Copyright
©The Author(s) 2020.
World J Cardiol. May 26, 2020; 12(5): 192-202
Published online May 26, 2020. doi: 10.4330/wjc.v12.i5.192
Published online May 26, 2020. doi: 10.4330/wjc.v12.i5.192
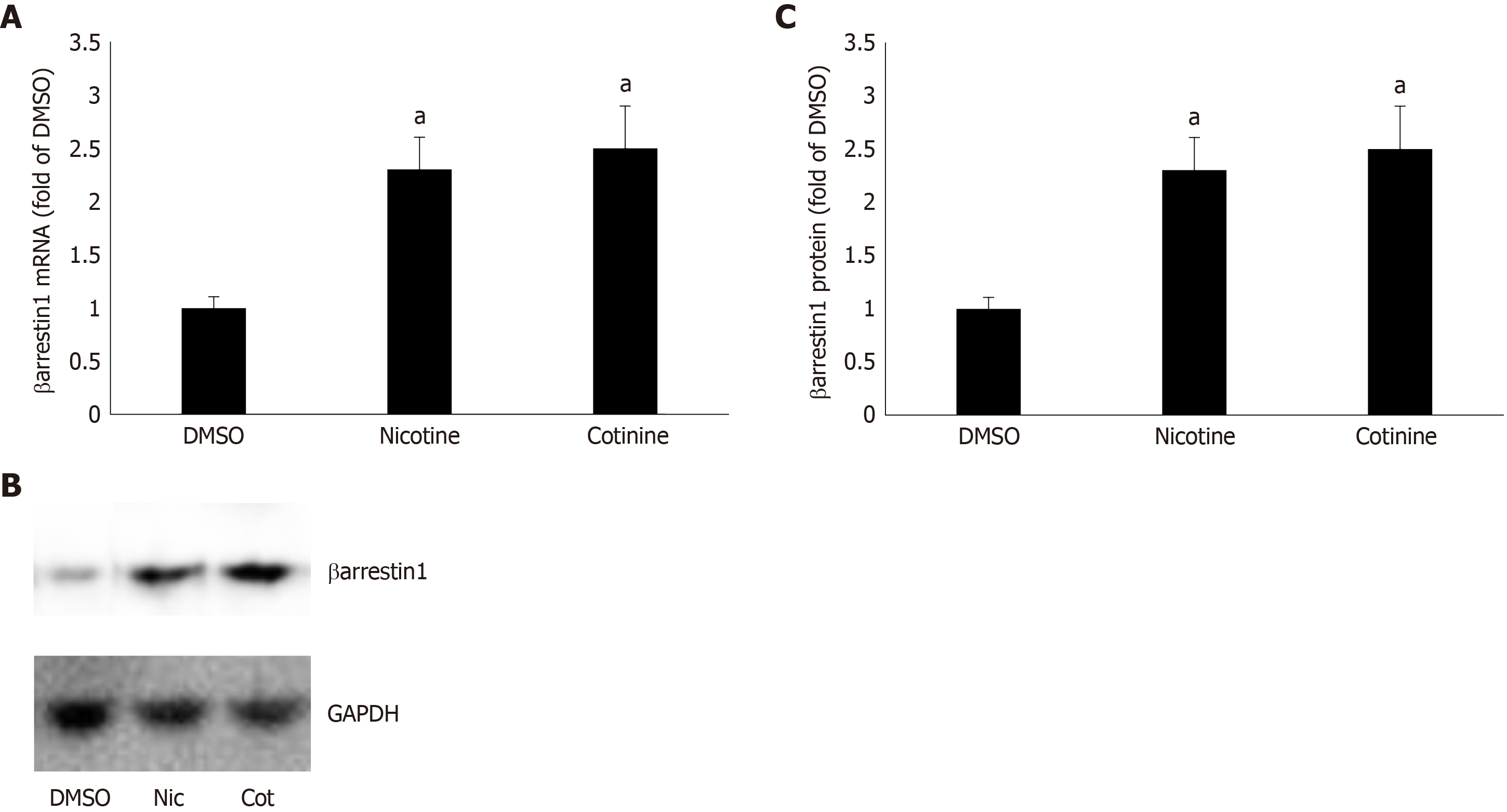
Figure 1 Effect of tobacco compounds on βarrestin1 levels in AZG cells.
