Copyright
©2011 Baishideng Publishing Group Co.
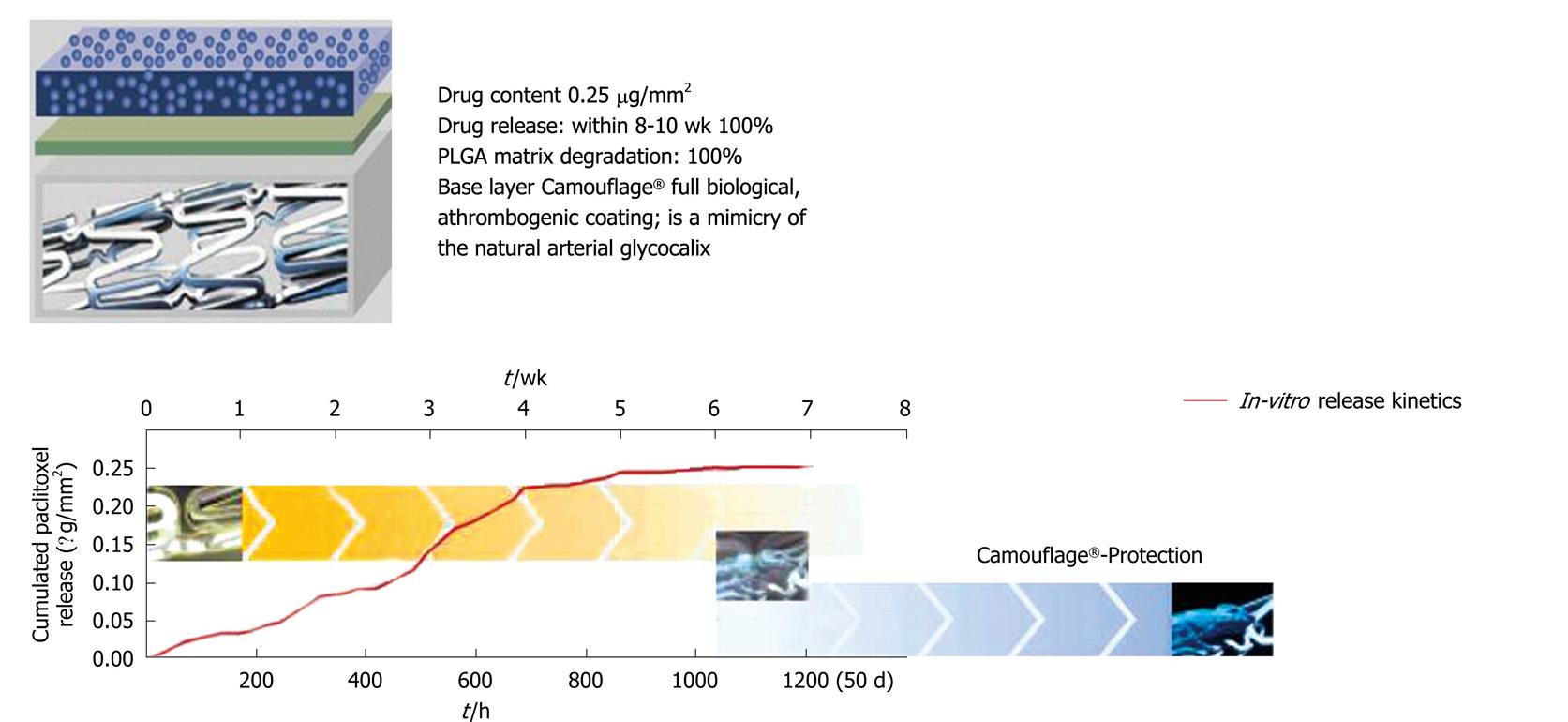
Figure 1 EUCATAX design and characteristics.
PLGA: Poly(D,L-lactic-co-glycolic acid).
