Peer-review started: July 14, 2016
First decision: September 12, 2016
Revised: October 29, 2016
Accepted: December 7, 2016
Article in press: December 9, 2016
Published online: February 26, 2017
Processing time: 233 Days and 8.3 Hours
Persistent postsurgical pain is a serious issue in public health, which has received increased interest in recent years. Previous studies have reported that psychological factors promote the development of chronic postsurgical pain. However, it is unclear how chronification of postsurgical pain occurs. The α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor (AMPA) phosphorylation in the central nervous system plays a critical role in synaptic plasticity and contributes to central sensitization and chronic pain development. Here, we discuss the role of AMPA receptor regulation in stress-induced pain chronification after surgery.
Core tip: The α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor phosphorylation contributes to stress-induced pain chronification after surgery.
- Citation: Liu S, Tao F. Role of α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor regulation in stress-induced pain chronification. World J Biol Chem 2017; 8(1): 1-3
- URL: https://www.wjgnet.com/1949-8454/full/v8/i1/1.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v8.i1.1
After surgery, patients experience either short-term or long-lasting pain. In some patients, pain post-surgery can persist even after surgical incision has recovered. Chronic postsurgical pain is primarily neuropathic in nature. As we know, acute postoperative pain is an adaptive response to surgical damage, but chronic postsurgical pain is maladaptive since it is not protective. However, the central mechanisms underlying pain chronification after surgery remain to be illustrated[1].
Pain chronification after surgery is a process involving multiple biological systems[2]. Chronic postsurgical pain provides a special opportunity to understand pathogenic mechanisms for the transition from acute to chronic pain. Previous studies have indicated that psychological factors promote the development of chronic postsurgical pain[3,4]. Psychological stress can disturb the physiological homeostasis of an organism[5,6]. People who experience stress in their early life or even before birth may have chronic susceptibility to developing pain in their whole life[7]. Thus, it is very possible that early stress could cause permanent changes in pain signaling in the central nervous system. In addition, surgery produces the release of inflammatory mediators, such as prostaglandins and cytokines[8], which can sensitize primary sensory afferents. Stress might increase sensitivity to the hyperalgesic effects of proinflammatory cytokines[9]. Moreover, stress-induced hyperalgesic priming, a neuroplastic change in primary afferent nociceptors, has been implicated in chronic generalized pain syndromes and other chronic pain conditions[10-15]. Therefore, stress may be involved in the development of chronic pain after surgery.
Psychological stress produces physiological and behavioral changes that cause long-term adaptive responses[5]. Two major reactions can occur in response to stress. One reaction after stress is rapid activation of the autonomic nervous system and subsequent release of the stress hormones epinephrine and norepinephrine[16]. Norepinephrine can activate cAMP-dependent protein kinase and calcium/calmodulin-dependent protein kinase II[17,18], which can phosphorylate α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor subunit GluA1 at the Ser845 and Ser831 sites, respectively[19-21]. Targeted mutant mice with the mutations of these phosphorylation sites that block phosphorylation at Ser845 and Ser831 sites of GluA1 display impairment in synaptic plasticity and learning[22]. Thus, stress through releasing the stress hormone norepinephrine can induce GluA1 phosphorylation at the Ser831 and Ser845 sites and then facilitate long-term potentiation induction[23]. Phosphorylation at these sites is sufficient to reduce the threshold for GluA1 synaptic incorporation during long-term potentiation[23]. Another reaction after stress is the stimulation of the hypothalamus-pituitary-adrenal axis and subsequent release of the stress hormone glucocorticoids (a type of corticosteroid hormone) from the adrenal glands[16]. Glucocorticoids are able to bind to two types of receptors in the central nervous system: Mineralocorticoid receptors and glucocorticoid receptors. Mineralocorticoid receptors have a high affinity for corticosterone (the main glucocorticoid in rodents) and are bound when the hormone level is low. Glucocorticoid receptors have a lower affinity for corticosterone than do mineralocorticoid receptors, which are activated only when the hormone level is high enough[16]. Both mineralocorticoid receptors and glucocorticoid receptors are expressed in the central nervous system[16,24]. By activating the two receptor subtypes, corticosterone rapidly and persistently regulates AMPA receptor GluA2 trafficking, which plays an important role in synaptic transmission and plasticity[5,25]. Therefore, the stress hormone corticosterone can effectively enhance the synaptic content of AMPA receptors and then produce synaptic potentiation[5,25].
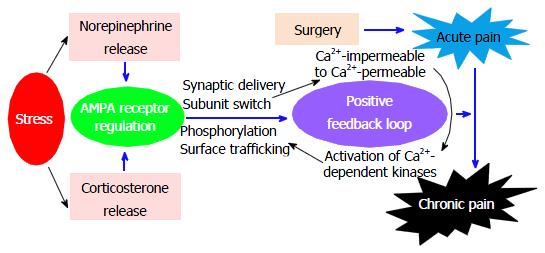
After injury and injury-induced pain, a “supersystem” consisting of nervous system, endocrine system, and immune system may act together to regulate the functional activities in these systems[26]. Thus, the development of chronic postsurgical pain could be caused by dysregulation of the supersystem. For instance, the nervous system and endocrine system can cooperate in the response to stress, which has been referred to as the neuroendocrine stress response[26]. Recently, we have utilized these concepts to develop a new animal model to study acute-to-chronic pain transition after surgery. In this model, we found that social defeat stress enhances plantar incision-induced spinal AMPA receptor phosphorylation and thereby prolongs incisional pain and that stress hormones regulate AMPA receptor activities in the spinal cord during the pain prolongation[27]. We also found that the social defeat stress not only increases GluA1 membrane expression, but also enhances GluA2 intracellular expression in the spinal dorsal horn neurons[27]. Our study identifies stress as a risk factor for pain chronification after surgery. Our recent study indicates that intrathecal injection of a Ca2+-permeable AMPA receptor blocker significantly inhibits the stress-induced postsurgical pain prolongation (unpublished data). Therefore, we hypothesize that by releasing two types of stress hormones (norepinephrine and corticosterone), stress regulates AMPA receptor activities (such as phosphorylation and trafficking), which leads to GluA1 membrane insertion and GluA2 internalization and causes a switch from Ca2+-impermeable (GluA2-containing) to Ca2+-permeable (GluA2-lacking) AMPA receptors. This switch will enhance Ca2+ influx and further activate Ca2+-dependent protein kinases, thereby promoting AMPA receptor phosphorylation and other phosphorylation-triggered activities (Figure 1). This positive feedback loop may contribute to the molecular mechanisms that underlie stress-induced pain chronification after surgery.
