Published online May 26, 2017. doi: 10.4331/wjbc.v8.i2.151
Peer-review started: August 29, 2016
First decision: November 17, 2016
Revised: February 20, 2017
Accepted: March 14, 2017
Article in press: March 15, 2017
Published online: May 26, 2017
Processing time: 264 Days and 1.4 Hours
To investigate the modulatory effect of B-1 cells on murine peritoneal macrophages infected with Leishmania major (L. major) in vitro.
Peritoneal macrophages obtained from BALB/c and BALB/c XID mice were infected with L. major and cultured in the presence or absence of B-1 cells obtained from wild-type BALB/c mice. Intracellular amastigotes were counted, and interleukin-10 (IL-10) production was quantified in the cellular supernatants using an enzyme-linked immunosorbent assay. The levels of the lipid mediator prostaglandin E2 (PGE2) were determined using a PGE2 enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI), and the number of lipid bodies was quantified in the cytoplasm of infected macrophages in the presence and absence of B-1 cells. Culturing the cells with selective PGE2-neutralizing drugs inhibited PGE2 production and confirmed the role of this lipid mediator in IL-10 production. In contrast, we demonstrated that B-1 cells derived from IL-10 KO mice did not favor the intracellular growth of L. major.
We report that B-1 cells promote the growth of L. major amastigotes inside peritoneal murine macrophages. We demonstrated that the modulatory effect was independent of physical contact between the cells, suggesting that soluble factor(s) were released into the cultures. We demonstrated in our co-culture system that B-1 cells trigger IL-10 production by L. major-infected macrophages. Furthermore, the increased secretion of IL-10 was attributed to the presence of the lipid mediator PGE2 in supernatants of L. major-infected macrophages. The presence of B-1 cells also favors the production of lipid bodies by infected macrophages. In contrast, we failed to obtain the same effect on parasite replication inside L. major-infected macrophages when the B-1 cells were isolated from IL-10 knockout mice.
Our results show that elevated levels of PGE2 and IL-10 produced by B-1 cells increase L. major growth, as indicated by the number of parasites in cell cultures.
Core tip:This original manuscript describes the modulatory effect of B-1 cells on Leishmania major-infected macrophages. We demonstrated the participation of soluble mediators in a co-culture system and characterized prostaglandin E2 and interleukin-10 (IL-10) as key factors involved in increased intracellular parasite replication. We also demonstrated that cell-cell contact is not important. The same effect was not observed when we used B-1 cells from IL-10 knockout mice, as no significant difference in parasite multiplication was observed. Thus, the current manuscript may be of interest for scientists working in the fields of immunoparasitology or immunomodulation.
- Citation: Arcanjo AF, Nunes MP, Silva-Junior EB, Leandro M, Rocha JDBD, Morrot A, Decote-Ricardo D, Freire-de-Lima CG. B-1 cells modulate the murine macrophage response to Leishmania major infection. World J Biol Chem 2017; 8(2): 151-162
- URL: https://www.wjgnet.com/1949-8454/full/v8/i2/151.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v8.i2.151
B-1 lymphocytes are murine cellular subpopulations that have their development and other characteristics that are distinct from the conventional B-2 cell populations[1]. The B-1 cell population is a minor B-cell compartment that has been identified in mice and is involved primarily in innate immune responses[2]. The interest in this cellular population has increased considerably due to its relationship with leukemia, autoimmunity and auto-reactivity[3-6]. Recently, several manuscripts demonstrated that B-1 cells are able to transform into a type of phagocyte called a B-1 cell-derived phagocyte (B-1CDP) and play a role as antigen presenting cells (APCs)[7-10]. Borrello and Phipps[11] demonstrated that B-1 lymphocytes from the peritoneum of mice differentiate into phagocytic cells that are similar to macrophages.
Leishmania major (L. major) is the causative agent of cutaneous leishmaniasis (CL) in the Old World and is transmitted by the bite of the female phlebotomine sandfly, which injects the infective metacyclic promastigote forms into the dermis of the host[12]. Cutaneous leishmaniasis is characterized by an ulcerative lesion that appears on the skin at the site of the sandfly bite and generally heals spontaneously. In experimental models of susceptibility, L. major infection induces a Th2-type immune response. In models of susceptibility to L. major infection, there is a production of anti-inflammatory mediators, which negatively modulate the response of the vertebrate host, favoring the establishment of infection[13-15]. Graf and collaborators demonstrated that B-1 cells express COX-1 and up-regulate COX-2 and prostaglandin production in response to inflammatory signals. Our group recently demonstrated that B-1 CDP cells are easily infected by L. major and exhibit high susceptibility to infection and that this mechanism is dependent on prostaglandin E2 (PGE2)/interleukin-10 (IL-10) production[16].
Based on these data, we investigated the interaction between B-1 cells and L. major-infected macrophages from BALB/c mice and BALB/c XID mice (a lineage that is genetically depleted of B-1 cells) to elucidate the possible influence of this minor B-cell population on the progression of infection in vitro. We performed experiments to investigate whether B-1 cells could mediate any effect on macrophage susceptibility to infection based on cell direct interaction or cytokine-driven modulation.
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (United States). The protocol was approved by the Committee on the Ethics of Animal Experiments of the Health Science Center of the Federal University of Rio de Janeiro (CEUA-CCS, Permit Number: IBCCF 062/14), and all efforts were made to minimize suffering.