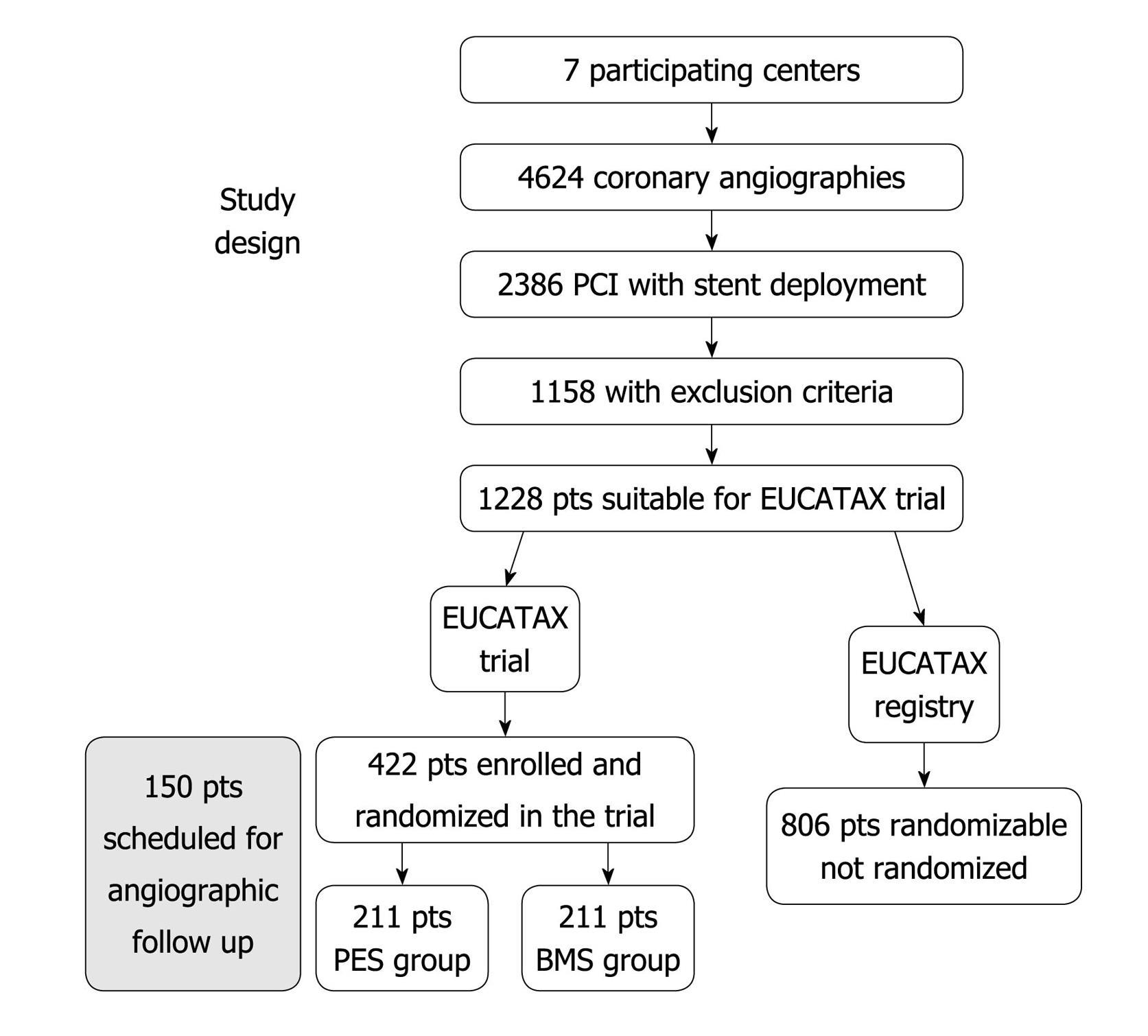
Figure 2 EUCATAX randomized trial design.
Modified form Rodriguez et al[39]. Pts: Patients; PCI: Percutaneous coronary interventions; PES: Paclitaxel eluting stent; BMS: Bare-metal stent.
