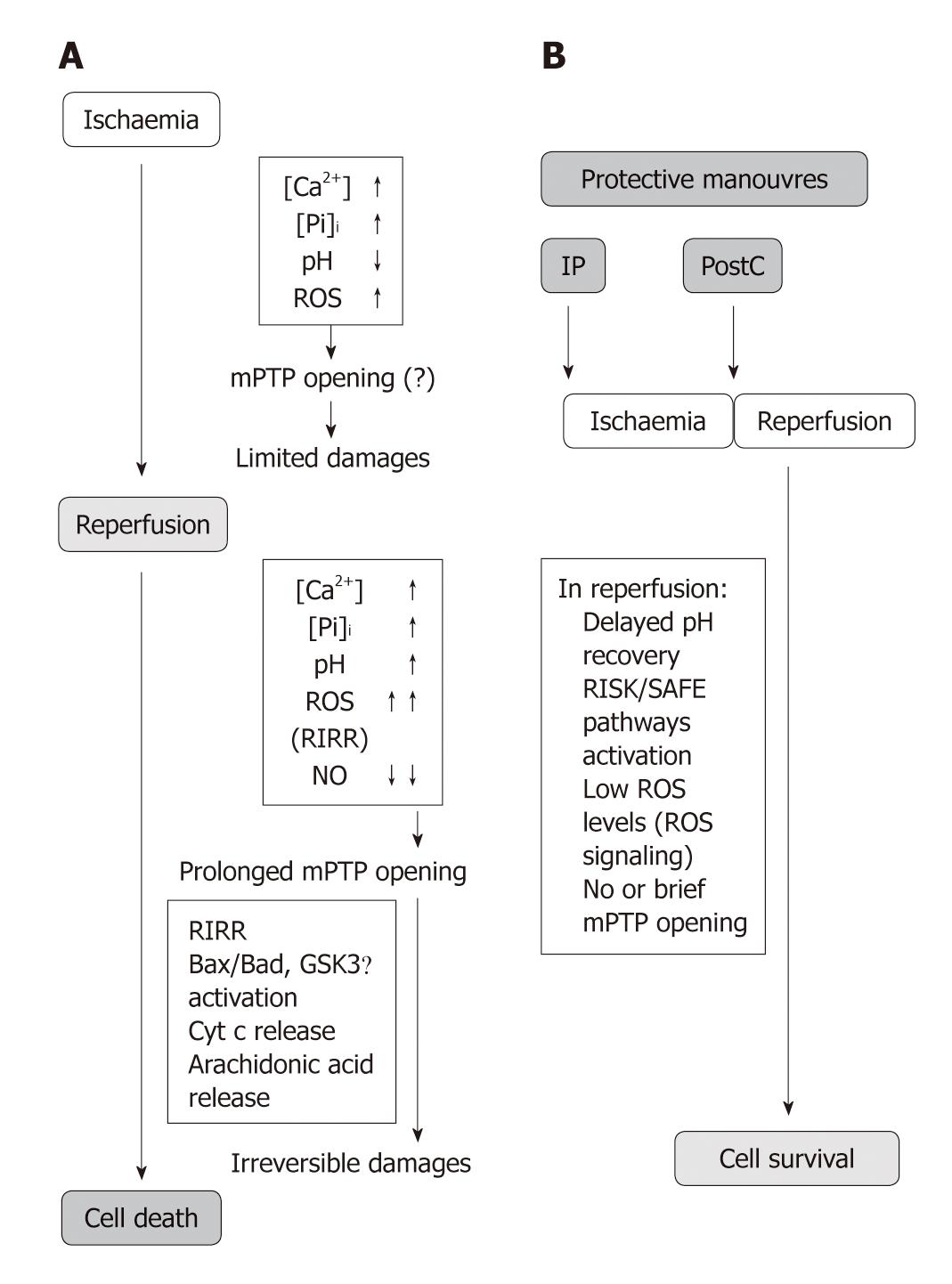
Figure 1 Flowchart depicting the variations of pH, ROS and mPTP opening during ischaemia and reperfusion phases in the control hearts, and in hearts protected by preconditioning or postconditioning.
A: In the control hearts reactive oxygen species (ROS) production slightly increases during the initial part of ischaemia until the O2 is exhausted. Then sharply increases in reperfusion. Formation of mitochondrial permeability transition pores (mPTP) had been limited during ischaemia by the low pH despite increased cellular levels of ROS, Ca2+ and Pi overload. But as pH returns to its baseline level and ROS formation increases prolonged opening occurs. The limited damages occurring during ischaemia are exacerbated by the prolonged mPTP opening which mediates irreversible cell damages in reperfusion. The opening effect, besides Ca2+ overload, is also due to indirect effects, such as phospholipase A2 (PLA2) and calpain activations and consequent arachidonic acid release after membrane phospholipids degradation. A part membrane depolarisation also inorganic phosphate (Pi), lower levels of nitric oxide (NO) contribute to mPTP opening. Other factors which regulate pore formation are Bcl-2–associated X protein (Bax)/Bcl-2-associated death promoter (Bad), B-cell lymphoma 2 (Bcl-2) and glycogen synthase kinase 3 β (GSK-3β). Pore opening leads to cell-death through the release of pro-apoptotic factors as cytochrome c (Cyt c) and via ROS-induced ROS release (RIRR); B: The pre/postconditioned hearts are characterized by delayed pH recovery, ROS signalling and activation of protective pathways (e.g. Reperfusion Injury Salvage Kinases (RISK)/Survivor Activating Factor Enhancement (SAFE). These conditions contribute to reduce mPTP opening and consequent cell death limitation. The details of the protective signalling (RISK/SAFE) can be seen in Figure 2 and Figure 3. For further explanations see the text.