BALB/c and BALB XID (a lineage that is genetically depleted of B-1 cells) mice of both sexes, aged 6-8 wk, were obtained from the Oswaldo Cruz Institute Animal Care Facility (Fiocruz, Rio de Janeiro, Brazil). C57BL/6 IL-10 knockout (KO) and C57BL/6 wild-type mice were kindly donated by Professor João Santana Silva from the Department of Pharmacology, School of Medicine, USP, Ribeirão Preto. L. major strain LV39 (MRHO/Sv/59/P) was isolated each month from the footpads of infected BALB/c mice and maintained in vitro as proliferating promastigotes. The parasites were maintained in Schneider’s medium (Gibco, Life Technologies) supplemented with 10% FCS, 1% glutamine and 2% human urine maintained in the animal facility at the Federal University of Rio de Janeiro (UFRJ).
B-1 cells were isolated from BALB/c mice using the protocol described by Abrahão et al[1]. Briefly, macrophages were harvested via peritoneal lavage of BALB/c mice using cold DMEM medium (Gibco, Life Technologies). The total population of cells from the peritoneum was plated into 25 cm2 tissue culture flasks (Corning) and incubated at 37 °C in a 5% CO2 atmosphere for 120 min. The non-adherent cells were discarded, and DMEM medium containing 2 mmol/L glutamine, 50 μmol/L 2-ME, 10 μg/mL of gentamicin, 1 mmol/L sodium pyruvate, and 100 µmol/L MEM nonessential amino acids plus 10% fetal calf serum (FCS) was added to the adherent monolayer. The cultures were maintained for 5 d without changing the medium under the conditions described above. The non-adherent cell population was composed primarily of B-1 cells, as indicated by flow cytometry (results not shown), whereas the adherent cells represented an enriched macrophage population.
Primary BALB/c or BALB/c XID peritoneal macrophages (2 × 105 cells/well) were cultured in 24-well plates (Corning) containing sterile round glass coverslips (13 mm) and allowed to attach for 2 h at 37 °C in 5% CO2. The adherent macrophages were infected for 24 h with stationary phase L. major promastigotes at a multiplicity of infection of 10:1 (parasite/macrophage) and were then incubated at 37 °C in 5% CO2. After 24 h, the monolayers were washed extensively with warm HBSS (Gibco, Life Technologies) to remove extracellular parasites. All cultures were maintained in medium containing 1% Nutridoma-SP (Roche, Basel, Switzerland) instead of FCS. B-1 cells were added at a 10:1 ratio (B-1 cell/macrophage) in the presence or absence of antibodies, solvents and reagents. After 3 d, infected macrophages monolayers were extensively washed to remove non-phagocytosed promastigotes, and medium was replaced by Schneider medium (Life Technologies), supplemented with 20% FCS and 2% human urine. Infected monolayers were cultured at 26 °C for additional 3 d. The number of motile promastigotes released into the cellular supernatant was evaluated using a Neubauer chamber.
The relative intracellular load of L. major was assessed by counting the number of motile extracellular promastigotes released in each well. Infected peritoneal macrophages cultured on glass coverslips in the presence or absence of B-1 cells for 3 d at 37 °C. After this time the cultures were washed and stained with May-Grunwald Giemsa (Sigma-Aldrich), and intracellular amastigotes were counted in 100 infected macrophages. The results are shown as the number of intracellular amastigotes per macrophages and as the percentage of infected macrophages. All results are presented as the mean and SE of triplicate cultures.
Peritoneal macrophage monolayers were treated with 10 μg/mL of aspirin (Sigma-Aldrich). Aspirin acts as an inhibitor of PGE2 production[17,18]. Neutralizing anti-transforming growth factor) (TGF)-β and normal chicken IgY (R and D System), anti-IL-10 and rat IgG1 isotype control (BioSource Europe, Nivelles) antibodies were used at a concentration of 10 µg/mL.
The concentrations of cytokines in the supernatants obtained from infected cell cultures were quantified after 24 h of incubation using the sandwich immunoassay (ELISA) method according to the methodology recommended by the manufacturer (R and D). The optical density was obtained by reading the absorbance in a plate spectrophotometer (Versamax Microplates Reader Molecular Devices, United States), with a filter of 405 nm. The concentrations of cytokines were calculated from a standard curve of recombinant cytokines. PGE2 was quantified using a PGE2-specific EIA kit, according to the methodology recommended by the manufacturer (Cayman Chemical, Ann Arbor, MI).
To observe lipid bodies (LBs), macrophages were fixed with 3.7% formaldehyde for 10 min and stained by osmium tetroxide. The morphology of fixed cells was observed, and osmium-stained lipid bodies were counted by light microscopy with a 100 × objective lens in 50 consecutively scanned leukocytes.
Statistical analysis was performed using the program GraphPad InStat version 3.01 (San Diego, CA, United States). The data were analyzed using a t-test. Differences with a P value of 0.05 or lower were considered to be significant.
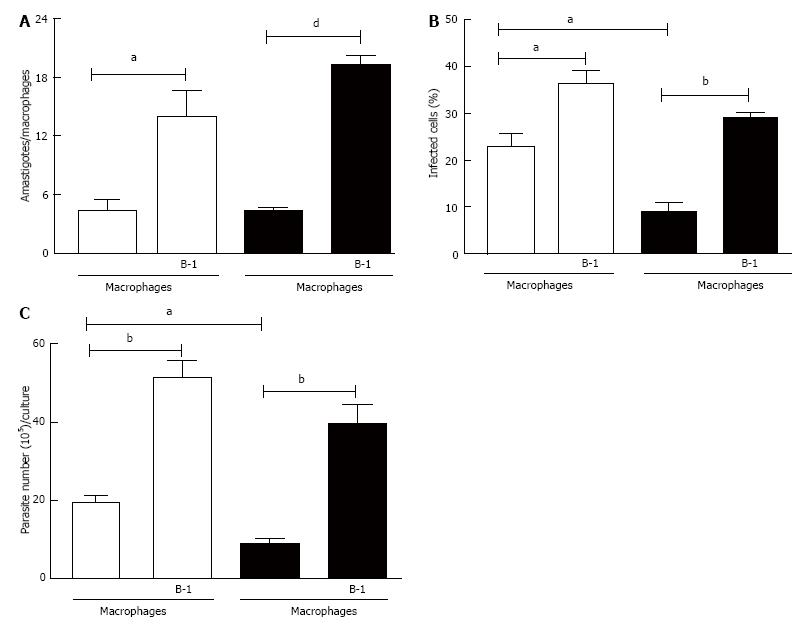
In this study, we analyzed the role of B-1 lymphocytes in modulating the replication of L. major in infected peritoneal macrophages in vitro. B-1 cells obtained from wild-type BALB/c mice and co-cultured with peritoneal macrophages from BALB/c and BALB XID mice infected with L. major promastigotes induced not only accentuated intracellular amastigote growth (Figure 1A) but also an increase in the percentage of infected cells (Figure 1B). The results obtained by counting the number of amastigotes in infected macrophages were confirmed via the quantification of motile promastigote forms in the supernatants after 5 d of interaction between B-1 cells and L. major-infected macrophages (Figure 1C). It is important to mention that XID macrophages were more resistant to infection with L. major than BALB/c macrophages (Figure 1).