Manuscript source: Invited manuscript
Specialty type: Biochemistry and molecular biology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chandra D, Fang YR, Liu SQ, Wang Y S- Editor: Qiu S L- Editor: A E- Editor: Li D
| 1. | Mifflin KA, Kerr BJ. The transition from acute to chronic pain: understanding how different biological systems interact. Can J Anaesth. 2014;61:112-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | McGreevy K, Bottros MM, Raja SN. Preventing Chronic Pain following Acute Pain: Risk Factors, Preventive Strategies, and their Efficacy. Eur J Pain Suppl. 2011;5:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 3. | Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert Rev Neurother. 2009;9:723-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 535] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 4. | Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618-1625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2613] [Cited by in RCA: 2690] [Article Influence: 141.6] [Reference Citation Analysis (0)] |
| 5. | Krugers HJ, Hoogenraad CC, Groc L. Stress hormones and AMPA receptor trafficking in synaptic plasticity and memory. Nat Rev Neurosci. 2010;11:675-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 6. | Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 882] [Cited by in RCA: 1022] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 7. | Maneyapanda SB, Venkatasubramanian A. Relationship between significant perinatal events and migraine severity. Pediatrics. 2005;116:e555-e558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Clark JD, Qiao Y, Li X, Shi X, Angst MS, Yeomans DC. Blockade of the complement C5a receptor reduces incisional allodynia, edema, and cytokine expression. Anesthesiology. 2006;104:1274-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009;32:611-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 360] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 10. | Van Houdenhove B, Egle UT. Fibromyalgia: a stress disorder? Piecing the biopsychosocial puzzle together. Psychother Psychosom. 2004;73:267-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 144] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 11. | Affleck G, Tennen H, Urrows S, Higgins P. Person and contextual features of daily stress reactivity: individual differences in relations of undesirable daily events with mood disturbance and chronic pain intensity. J Pers Soc Psychol. 1994;66:329-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 132] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Coutinho SV, Plotsky PM, Sablad M, Miller JC, Zhou H, Bayati AI, McRoberts JA, Mayer EA. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282:G307-G316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 322] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 13. | Amital D, Fostick L, Polliack ML, Segev S, Zohar J, Rubinow A, Amital H. Posttraumatic stress disorder, tenderness, and fibromyalgia syndrome: are they different entities? J Psychosom Res. 2006;61:663-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Cohen H, Neumann L, Haiman Y, Matar MA, Press J, Buskila D. Prevalence of post-traumatic stress disorder in fibromyalgia patients: overlapping syndromes or post-traumatic fibromyalgia syndrome? Semin Arthritis Rheum. 2002;32:38-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 124] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Dina OA, Khasar SG, Alessandri-Haber N, Green PG, Messing RO, Levine JD. Alcohol-induced stress in painful alcoholic neuropathy. Eur J Neurosci. 2008;27:83-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3042] [Cited by in RCA: 3201] [Article Influence: 160.1] [Reference Citation Analysis (0)] |
| 17. | Hall RA. Beta-adrenergic receptors and their interacting proteins. Semin Cell Dev Biol. 2004;15:281-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Wang W, Zhu W, Wang S, Yang D, Crow MT, Xiao RP, Cheng H. Sustained beta1-adrenergic stimulation modulates cardiac contractility by Ca2+/calmodulin kinase signaling pathway. Circ Res. 2004;95:798-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 151] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 19. | Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276:2042-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 768] [Cited by in RCA: 831] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 20. | Mammen AL, Kameyama K, Roche KW, Huganir RL. Phosphorylation of the alpha-amino-3-hydroxy-5-methylisoxazole4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J Biol Chem. 1997;272:32528-32533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 356] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 21. | Roche KW, O’Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 645] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 22. | Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, Yu S, Ding L, He C, Petralia RS. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell. 2003;112:631-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 635] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 23. | Hu H, Real E, Takamiya K, Kang MG, Ledoux J, Huganir RL, Malinow R. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell. 2007;131:160-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 378] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 24. | Joëls M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. Learning under stress: how does it work? Trends Cogn Sci. 2006;10:152-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 617] [Cited by in RCA: 624] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 25. | Groc L, Choquet D, Chaouloff F. The stress hormone corticosterone conditions AMPAR surface trafficking and synaptic potentiation. Nat Neurosci. 2008;11:868-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 208] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 26. | Chapman CR, Tuckett RP, Song CW. Pain and stress in a systems perspective: reciprocal neural, endocrine, and immune interactions. J Pain. 2008;9:122-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 368] [Cited by in RCA: 328] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 27. | Li C, Yang Y, Liu S, Fang H, Zhang Y, Furmanski O, Skinner J, Xing Y, Johns RA, Huganir RL. Stress induces pain transition by potentiation of AMPA receptor phosphorylation. J Neurosci. 2014;34:13737-13746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |