Copyright
©The Author(s) 2024.
World J Cardiol. Aug 26, 2024; 16(8): 469-483
Published online Aug 26, 2024. doi: 10.4330/wjc.v16.i8.469
Published online Aug 26, 2024. doi: 10.4330/wjc.v16.i8.469
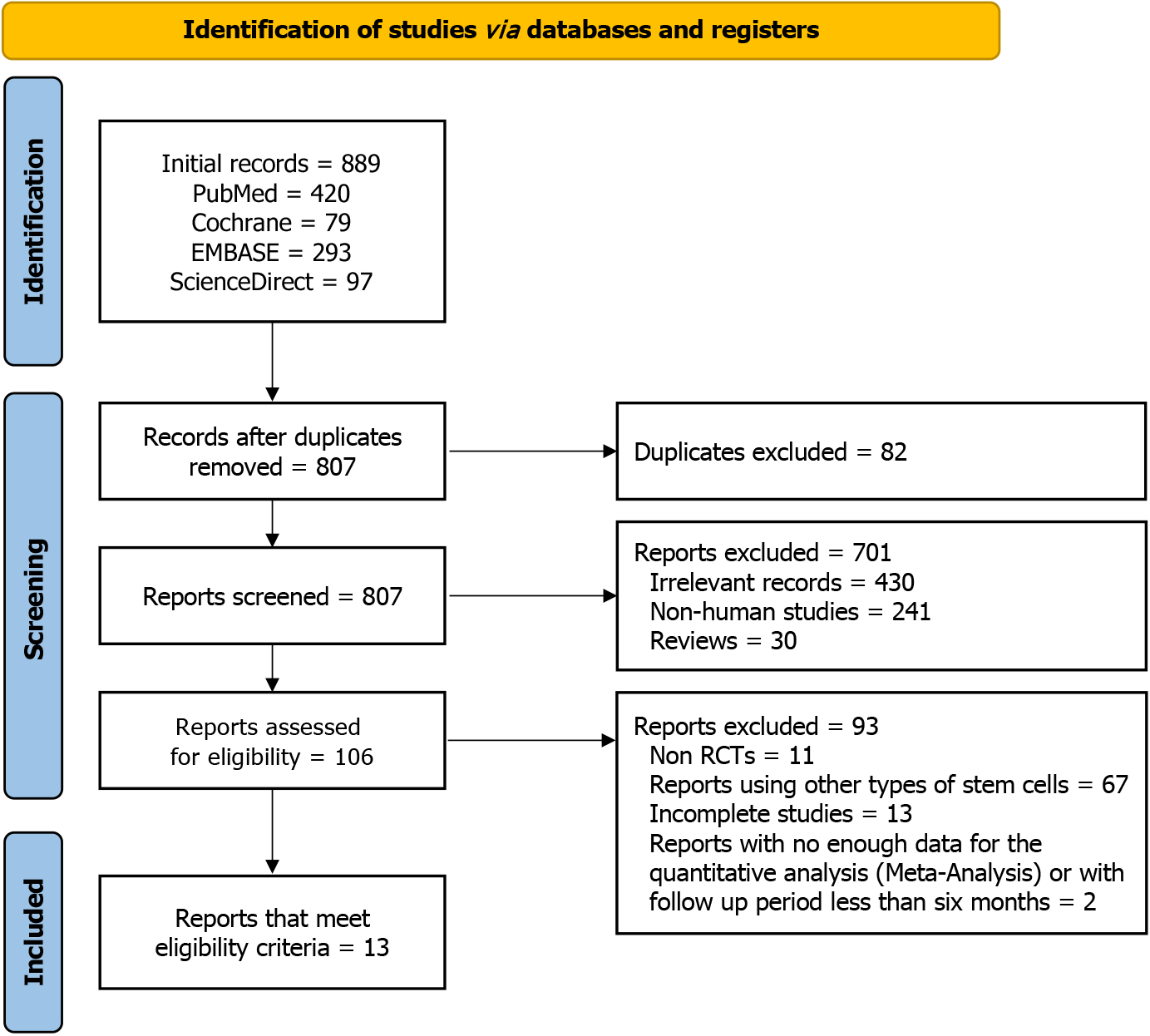
Figure 1 Study selection flow diagram of preferred reporting item for systematic reviews and meta-analysis.
RCTs: Randomized controlled trials.
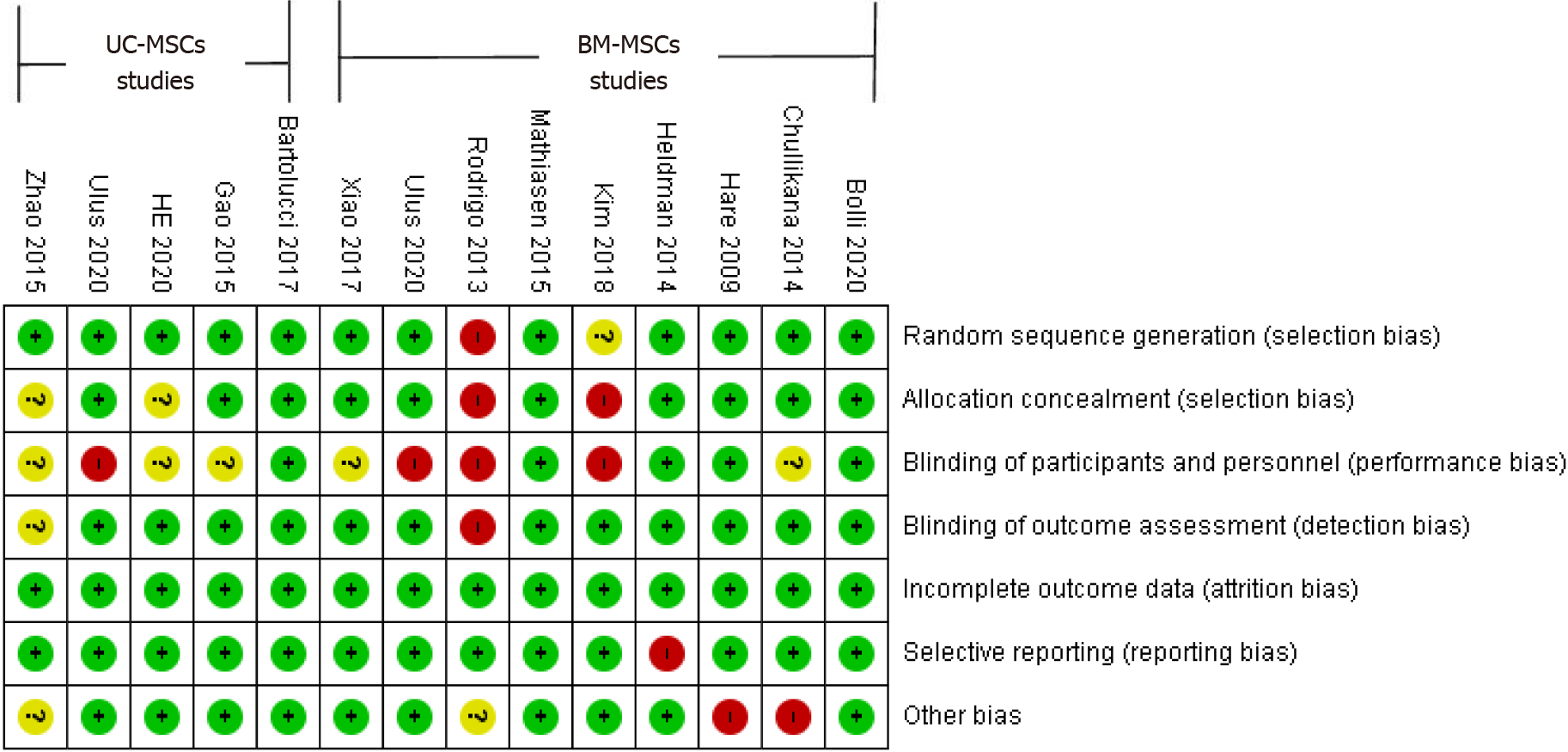
Figure 2 Risk of bias assessment graph.
UC-MSCs: Umbilical-cord-derived mesenchymal stem cells; BM-MSCs: Bone-marrow-derived mesenchymal stem cell.
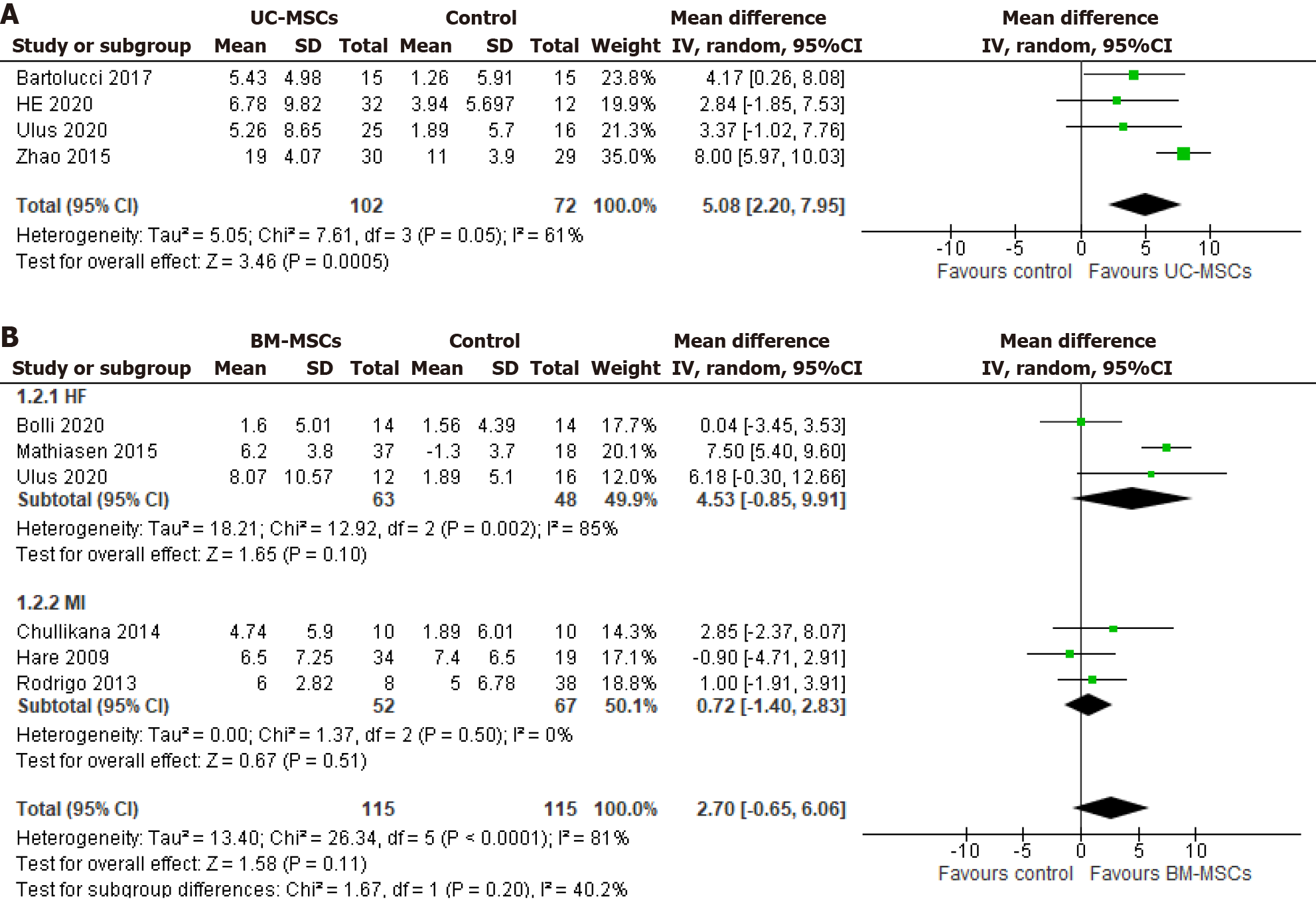
Figure 3 Forest plot of left ventricular ejection fraction change from baseline to 6 mo of follow-up.
A: Umbilical-cord-derived mesenchymal stem cells; B: Bone-marrow-derived mesenchymal stem cells. UC-MSCs: Umbilical cord-derived mesenchymal stem cells; BM-MSCs: Bone marrow-derived mesenchymal stem cell; MI: Myocardial infarction; HF: Heart failure.
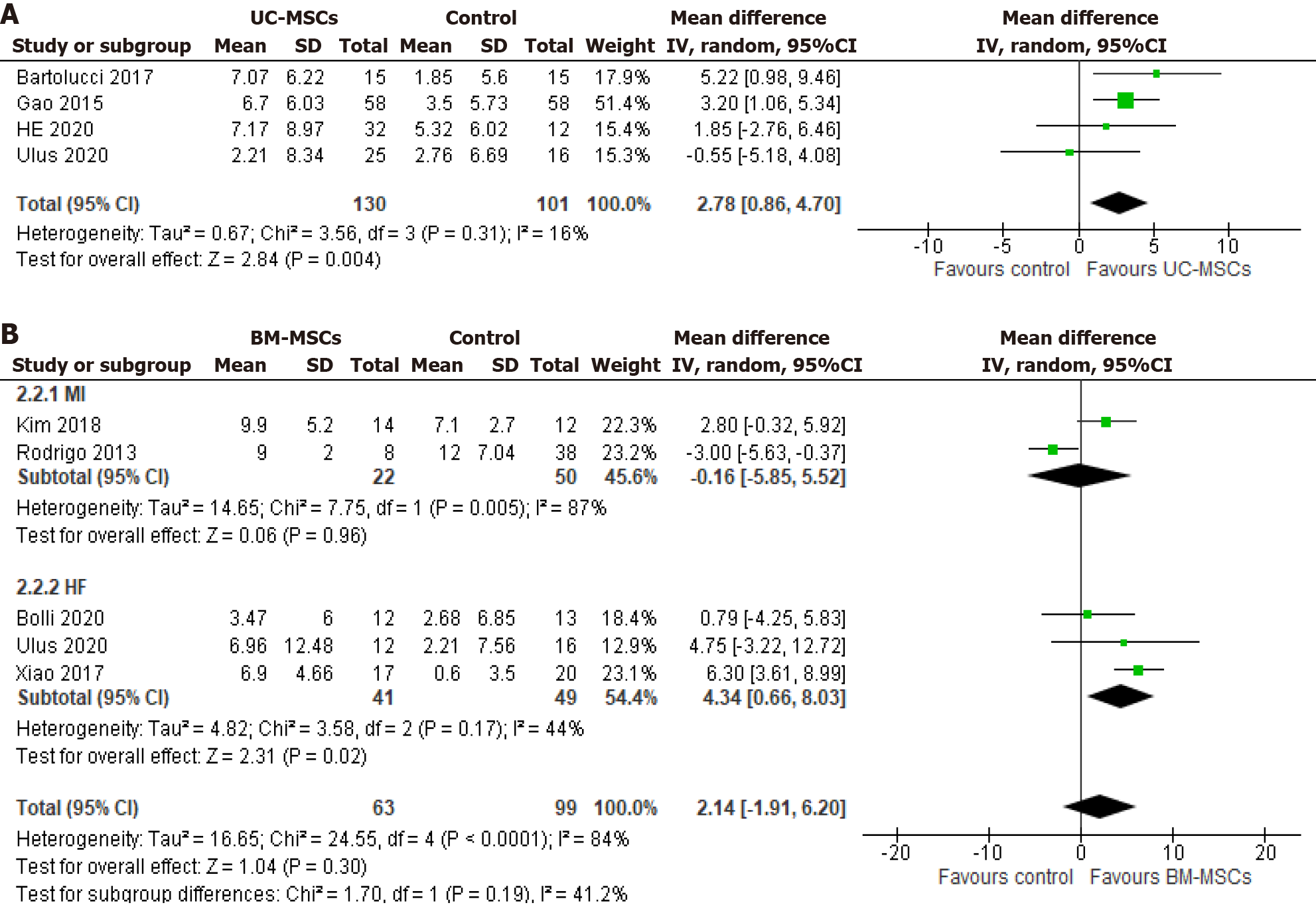
Figure 4 Forest plot of left ventricular ejection fraction change from baseline to 12 mo follow-up.
A: Umbilical cord-derived mesenchymal stem cells; B: Bone marrow-derived MSCs. UC-MSCs: Umbilical-cord-derived mesenchymal stem cells; BM-MSCs: Bone-marrow-derived mesenchymal stem cell; MI: Myocardial infarction; HF: Heart failure.
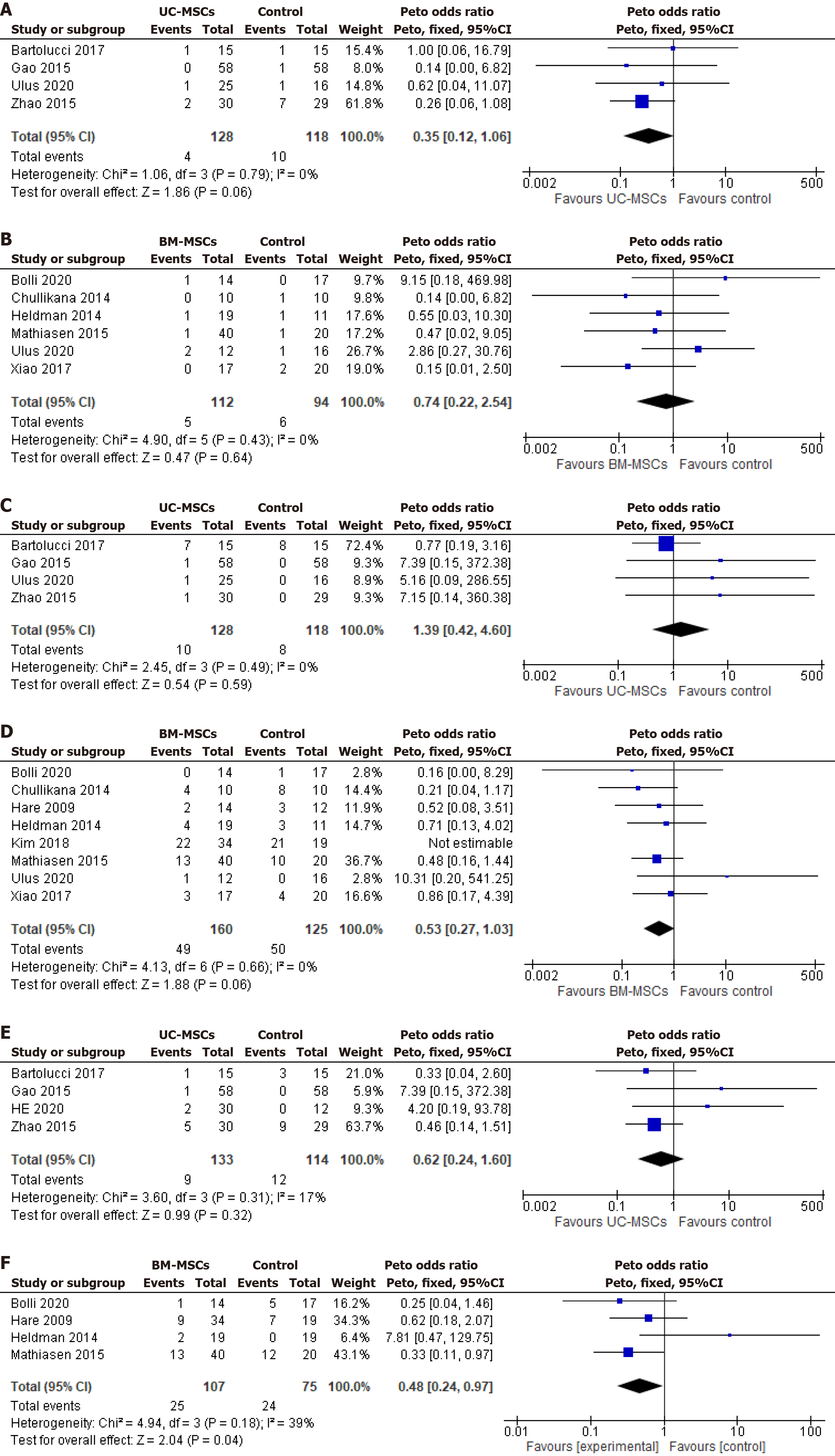
Figure 5 Forest Plot of major adverse events.
A and B: Mortality in umbilical-cord-derived mesenchymal stem cells (A) and bone-marrow-derived mesenchymal stem cells (B); C and D: Major cardiac adverse events in UC-MSCs (C) and BM-MSCs (D); E and F: Rehospitalization in UC-MSCs (E) and BM-MSCs (F). UC-MSCs: Umbilical-cord-derived mesenchymal stem cells; BM-MSCs: Bone-marrow-derived mesenchymal stem cells.
- Citation: Safwan M, Bourgleh MS, Aldoush M, Haider KH. Tissue-source effect on mesenchymal stem cells as living biodrugs for heart failure: Systematic review and meta-analysis. World J Cardiol 2024; 16(8): 469-483
- URL: https://www.wjgnet.com/1949-8462/full/v16/i8/469.htm
- DOI: https://dx.doi.org/10.4330/wjc.v16.i8.469