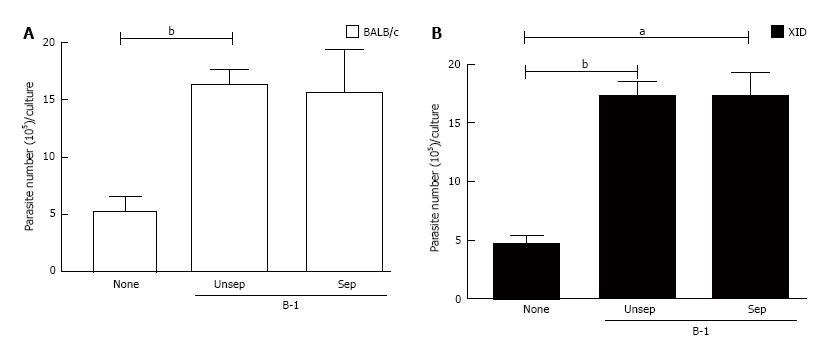
We then assessed whether B-1/macrophage physical contact was required for the increased intracellular amastigote replication that was observed in our co-cultures. Increasing T. cruzi replication in macrophages requires recognition with apoptotic cells, thus directing the process of adhesion and phagocytosis[19-21]. However, when using a Trans-well system in our model of intracellular L. major growth, we found that parasite multiplication was independent of cell contact between B-1 cells and infected macrophages from both types of mice (Figure 2). These results indicate that in our system, the observed parasite growth is mediated by a soluble factor(s) that is released into the cultures.
B-1 cells are known to produce high levels of IL-10[13,22,23]. However, the importance of B-1-derived IL-10 in infected macrophages must be clarified. Recently, our group demonstrated that the IL-10 produced by B-1 CDP cells is important to facilitate intracellular infection and increase the number of motile L. major promastigotes in the supernatants[13]. Based on this information, we investigated whether B-1 cell-derived IL-10 should be related in the susceptibility of macrophages to intracellular infection.
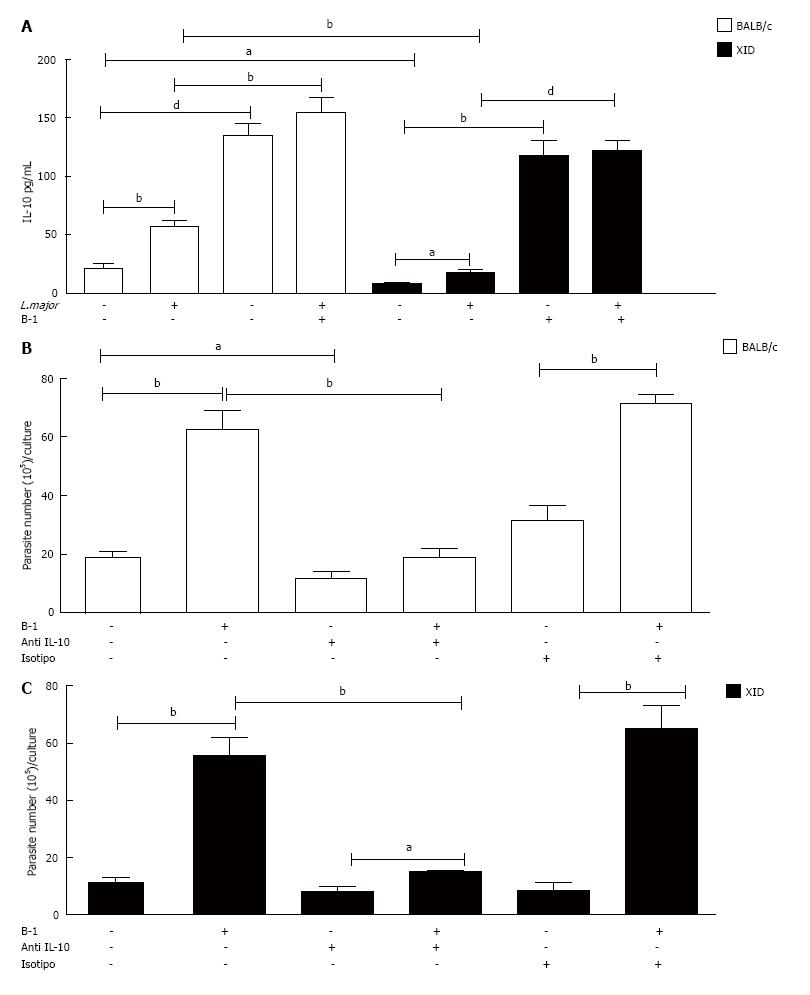
Our data show that the amount of IL-10 in the supernatants of macrophages infected with L. major is increased in the presence of B-1 cells (Figure 3A). The cytokine TGF-β, which is another important mediator involved in the modulation of macrophages infected with intracellular parasites[19-20], was not detected in the present study model (data not shown).
