Copyright
©The Author(s) 2021.
World J Cardiol. Mar 26, 2021; 13(3): 55-67
Published online Mar 26, 2021. doi: 10.4330/wjc.v13.i3.55
Published online Mar 26, 2021. doi: 10.4330/wjc.v13.i3.55
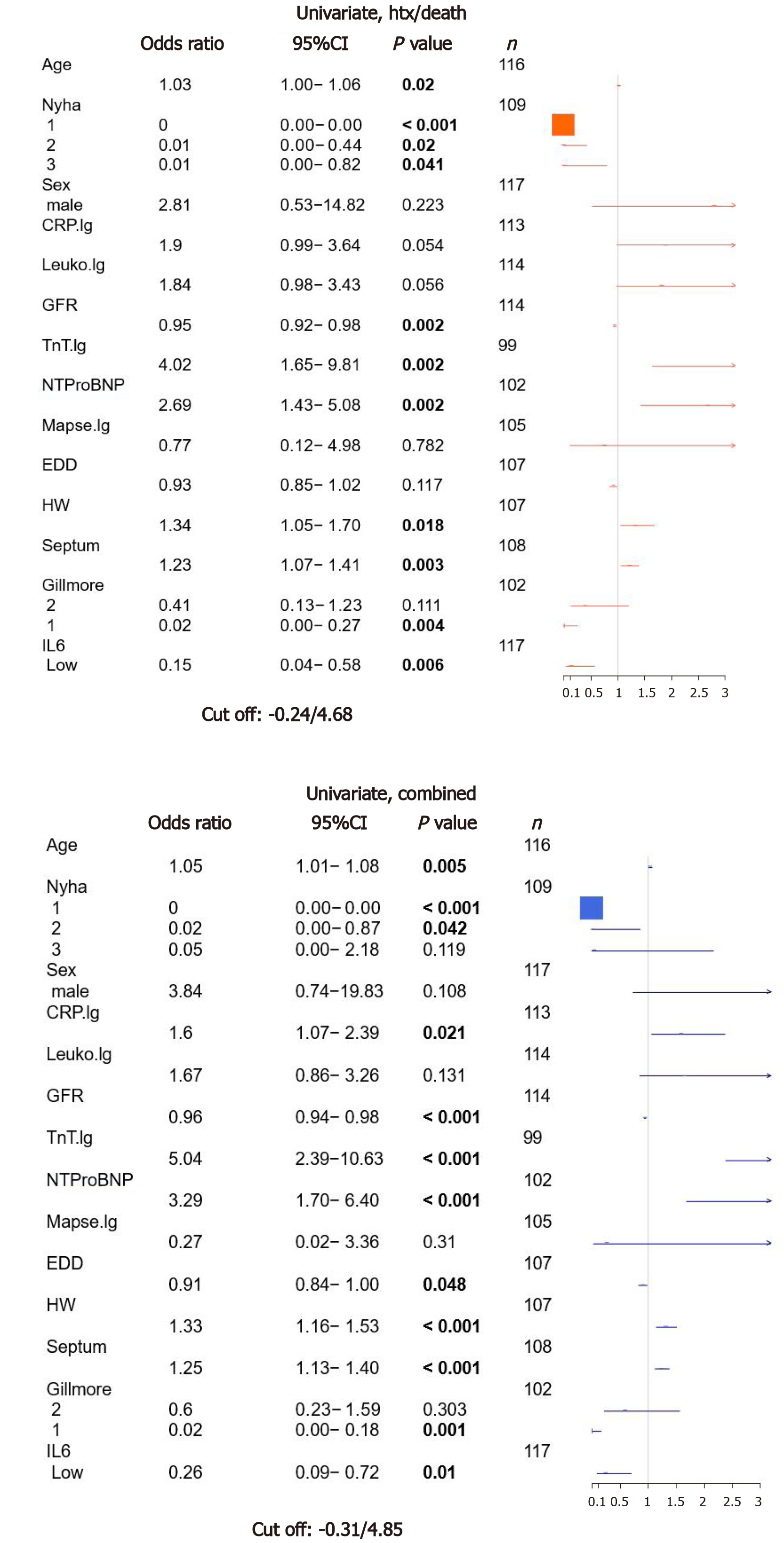
Figure 1 Univariate analysis for clinical/laboratory/risk score parameters and interleukin-6.
Parametric survival regression, loglogistic distribution, wild-type P values. CRP: C-reactive protein; IL6: Interleukin-6; NTProBNP: N-terminal pro-brain natriuretic peptide; GFR: Glomerular filtration rate.
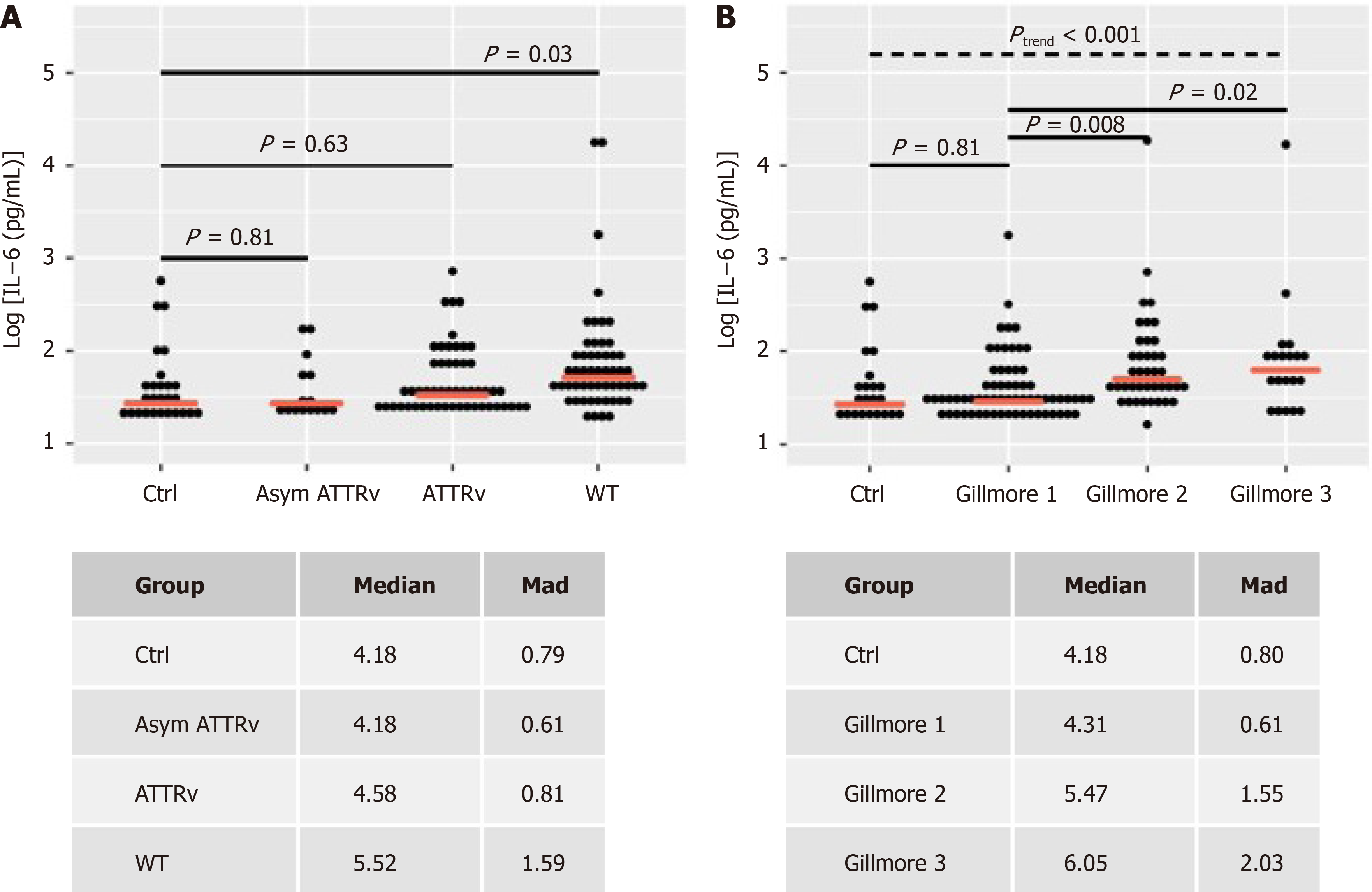
Figure 2 Interleukin-6 values are depicted according to study group or Gillmore class.
A: Study group; B: Gillmore class. Ctrl: Control group; asym ATTRv: Asymptomatic ATTRv; ATTRv: Symptomatic ATTRv amyloidosis; WT: Wild-type ATTR amyloidosis. Linear model P values for two-group comparisons and trend-test (Jonckheere-Terpstra). Median and median absolute deviation (mad) of non-log and non-z transformed data (pg/mL). Red dashed line: Median interleukin-6 value per group.
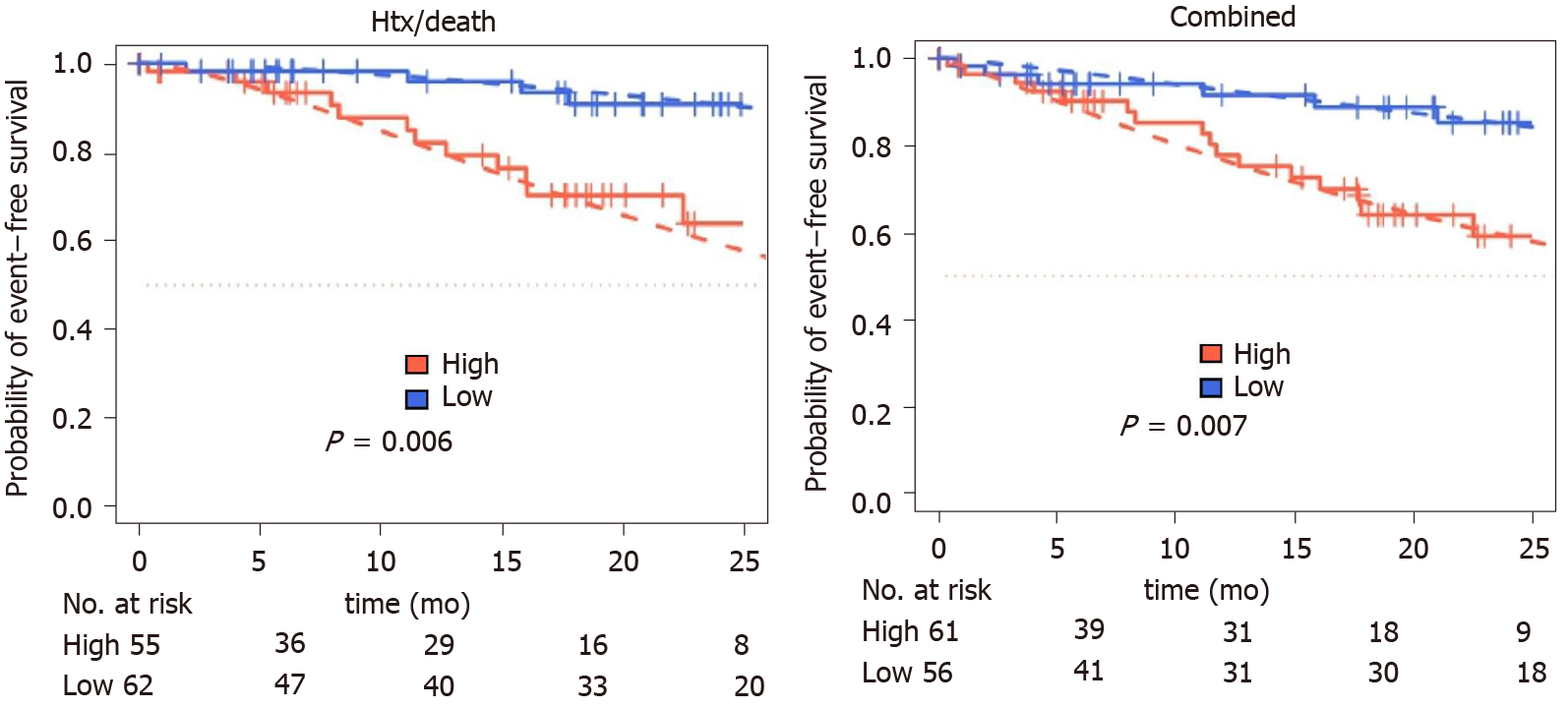
Figure 3 Kaplan-Meier curves for combined, decompensation and death or heart transplantation endpoints for dichotomized interleukin-6.
Dashed: Parametric survival regression (log logistic distribution), wild-type P values.
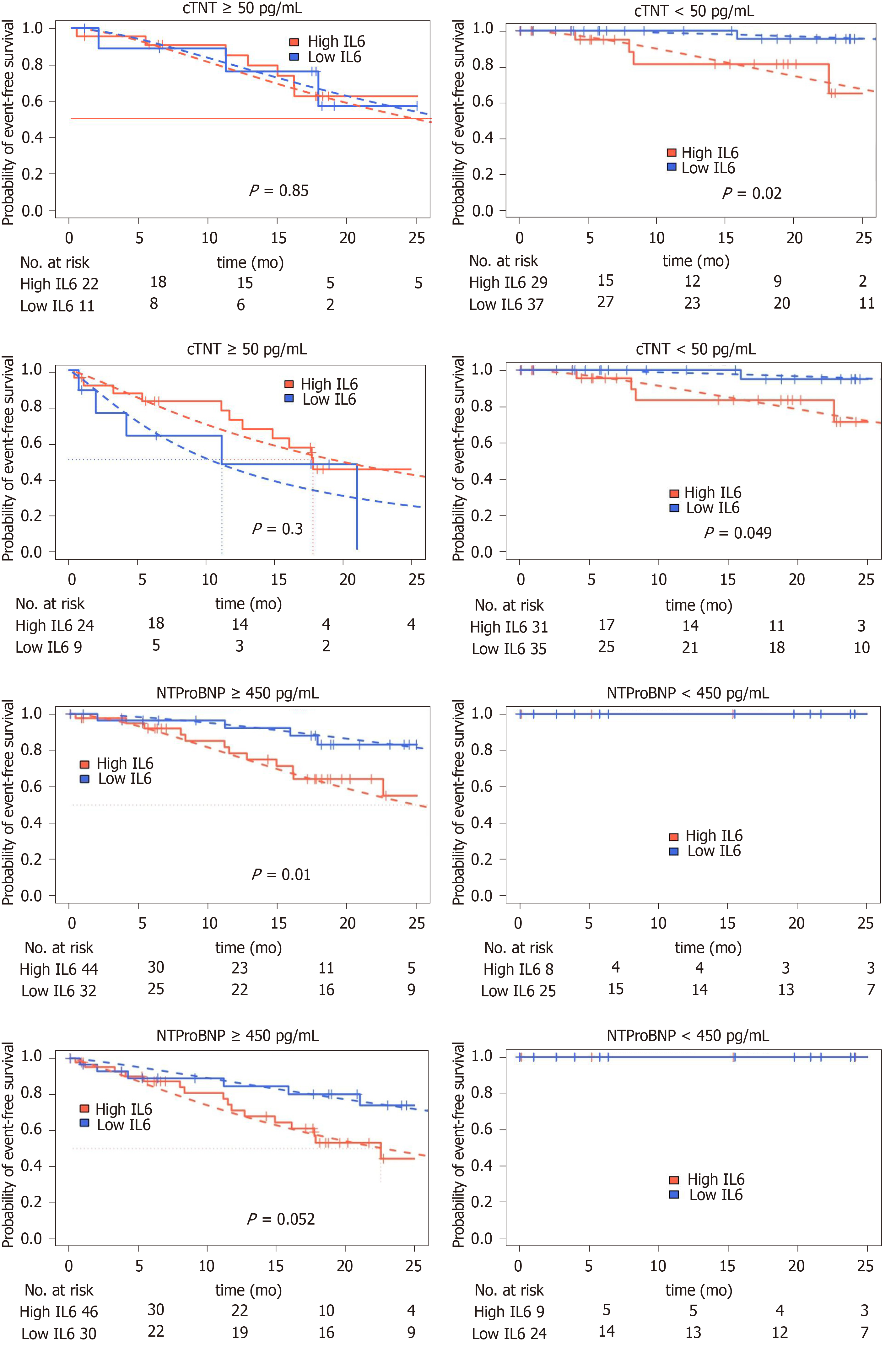
Figure 4 Kaplan-Meier curves for combined, decompensation and death or heart transplantation endpoints for dichotomized interleukin-6 in N-terminal pro-brain natriuretic peptide and TroponinT stratified patients.
Dashed: Parametric survival regression (loglogistic distribution), likelihood ratio P values. cTNT: cardiac TroponinT; IL6: Interleukin-6; NTProBNP: N-terminal pro-brain natriuretic peptide.
- Citation: Hein SJ, Knoll M, Aus dem Siepen F, Furkel J, Schoenland S, Hegenbart U, Katus HA, Kristen AV, Konstandin M. Elevated interleukin-6 levels are associated with impaired outcome in cardiac transthyretin amyloidosis. World J Cardiol 2021; 13(3): 55-67
- URL: https://www.wjgnet.com/1949-8462/full/v13/i3/55.htm
- DOI: https://dx.doi.org/10.4330/wjc.v13.i3.55