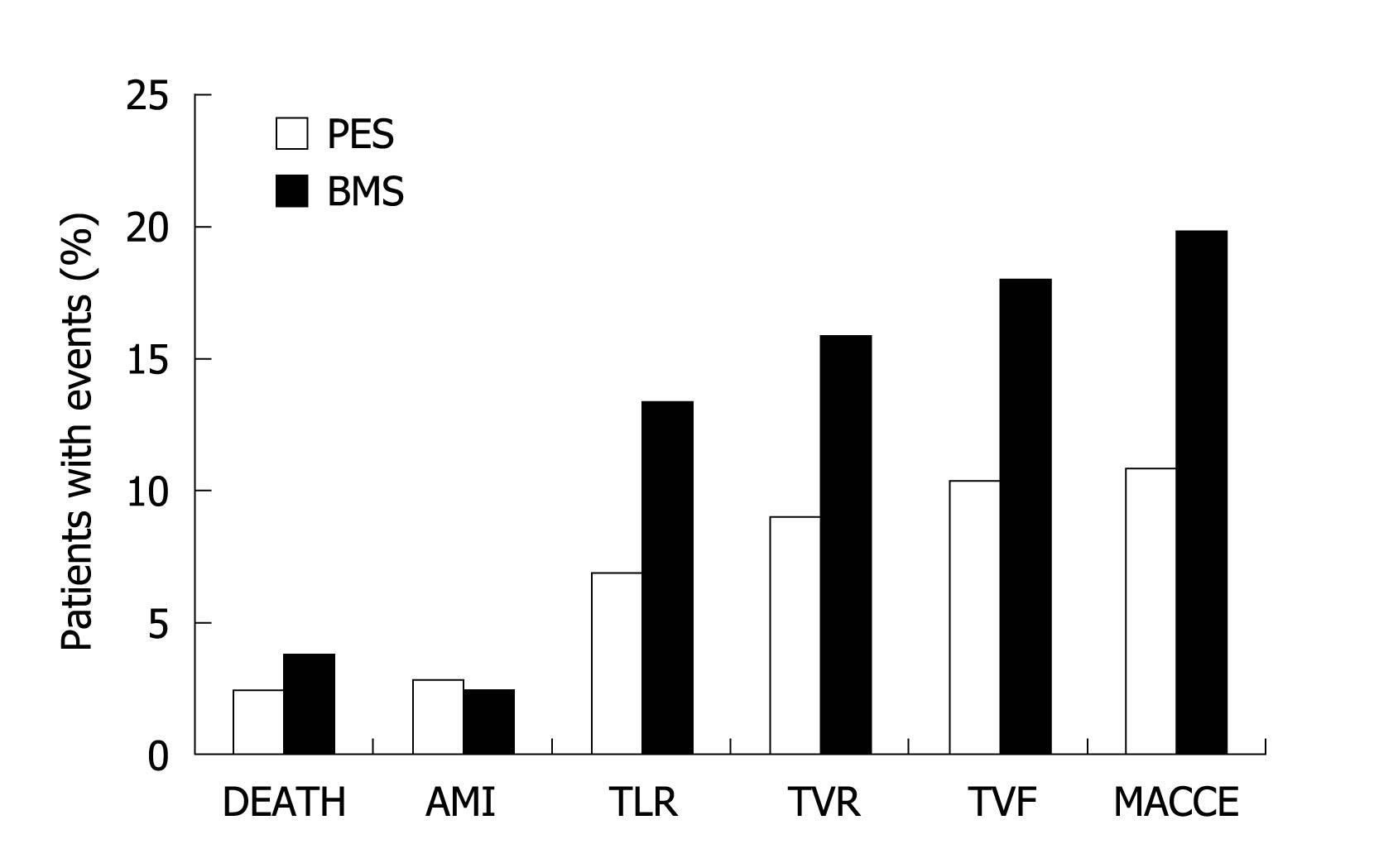
Figure 3 Results at 18 mo.
EUCATAX trial. AMI: Acute myocardial infarction; MACCE: Major adverse cardiovascular events; TVF: Target vessel failure; TLR: Target lesion revascularisation; TVR: Target vessel revascularisation; PES: Paclitaxel-eluting stents; BMS: Bare-metal stent.
- Citation: Rodriguez-Granillo A, Rubilar B, Rodriguez-Granillo G, Rodriguez AE. Advantages and disadvantages of biodegradable platforms in drug eluting stents. World J Cardiol 2011; 3(3): 84-92
- URL: https://www.wjgnet.com/1949-8462/full/v3/i3/84.htm
- DOI: https://dx.doi.org/10.4330/wjc.v3.i3.84