To determine the real importance of B-1-derived IL-10 in L. major-infected macrophages, we added a neutralizing anti-IL-10 antibody to the cell cultures. Our results demonstrated that the neutralization of IL-10 induced an important reduction in the number of promastigotes released into the supernatant (Figure 3B and C). The data previously described demonstrate that the production of this cytokine by B-1 lymphocytes is relevant for increasing parasite growth in peritoneal macrophages.
Lipid bodies (LBs) are organelles that are related in arachidonic acid metabolism and produce lipid mediators in response to inflammatory stimulus[24,25]. Infection with intracellular pathogens alters and increases the number of the lipid bodies[26,27] in infected cells. In recent years, we have described a progress of the number of lipid bodies in phagocytic cells infected with Trypanosoma cruzi and L. major and evidenced the role of these lipid bodies in disease development[13,26].
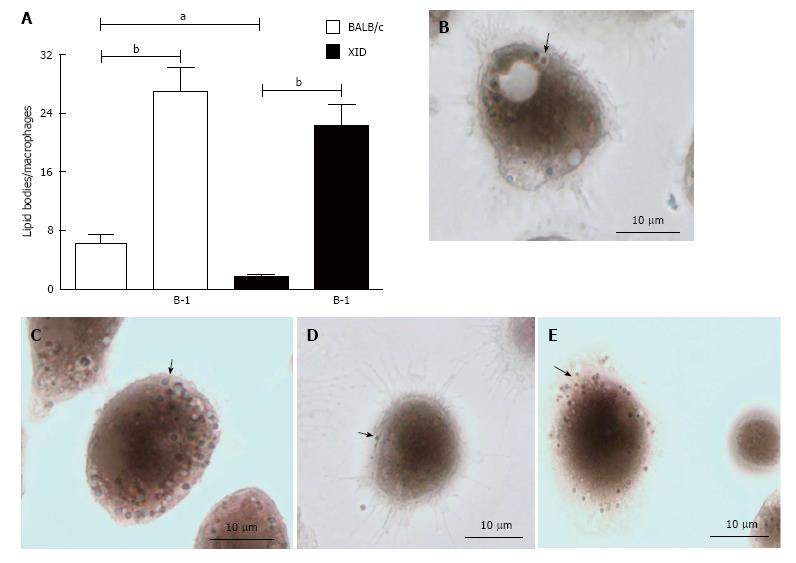
The results shown in Figure 4 demonstrate that infected macrophages co-cultured with B-1 cells contain a large number of lipid bodies in comparison to infected macrophages cultivated without B-1 cells (Figure 4). Our data demonstrate that macrophages from BALB/c mice contain more lipid bodies than XID macrophages, even in absence of a stimulus (Figure 4).
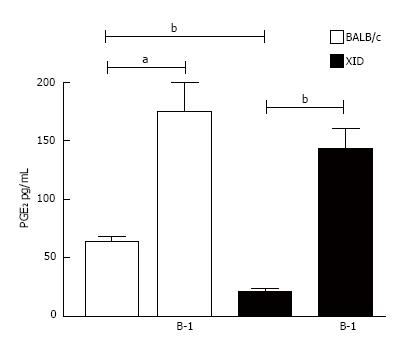
The mechanism of LB formation and PGE2 synthesis during L. major infection in co-cultures of macrophages and B-1 cells was investigated. Phagocytes derived from B-1 cells (B-1CDP cells) constitutively express COX enzymes and produce large amounts of PGE2 in response to inflammatory signals or infection with Leishmania spp.[13]. However, the production of the lipid mediator PGE2 by B-1 cells has not yet been described. Based on this information, we investigated the production of PGE2 by L. major-infected peritoneal macrophages obtained from BALB/c and XID mice. As described in Figure 5, the presence of B-1 cells induced considerable amounts of PGE2 in both types of infected macrophages. Our data also demonstrate that infected macrophages from XID mice produce less PGE2 than macrophages from BALB/c mice, even in the absence of B-1 cells (Figures 5 and 6).
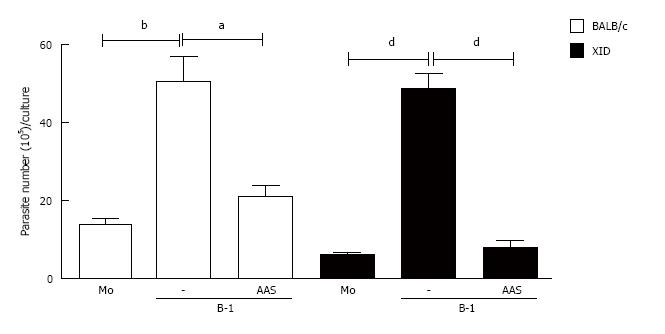
To characterize the importance of PGE2 for parasite load, we added two different non-steroidal anti-inflammatory drugs (NSAIDs) to the B-1 cell/macrophage cultures: aspirin and indomethacin. These two drugs are recognized as potent inhibitors of PGE2 production[19,20]. Our data demonstrate that the blockade of PGE2 production affected the parasite burden inside the macrophages (Figure 6).
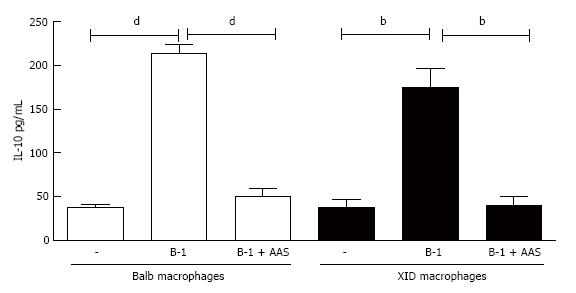
Following the rationale that the inhibition of PGE2 decreased the release of promastigotes into the supernatant of L. major-infected macrophages co-cultured with B-1 cells, we investigated whether that lipid mediator has an effect on IL-10 production. The results shown in Figure 7 reveal that an important reduction of IL-10 production occurred when the cultures were treated with NSAIDs (Figure 7).