A: βarrestin1 mRNA levels in H295R cells treated for 24 h with 10 μmol/L nicotine or 10 μmol/L cotinine or vehicle. B-C: Protein levels in H295R cells treated for 24 h with 10 μmol/L nicotine or 10 μmol/L cotinine or vehicle. Representative western blots are shown in (B), along with glyceraldehyde 3-phosphate dehydrogenase as loading control, and the densitometric quantitation of three independent cell extracts per condition run in duplicate (and normalized with glyceraldehyde 3-phosphate dehydrogenase levels) is shown in (C). aP < 0.05 vs vehicle, n = 3 independent experiments/treatment in duplicate. DMSO: Vehicle; Cot: Cotinine; Nic: Nicotine; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase.
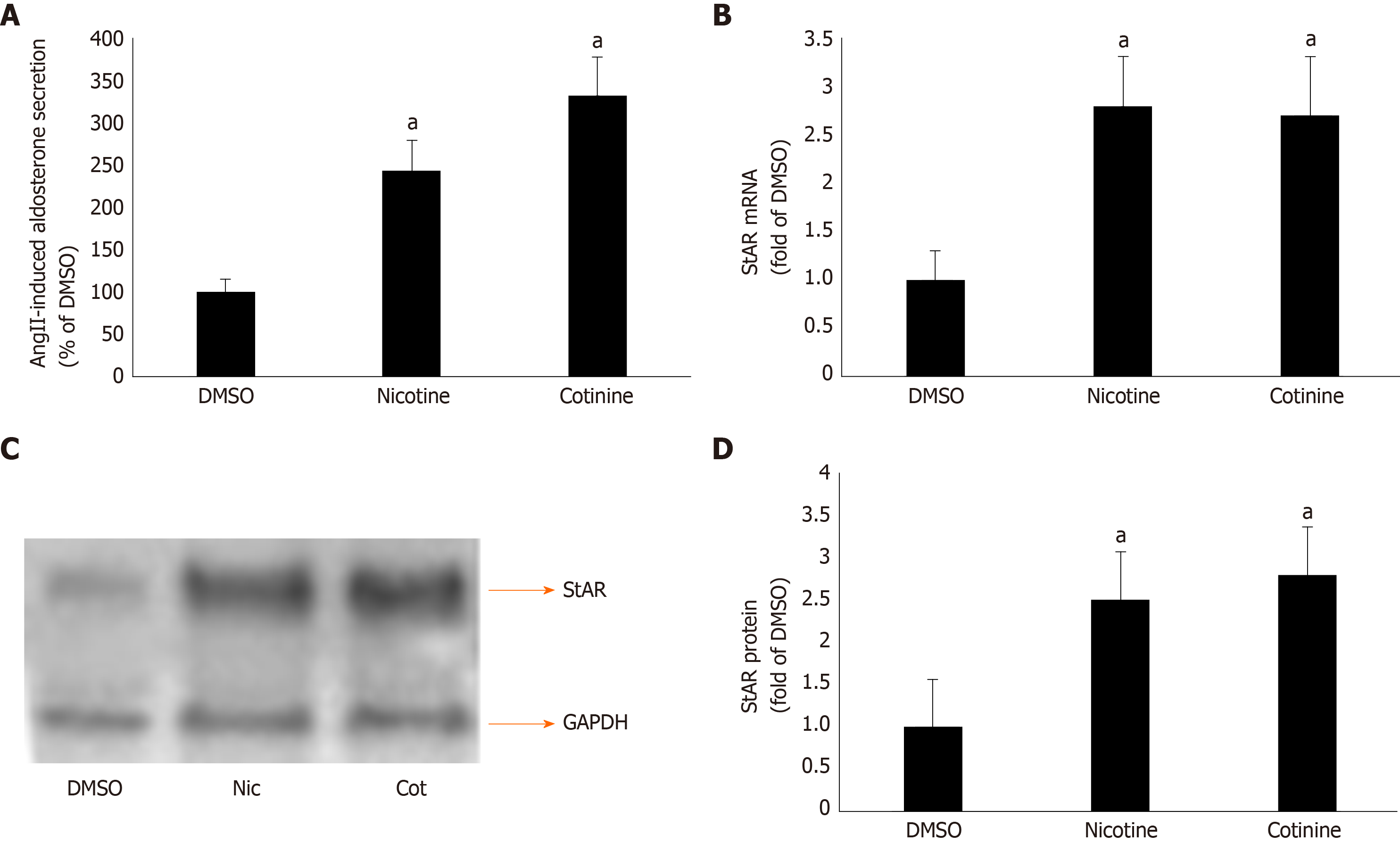
Figure 2 Effect of tobacco compounds on AngII-dependent aldosterone synthesis and secretion in adrenocortical zona glomerulosa cells.
A: Aldosterone secretion into the culture medium from H295R cells in response to a 6-hour-long 100 mmol/L AngII challenge, as measured 24 h post-treatment with 10 μmol/L nicotine or 10 μmol/L cotinine or vehicle (DMSO). Data are expressed as % of the AngII response in DMSO-treated cells. aP < 0.05, vs DMSO; n = 5 independent measurements per treatment performed in duplicate; B: StAR mRNA levels in H295R cells treated for 24 h with 10 μmol/L nicotine (Nic) or 10 μmol/L cotinine (Cot) or vehicle (DMSO). C-D: Protein levels in H295R cells treated for 24 h with 10 mol/L nicotine (Nic) or 10 μmol/L cotinine or DMSO. Representative western blots are shown in (C), along with glyceraldehyde 3-phosphate dehydrogenase as loading control, and the densitometric quantitation of three independent cell extracts per condition run in duplicate (and normalized to glyceraldehyde 3-phosphate dehydrogenase) is shown in (D). aP < 0.05, vs DMSO; n = 3 independent experiments/treatment in duplicate. DMSO: Vehicle; Cot: Cotinine; Nic: Nicotine; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase.
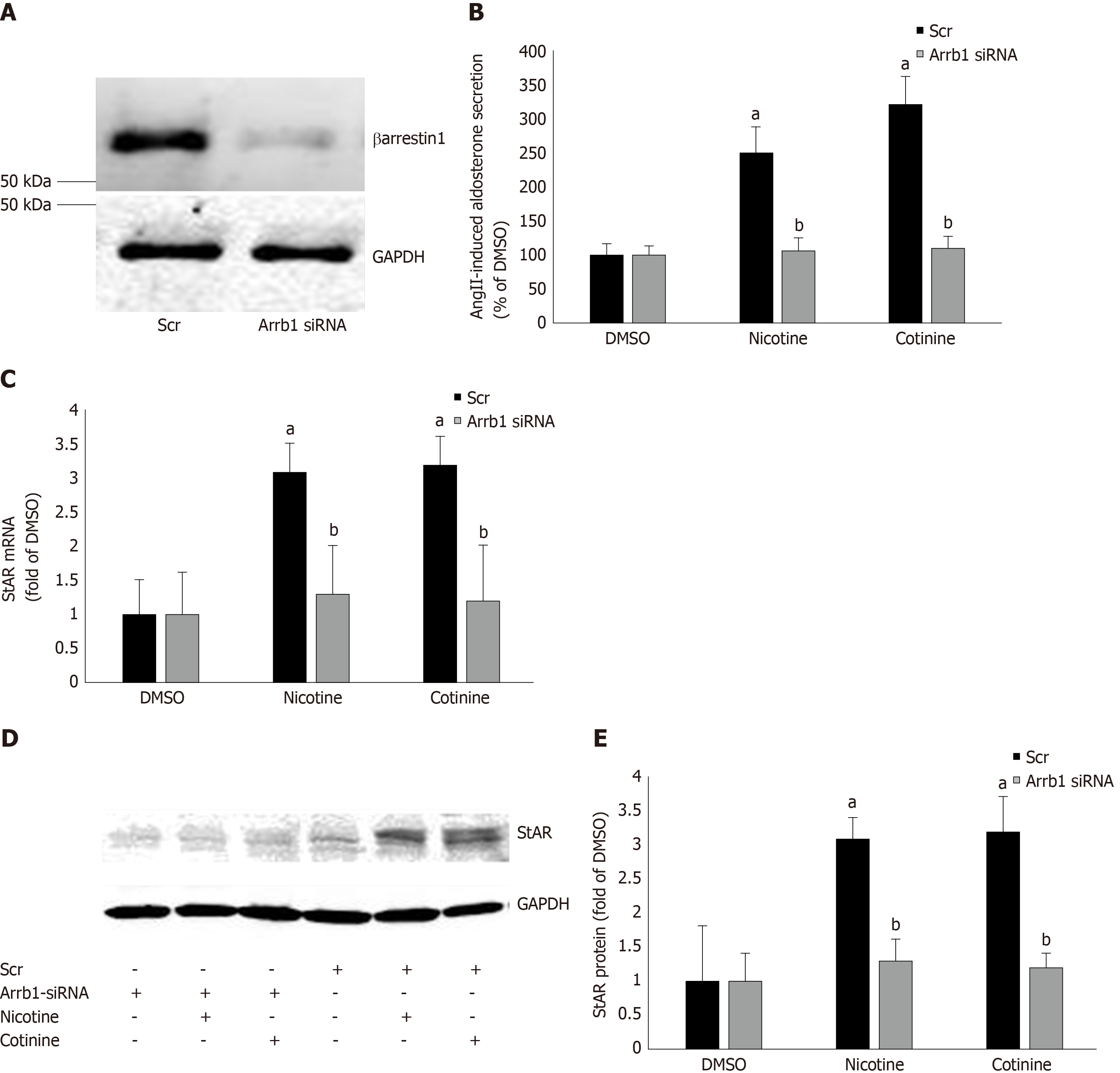
Figure 3 Effect of βarrestin1 knockdown on tobacco-induced aldosterone production in adrenocortical zona glomerulosa cells in vitro.
A: Immunoblotting for βarrestin1 in H295R cell extracts 48 h post-transfection with βarrestin1-sepcific (Arrb1) siRNA or scrambled (Scr) siRNA to test the efficiency of the βarrestin1 siRNA-mediated knockdown. A representative blot of three independent cell extracts run in duplicate is shown, along with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as loading control, confirming an > 80% βarrestin1 protein knockdown; B: Aldosterone secretion into the culture medium from H295R cells in response to a 6-hour-long 100 nmol/L AngII challenge, as measured 24 h post-treatment with 10 µmol/L nicotine or 10µmol/L cotinine or vehicle (DMSO) in H295R cells having Arrb1 siRNA or not (scrambled siRNA-transfected, Scr). Drug treatments were carried out 48 h after siRNA transfection (i.e., AngII was added at 72 h post-transfection). Data are expressed as % of the AngII response in DMSO-treated cells. aP < 0.05, vs DMSO (Arrb1 siRNA or Scr); bP < 0.05, vs Scr; n = 5 independent measurements per treatment performed in duplicate; C: StAR mRNA levels in these cells; D-E: Protein levels in these cells. For StAR immunoblotting, representative blots are shown in (D), along with GAPDH as loading control, and the densitometric quantitation of three independent cell extracts per treatment condition run in duplicate (and normalized to GAPDH) is shown in (E). aP < 0.05, vs DMSO (vehicle); bP < 0.05, vs Scr; n = 3 independent experiments/treatment in duplicate. DMSO: Vehicle; Scr: Scrambled; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase; Arrb1: Arrestin1-sepcific.
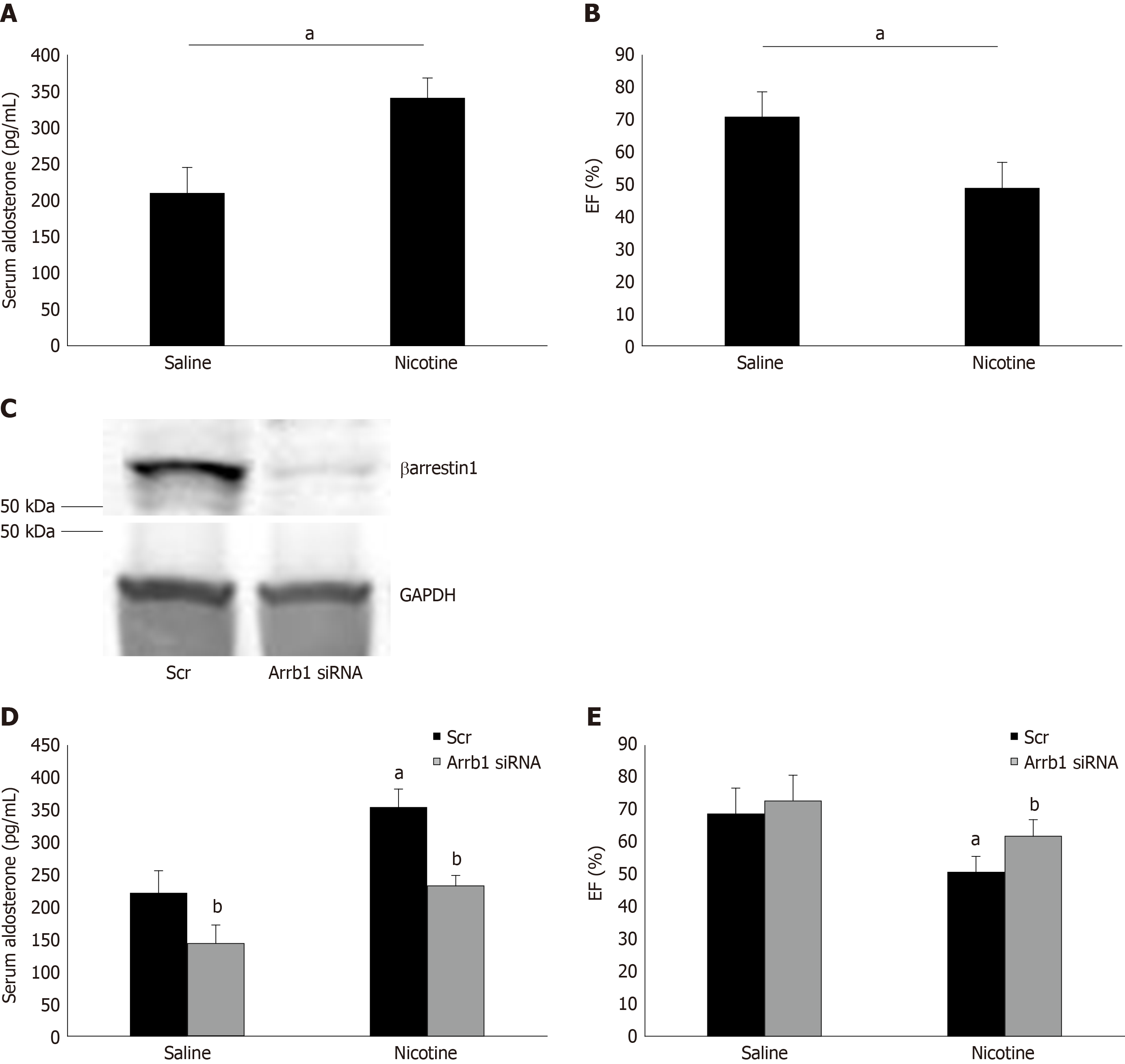
Figure 4 Effect of adrenal βarrestin1 knockdown on nicotine-dependent hyperaldosteronism and cardiac dysfunction in vivo.