Figure 2 Flowchart depicting the main factors involved in cardioprotective pathways triggered by pre and postconditioning.
Activation of cell-surface receptors in response to an ischaemic conditioning stimulus recruits cGMP/PKG, RISK and SAFE pathways. In particular, iNOS seems to be involved in SAFE pathway[77]. These signal transduction pathways, together with acidosis, activated at the time of reperfusion will crosstalk and will terminate on mitochondria to activate protective pathways. Akt: Serine/threonine protein kinase; cGMP/PKG: Cyclic guanosin monophosphate/protein kinase G; eNOS: Endothelial NO synthase; ERK1/2: Extracellular regulated kinase 1/2; gp130: Glycoprotein 130; GPCR: G-protein-coupled receptor; GSK3β: Glycogen synthase kinase 3 β; IL-6: Interleukin 6; iNOS: Inducible NO synthase; JAK: Janus kinase; MEK: Mitogen-activated protein kinase kinase; mPTP: Mitochondrial permeability transition pore; NO: Nitric oxide; P70S6K: p70 ribosomal S6 protein kinase; PI3K: Phosphoinositide 3-kinase; PKG: Protein kinase G; RISK: Reperfusion injury salvage kinases; SAFE: Survivor activating factor enhancement; STAT-3: Signal transducer and activator of transcription 3; TNFα: Tumour necrosis factor α; TNF-R2: Tumour necrosis factor receptor 2.
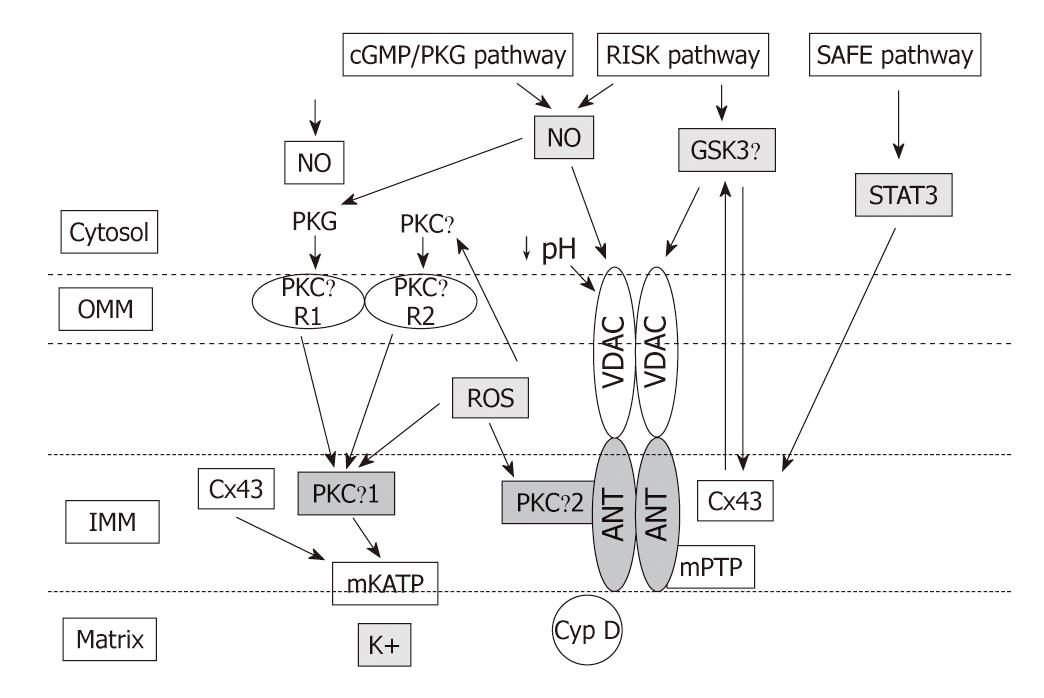
Figure 3 Extra- and intra-mitochondrial signalling: interactions among Cx43, mKATP, PKCε, ROS, and mPTP.
The mPTP is believed to be composed of the adenine nucleotide transporter (ANT) in the inner membrane (IMM), the voltage-dependent anion channel (VDAC) of the outer membrane (OMM), and cyclophilin D (Cyp D) in the matrix. Signals arising from RISK and SAFE pathways are delivered to mitochondrial permeability transition pore (mPTP) via nitric oxide (NO)/glycogen synthase kinase 3 β (GSK3β) and signal transducer and activator of transcription 3 (STAT-3), respectively, and then to connexin 43 (Cx43) on the IMM. Signals arising from cGMP/PKG are delivered to mitochondria via the terminal protein kinase G (PKG). PKG phosphorylates an unknown OMM receptor, “R1”, whereas PKCε act in conjunction to activate a distinct OMM receptor, “R2”. These OMM receptors transmit the signal by an unknown mechanism to PKCε1 located at the IMM. The activated PKCε1 phosphorylates and opens mitochondrial ATP-sensitive potassium (mKATP). Also Cx43 located at the IMM regulated mKATP opening. The mKATP opening via PKCε1 causes K+ uptake and increased ROS production from respiratory chain. ROS produced by mKATP activation now diffuses and activates both PKCε1 and PKCε2 on the IMM and PKCε in the cytosol. ROS signalling may represent the link between mitochondria and cytosol. mPTP opening is prevented by cytosolic pH lowering, ROS signalling and PKCε2 activation.
- Citation: Perrelli MG, Pagliaro P, Penna C. Ischemia/reperfusion injury and cardioprotective mechanisms: Role of mitochondria and reactive oxygen species. World J Cardiol 2011; 3(6): 186-200
- URL: https://www.wjgnet.com/1949-8462/full/v3/i6/186.htm
- DOI: https://dx.doi.org/10.4330/wjc.v3.i6.186