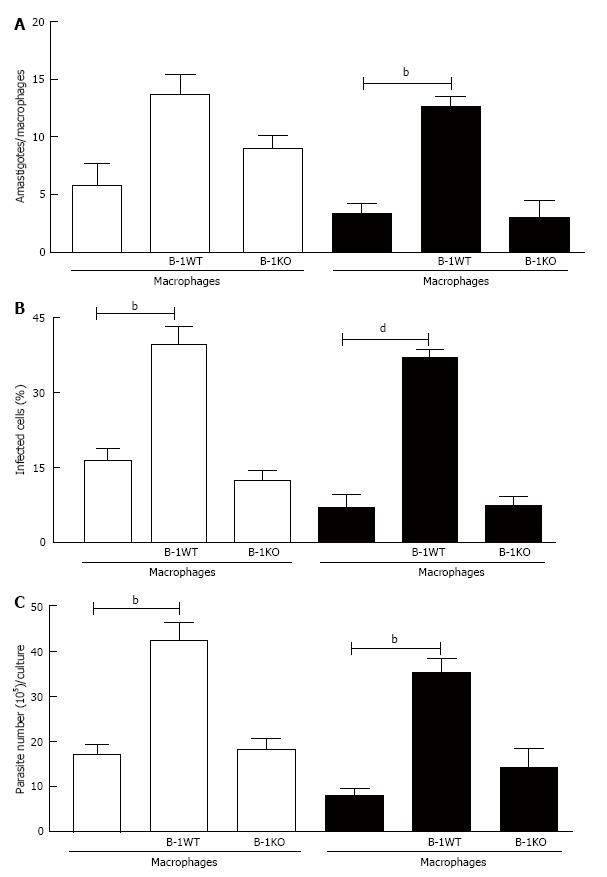
To demonstrate that the presence of the soluble cytokine IL-10 was essential for favoring intracellular infection, we used murine B-1 lymphocytes from IL-10 KO animals. When L. major-infected macrophages were co-cultured with B-1 cells from wild-type mice, the number of intracellular amastigotes (Figure 8A), the percentage of infected macrophages (Figure 8B) and the number of promastigotes released (Figure 8C) all increased. On the other hand, when infected macrophages were co-cultivated with B-1 cells from IL-10 KO mice, the modulatory effect that favors the parasite was not observed. These results confirm the importance of IL-10 released by B-1 cells in the modulation of macrophage infection by L. major.
The findings reported in this manuscript are strengthened by experimental data. We co-cultivated B-1 cells with L. major-infected peritoneal macrophages from BALB/c mice and BALB XID mice, which are devoid of B-1 cells.
B-1 cells are of interest due to the immunomodulatory effects that these cells have exerted in different models of infection and inflammation[2,13,23,28-31]. B-1 cells from the peritoneal cavity migrate to inflammatory and/or infectious sites and release a series of immunomodulatory factors into the environment and also have the ability to differentiate into phagocytes[2,32]. Our group recently demonstrated a role for B-1 cell-derived phagocytes (B-1CDP) in L. major infection in vitro[13].
In the present study, we demonstrate that B-1 lymphocytes are efficient at inducing parasite replication when co-cultured with L. major-infected macrophages. The increased number of amastigotes observed in infected macrophages co-cultured with B-1 cells in our system is related to the number of motile promastigotes in the supernatants. Peritoneal macrophages isolated from XID mice exhibit resistance to infection with L. major when compared to BALB/c mice. However, when B-1 lymphocytes from BALB/c were added to the cultures of infected XID peritoneal macrophages in vitro, the cells became susceptible to infection, in comparison to co-cultures without B-1 cells. B-1 cells are known to produce large amounts of cytokines that could modulate macrophages and promote the intracellular infection of macrophages[13,23]. To determine if cell-cell physical contact was responsible for macrophage infection and parasite replication, we used a cell-impermeable membrane and observed that macrophage infection occurred in the absence of cell contact. This result demonstrates that soluble factors were being secreted into the medium by B-1 cells.
As reported in several studies, B-1 cells can produce large amounts of IL-10, which plays an important immunomodulatory action in different cell types[13,23,33]. IL-10 is an important mediator, which when released acts directly on different cell populations and is known to be an inhibitor of different types of cytokines, which are important factors for the activation of phagocytes[34-37] and consequently suppress the secretion of nitric oxide (NO). NO is an important factor that has leishmanicidal activity and favors the arginase pathway, which is involved in the activity of ornithine decarboxylase[19,38-40]. Arginase would facilitate the intracellular growth of pathogens[19,20].
In addition to IL-10, TGF-β is another cytokine that is important in modulating the intracellular growth of parasites[19,20,26]. TGF-β has been implicated in increased parasite replication in different in vivo and in vitro models of experimental Leishmania infection[41-44]. The results reported in this manuscript strongly suggest that macrophage susceptibility to infection with L. major was related to the production of IL-10 by B-1 cells. Our data also indicated that the TGF-β was not related in the increased intracellular infection (data not shown). The importance of IL-10 produced by B-1 cells was confirmed in our study when we added a neutralizing antibody anti-IL-10 and observed a marked decrease in the number of parasites released into the culture supernatants.
We also found a large number of lipid bodies in the cytoplasm of L. major-infected macrophages co-cultured with B-1 cells. Lipid bodies are lipid-rich cytoplasmic organelles that control the accumulation and hydrolysis of neutral lipids and are found primarily in adipocytes[45,46]. Macrophages were previously reported to have few lipid bodies in their cytoplasm, but the number of lipid bodies in these phagocytic cells can increase after stimulation[13,26]. T. cruzi and Leishmania are able to induce lipid bodies in the cytoplasm of infected macrophages[13,26]. It is already known that lipid bodies can be used as lipid sources that favor parasite replication or contribute to the production of soluble mediators involved in inflammation[47]. Lipid bodies store arachidonic acid, which is important for the production of lipid mediators[47].
Our data demonstrate that the number of lipid bodies in the cytoplasm of infected macrophages increased when these cells were co-cultured with B-1 cells. In addition to the increased number of lipid bodies, we also observed strong production of PGE2 in the co-cultures. Our data also show that the inhibition of PGE2 modulated the infection, resulting in a decrease in the number of promastigote forms released in co-cultures of infected macrophages and B-1 cells. PGE2 is a lipid mediator that plays an important role in the production of factors, such as TGF-β[19] and IL-10[13].
Our group recently described the production of anti-inflammatory mediators in experimental T. cruzi and Leishmania models, resulting in the inhibition of leishmanicidal products[13,19].
PGE2 is an important modulator of the T lymphocytes activation and the production of NO by phagocytes and favors the infection of intracellular parasites[19,21]. In addition, PGE2 is essential for increasing parasite growth in macrophages that have ingested apoptotic cells[19,21]. The presence of the lipid mediator PGE2 acts as a potent inhibitor of not only the immune response mediated by T lymphocytes but also the production of microbicidal factors by macrophages, favoring the growth of intracellular parasites[13,19,21]. In addition, PGE2 is a key lipid mediator that has been implicated in T. cruzi amastigote proliferation inside macrophages that have phagocytosed apoptotic bodies[19-21,26].
Our results demonstrate that the production of IL-10 by B-1 cells is involved in the immunomodulatory mechanism in infected macrophages and consequently favors the replication of intracellular parasites. This result clarifies the involvement of IL-10 produced by B-1 cells as the key factor for the modulation of macrophage activation. A similar result was obtained by Arcanjo et al[13], who used B-1CDP phagocytes from IL-10 KO mice and observed that intracellular infection with L. major was impaired. Another elegant work reported similar data in the context of in vitro infection by Coxiella burnetii. The authors observed that the macrophages of XID mice, which present a defect in the production of B-1 lymphocytes, have a better resistance to intracellular infection by C. burnetti when compared to the macrophages of wild-type mice[48].
In summary, our work demonstrates that one role of the B-1 cell population is to produce an anti-inflammatory cytokine and a lipid mediator that exert their effects on macrophages of the innate immune system, contributing to L. major growth and replication in vitro, probably by inhibition of NO and ROS production. Further studies should be addressed to investigate the importance of B-1 cells in the lesion sites highlighting a possible clinical significance of these cells in the infection.
During infections the pathogens subvert the immune responses to their purpose. This is well demonstrated in chronic persistent infection mediated by Leishmania species. This parasite is adapted to grow inside macrophage where they are maintained during the parasitism of the host. Herein, the authors show that infection by Leishmania major (L. major) triggers the suppressive role of B-1 cells responsible to secrete interleukin-10 (IL-10) cytokine upon contact with infected macrophages. This immunomodulatory cytokine is shown to deactivate macrophage innate responses thus favoring the parasite burst in the host.
The findings shown in the present study are of relevance to the immunoparasitology field.
The knowledge of the host-parasite interplay is crucial to design therapies aimed at controlling pathogen infections. Here the authors show that L. major parasites induce immunosuppression of macrophage cells which is important to their maintenance in the host.
The authors’ main findings point to a role of B-1 cells mediating the parasite subversion of the host´s immune defenses. Further studies should be focused to investigate the molecular mechanisms involved in this immunomodulatory response.
Parasite subversion: Inhibition of protective immunity of the host to favor the parasitism; Chronic persistent infection: A sort of pathogens are able to subvert the host immune responses in order to establish latent infections; Immunomodulatory response: The immune system is counter balanced by homeostatic responses in order to avoid its overactivity; B-1 cells: B lymphocyte subset displaying functional properties different from the conventional B cell population (B-2 cells). B-1 cells are thought to mediate immunomodulatory responses by IL-10 and prostaglandin E2 (PGE2) secretion.
The authors investigate the immunomodulatory effect of B-1 cells in L. major infected macrophages. Results suggest that PGE2 and IL-10 released from B-1 cells increase intracellular parasite replication. The manuscript is generally well-written.
Manuscript source: Invited manuscript
Specialty type: Biochemistry and molecular biology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Capasso R, Singh AP, Tabarkiewicz J, Yeligar SM, Zhang HT S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Abrahão TB, Freymüller E, Mortara RA, Lopes JD, Mariano M. Morphological characterization of mouse B-1 cells. Immunobiology. 2003;208:401-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Lopes JD, Mariano M. B-1 cell: the precursor of a novel mononuclear phagocyte with immuno-regulatory properties. An Acad Bras Cienc. 2009;81:489-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Hardy RR. B-1 B cell development. J Immunol. 2006;177:2749-2754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 176] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 4. | Hayakawa K, Hardy RR, Parks DR, Herzenberg LA. The “Ly-1 B” cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med. 1983;157:202-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 589] [Cited by in RCA: 646] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 5. | Boumsell L, Bernard A, Lepage V, Degos L, Lemerle J, Dausset J. Some chronic lymphocytic leukemia cells bearing surface immunoglobulins share determinants with T cells. Eur J Immunol. 1978;8:900-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 98] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Casali P, Burastero SE, Nakamura M, Inghirami G, Notkins AL. Human lymphocytes making rheumatoid factor and antibody to ssDNA belong to Leu-1+ B-cell subset. Science. 1987;236:77-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 381] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 7. | Popi AF, Motta FL, Mortara RA, Schenkman S, Lopes JD, Mariano M. Co-ordinated expression of lymphoid and myeloid specific transcription factors during B-1b cell differentiation into mononuclear phagocytes in vitro. Immunology. 2009;126:114-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Almeida SR, Aroeira LS, Frymuller E, Dias MA, Bogsan CS, Lopes JD, Mariano M. Mouse B-1 cell-derived mononuclear phagocyte, a novel cellular component of acute non-specific inflammatory exudate. Int Immunol. 2001;13:1193-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 96] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Barbeiro DF, Barbeiro HV, Faintuch J, Ariga SK, Mariano M, Popi AF, de Souza HP, Velasco IT, Soriano FG. B-1 cells temper endotoxemic inflammatory responses. Immunobiology. 2011;216:302-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Popi AF, Osugui L, Perez KR, Longo-Maugéri IM, Mariano M. Could a B-1 cell derived phagocyte “be one” of the peritoneal macrophages during LPS-driven inflammation? PLoS One. 2012;7:e34570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Borrello MA, Phipps RP. Fibroblast-secreted macrophage colony-stimulating factor is responsible for generation of biphenotypic B/macrophage cells from a subset of mouse B lymphocytes. J Immunol. 1999;163:3605-3611. [PubMed] |
| 12. | Cunningham AC. Parasitic adaptive mechanisms in infection by leishmania. Exp Mol Pathol. 2002;72:132-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 153] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Arcanjo AF, LaRocque-de-Freitas IF, Rocha JD, Zamith D, Costa-da-Silva AC, Nunes MP, Mesquita-Santos FP, Morrot A, Filardy AA, Mariano M. The PGE2/IL-10 Axis Determines Susceptibility of B-1 Cell-Derived Phagocytes (B-1CDP) to Leishmania major Infection. PLoS One. 2015;10:e0124888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Filardy AA, Pires DR, Nunes MP, Takiya CM, Freire-de-Lima CG, Ribeiro-Gomes FL, DosReis GA. Proinflammatory clearance of apoptotic neutrophils induces an IL-12(low)IL-10(high) regulatory phenotype in macrophages. J Immunol. 2010;185:2044-2050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 175] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 15. | Liese J, Schleicher U, Bogdan C. The innate immune response against Leishmania parasites. Immunobiology. 2008;213:377-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Graf BA, Nazarenko DA, Borrello MA, Roberts LJ, Morrow JD, Palis J, Phipps RP. Biphenotypic B/macrophage cells express COX-1 and up-regulate COX-2 expression and prostaglandin E(2) production in response to pro-inflammatory signals. Eur J Immunol. 1999;29:3793-3803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Pinheiro RO, Nunes MP, Pinheiro CS, D’Avila H, Bozza PT, Takiya CM, Côrte-Real S, Freire-de-Lima CG, DosReis GA. Induction of autophagy correlates with increased parasite load of Leishmania amazonensis in BALB/c but not C57BL/6 macrophages. Microbes Infect. 2009;11:181-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Ribeiro-Gomes FL, Otero AC, Gomes NA, Moniz-De-Souza MC, Cysne-Finkelstein L, Arnholdt AC, Calich VL, Coutinho SG, Lopes MF, DosReis GA. Macrophage interactions with neutrophils regulate Leishmania major infection. J Immunol. 2004;172:4454-4462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 172] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 19. | Freire-de-Lima CG, Nascimento DO, Soares MB, Bozza PT, Castro-Faria-Neto HC, de Mello FG, DosReis GA, Lopes MF. Uptake of apoptotic cells drives the growth of a pathogenic trypanosome in macrophages. Nature. 2000;403:199-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 344] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 20. | Luna-Gomes T, Filardy AA, Rocha JD, Decote-Ricardo D, LaRocque-de-Freitas IF, Morrot A, Bozza PT, Castro-Faria-Neto HC, DosReis GA, Nunes MP. Neutrophils increase or reduce parasite burden in Trypanosoma cruzi-infected macrophages, depending on host strain: role of neutrophil elastase. PLoS One. 2014;9:e90582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Lopes MF, Freire-de-Lima CG, DosReis GA. The macrophage haunted by cell ghosts: a pathogen grows. Immunol Today. 2000;21:489-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Popi AF, Lopes JD, Mariano M. Interleukin-10 secreted by B-1 cells modulates the phagocytic activity of murine macrophages in vitro. Immunology. 2004;113:348-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Geraldo MM, Costa CR, Barbosa FM, Vivanco BC, Gonzaga WF, Novaes E Brito RR, Popi AF, Lopes JD, Xander P. In vivo and in vitro phagocytosis of Leishmania (Leishmania) amazonensis promastigotes by B-1 cells. Parasite Immunol. 2016;38:365-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Bozza PT, Bakker-Abreu I, Navarro-Xavier RA, Bandeira-Melo C. Lipid body function in eicosanoid synthesis: an update. Prostaglandins Leukot Essent Fatty Acids. 