A: Circulating aldosterone levels in rats injected i.p. with 1 mg/kg per day nicotine or saline (control) for 7 consecutive d. aP < 0.05; n = 5 rats/group; B: Ejection fraction [EF (%)] of these animals at the end of the saline or nicotine treatments. aP < 0.05; n = 5 rats/group; C: Immunoblotting for βarrestin1 in adrenal protein extracts isolated from rats at 7 d post-injection with βarrestin1-specific siRNA or scrambled (Scr) siRNA directly into their adrenal glands. A representative blot of three independent rat adrenal protein extracts is shown, along with glyceraldehyde 3-phosphate dehydrogenase as loading control, confirming an > 90% βarrestin1 protein knockdown in the adrenal glands in vivo; D: Circulating aldosterone levels of rats having adrenal βarrestin1 knocked-down (rrestin1-specific siRNA) or not (scrambled siRNA-injected, Scr) and treated with 1 mg/ kg per day i.p. nicotine or saline (control) for 7 consecutive d, at the end of these treatments. E: EF (%) of rats having adrenal βarrestin1 knocked-down (rrestin1-specific siRNA) or not (scrambled siRNA-injected, Scr) and treated with 1 mg/kg per day i.p. nicotine or saline (control) for 7 consecutive d, at the end of these treatments. aP < 0.05 vs Saline; bP < 0.05 vs Scr; n = 5 rats/group. Scr: Scrambled; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase; Arrb1: Arrestin1-sepcific; EF (%): Ejection fraction.
- Citation: Cora N, Ghandour J, Pollard CM, Desimine VL, Ferraino KE, Pereyra JM, Valiente R, Lymperopoulos A. Nicotine-induced adrenal beta-arrestin1 upregulation mediates tobacco-related hyperaldosteronism leading to cardiac dysfunction. World J Cardiol 2020; 12(5): 192-202
- URL: https://www.wjgnet.com/1949-8462/full/v12/i5/192.htm
- DOI: https://dx.doi.org/10.4330/wjc.v12.i5.192