2011;85:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 25. | Toledo DA, Roque NR, Teixeira L, Milán-Garcés EA, Carneiro AB, Almeida MR, Andrade GF, Martins JS, Pinho RR, Freire-de-Lima CG. Lipid Body Organelles within the Parasite Trypanosoma cruzi: A Role for Intracellular Arachidonic Acid Metabolism. PLoS One. 2016;11:e0160433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | D’Avila H, Freire-de-Lima CG, Roque NR, Teixeira L, Barja-Fidalgo C, Silva AR, Melo RC, Dosreis GA, Castro-Faria-Neto HC, Bozza PT. Host cell lipid bodies triggered by Trypanosoma cruzi infection and enhanced by the uptake of apoptotic cells are associated with prostaglandin E₂ generation and increased parasite growth. J Infect Dis. 2011;204:951-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 27. | Araújo-Santos T, Prates DB, Andrade BB, Nascimento DO, Clarêncio J, Entringer PF, Carneiro AB, Silva-Neto MA, Miranda JC, Brodskyn CI. Lutzomyia longipalpis saliva triggers lipid body formation and prostaglandin E₂ production in murine macrophages. PLoS Negl Trop Dis. 2010;4:e873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Oliveira HC, Popi AF, Bachi AL, Nonogaki S, Lopes JD, Mariano M. B-1 cells modulate the kinetics of wound-healing process in mice. Immunobiology. 2010;215:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Russo RT, Mariano M. B-1 cell protective role in murine primary Mycobacterium bovis bacillus Calmette-Guerin infection. Immunobiology. 2010;215:1005-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Noal V, Santos S, Ferreira KS, Almeida SR. Infection with Paracoccidioides brasiliensis induces B-1 cell migration and activation of regulatory T cells. Microbes Infect. 2016;18:798-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Gambero M, Teixeira D, Butin L, Ishimura ME, Mariano M, Popi AF, Longo-Maugéri IM. Propionibacterium acnes induces an adjuvant effect in B-1 cells and affects their phagocyte differentiation via a TLR2-mediated mechanism. Immunobiology. 2016;221:1001-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Popi AF, Longo-Maugéri IM, Mariano M. An Overview of B-1 Cells as Antigen-Presenting Cells. Front Immunol. 2016;7:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 33. | De-Gennaro LA, Popi AF, Almeida SR, Lopes JD, Mariano M. B-1 cells modulate oral tolerance in mice. Immunol Lett. 2009;124:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4725] [Cited by in RCA: 4961] [Article Influence: 206.7] [Reference Citation Analysis (0)] |
| 35. | Kamanaka M, Kim ST, Wan YY, Sutterwala FS, Lara-Tejero M, Galán JE, Harhaj E, Flavell RA. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity. 2006;25:941-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 343] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 36. | Joss A, Akdis M, Faith A, Blaser K, Akdis CA. IL-10 directly acts on T cells by specifically altering the CD28 co-stimulation pathway. Eur J Immunol. 2000;30:1683-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 37. | Padigel UM, Alexander J, Farrell JP. The role of interleukin-10 in susceptibility of BALB/c mice to infection with Leishmania mexicana and Leishmania amazonensis. J Immunol. 2003;171:3705-3710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 38. | Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1219] [Cited by in RCA: 1357] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 39. | Raber P, Ochoa AC, Rodríguez PC. Metabolism of L-arginine by myeloid-derived suppressor cells in cancer: mechanisms of T cell suppression and therapeutic perspectives. Immunol Invest. 2012;41:614-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 546] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 40. | Munder M, Choi BS, Rogers M, Kropf P. L-arginine deprivation impairs Leishmania major-specific T-cell responses. Eur J Immunol. 2009;39:2161-2172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 41. | Chowdhury BP, Das S, Majumder S, Halder K, Ghosh S, Biswas S, Bandyopadhyay S, Majumdar S. Immunomodulation of host-protective immune response by regulating Foxp3 expression and Treg function in Leishmania-infected BALB/c mice: critical role of IRF1. Pathog Dis. 2015;73:ftv063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Clemente AM, Severini C, Castronovo G, Tanturli M, Perissi E, Cozzolino F, Torcia MG. Effects of soluble extracts from Leishmania infantum promastigotes, Toxoplasma gondii tachyzoites on TGF-β mediated pathways in activated CD4+ T lymphocytes. Microbes Infect. 2014;16:778-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | França-Costa J, Van Weyenbergh J, Boaventura VS, Luz NF, Malta-Santos H, Oliveira MC, Santos de Campos DC, Saldanha AC, dos-Santos WL, Bozza PT. Arginase I, polyamine, and prostaglandin E2 pathways suppress the inflammatory response and contribute to diffuse cutaneous leishmaniasis. J Infect Dis. 2015;211:426-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 44. | Barral A, Teixeira M, Reis P, Vinhas V, Costa J, Lessa H, Bittencourt AL, Reed S, Carvalho EM, Barral-Netto M. Transforming growth factor-beta in human cutaneous leishmaniasis. Am J Pathol. 1995;147:947-954. [PubMed] |
| 45. | Li L, Zhang H, Wang W, Hong Y, Wang J, Zhang S, Xu S, Shu Q, Li J, Yang F. Comparative proteomics reveals abnormal binding of ATGL and dysferlin on lipid droplets from pressure overload-induced dysfunctional rat hearts. Sci Rep. 2016;6:19782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 46. | Shapiro H, Pecht T, Shaco-Levy R, Harman-Boehm I, Kirshtein B, Kuperman Y, Chen A, Blüher M, Shai I, Rudich A. Adipose tissue foam cells are present in human obesity. J Clin Endocrinol Metab. 2013;98:1173-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 47. | Bozza PT, Payne JL, Morham SG, Langenbach R, Smithies O, Weller PF. Leukocyte lipid body formation and eicosanoid generation: cyclooxygenase-independent inhibition by aspirin. Proc Natl Acad Sci USA. 1996;93:11091-11096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 94] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 48. | Freire-de-Lima CG, Xiao YQ, Gardai SJ, Bratton DL, Schiemann WP, Henson PM. Apoptotic cells, through transforming growth factor-beta, coordinately induce anti-inflammatory and suppress pro-inflammatory eicosanoid and NO synthesis in murine macrophages. J Biol Chem. 2006;281:38376-38384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 248] [Article Influence: 13.1] [Reference Citation Analysis (0)] |