REGIONALIZATIONS IN THE CNS
During development of the CNS the formation of regional patterning of the neural tube along anteroposterior and dorsoventral axes is one of the earliest events. At this step, hundreds of distinct neuronal subtypes are generated from a single layer of multipotent neuroepithelial progenitor cells with a positional identity (see reviews[3-5]). The telencephalon, where the cerebral cortex and the basal ganglia originate, is initially regionalized. Based on findings in research of the development of the cortex, it has been proposed that the structure patterning is intrinsically encoded in progenitors[5,11,12]. It is found that interneurons related clonally tend to cluster together and act synchronously once they reach the cortex[13,14]. The area identity has been established before any functional diversification is apparent[15]. Although it has also been found that the areal specificity can be affected by afferent connections in a neuronal activity-dependent manner[5,15], a stronger genetic prepatterning is indicated by findings that the region-specific thalamocotical connections are maintained while the mutations of the genes Emx2, COUP-TFI and Sp8 cause changes in cortical area identity[16-18].
Data obtained from cultures of neural progenitors in vitro have revealed a high degree of plasticity in cell fate choices[19-22]. Mature neurons can be produced from multiple cell sources such as fibroblasts by using the technique “direct reprogramming” or “trans differentiation”[19-23]. The sequential generation of projection neurons observed in vivo can also been found in cultures of neuron precursors in vitro[24]. Taken together, it has been proposed that genetic factors play primary roles in neuronal development from progenitor cells, while the environmental factors acting in vivo are secondary[3-5].
Developmental changes may also occur in non-neural cells in the CNS such as glial cells including both astrocytes and microglial. Astrocytes are the most abundant cell type in the mammalian CNS and have been viewed as relatively homogeneous, interchangeable and the least migratory. Recently, however, there is accumulating evidence of morphological and molecular differences between astrocytes in different brain regions[25,26]. Culture and transplantation experiments have also demonstrated functional differences among astrocytes from different brain regions[25,27,28]. These differences may be related to astrocyte derivation from regionally patterned radial glial cells[29].
The spatial patterning in the spinal cord is organized by neural progenitors with position identities, which create a segmental template that underlies neuronal diversity[25]. In the spinal cord, astrocytes are also developed in a position-dependent manner and regionalized in a segmental template[25,30]. By providing positional guidance cues for neuronal migrating and axon path finding, astrocytes are involved in the formation of neuronal circuits[25,31]. Several genes encoding extracellular matrix components or axon and cell migration factors have been found to be expressed differentially in astrocytes in the dorsal vs ventral spinal cord[32]. This suggests that regional astrocytic subtypes may encode positional functions during development.
THE REGION-SPECIFIC REGULATION OF NEURONAL DEVELOPMENT IN Vc VS SDH
Vc represents the most caudal component of the trigeminal system. Both Vc and SDH contain second-order sensory neurons receiving low- and/or high-threshold primary afferent inputs from the orofacial region and other somatic regions of the body, respectively. Most small-diameter nociceptive primary afferents terminate in the superficial laminae of SDH and Vc. Nociceptive neurons in the superficial and deeper laminae relay the nociceptive input to higher brain centers[6,7,9,10,33]. According to their mechanoreceptive field properties, sensory neurons in Vc and SDH have been classified into low-threshold mechanoreceptive neurons which only respond to innocuous stimuli (e.g., tactile); wide-dynamic range neurons which respond to both innocuous and noxious stimulation; and nociceptive-specific neurons (NS) which only respond to noxious stimuli[6,7,9,10,33]. Astrocytes and microglial in Vc and SDH have been found to modulate the activity of the nociceptive neurons[34,35].
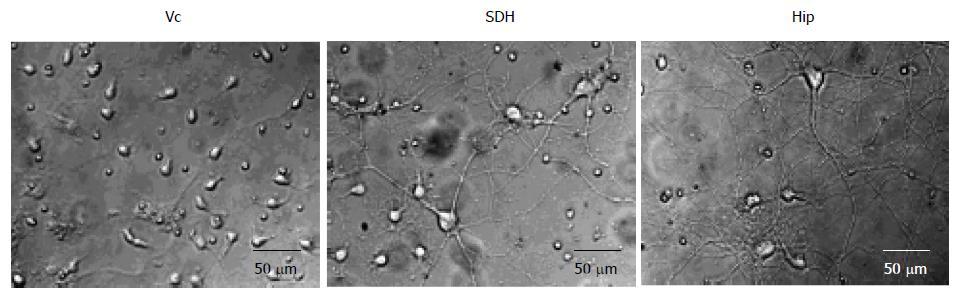
However, despite the several morphological and functional similarities between Vc and SDH noted above, there are some differences. For example, the distribution of afferent fibers releasing substance P and calcitonin gene related peptide in Vc of adult animals shows a different pattern than that in SDH, a dual representation of peripheral tissues can be found in the rostral and caudal regions of Vc, and there is a transitional zone between Vc and trigeminal subnucleus interpolaris (of the trigeminal spinal tract nucleus) that has a special role in orofacial sensorimotor function and appears to have no clear parallel in the SDH or spinal system[33,36,37]. In addition, it was recently found that cultured neurons from Vc of rat embryos (18 d gestation) grew much slower, the number of primary processes was less and the length of the processes was shorter when compared with cultured SDH and hippocampus (Hip) neurons[38]. Moreover, two weeks after plating for culture, Vc neurons also remained separated while the processes of a large number of neurons in Hip and SDH cultures had merged and formed networks[38] (Figure 1). A question raised by these findings is: What is the functional significance of these differences between Vc and SDH or Hip neurons?
Figure 1 Neuronal growth in trigeminal subnucleus caudalis, spinal dorsal horn and hippocampus cultures.
Examples of images were recorded at day 14 after plating. Vc: Trigeminal subnucleus caudalis; SDH: Spinal dorsal horn; Hip: Hippocampus.
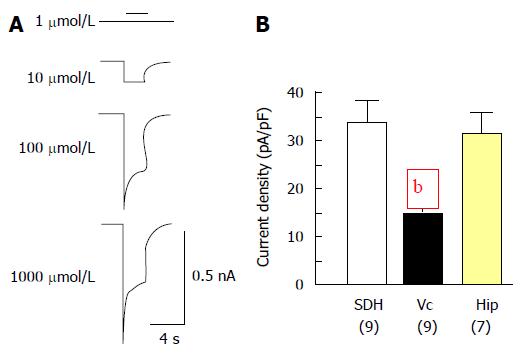
To answer this question, glutamate-mediated whole-cell currents in cultured Vc neurons were recorded at day 14 after plating[38]. All neurons tested showed glutamate concentration-dependent responses (see an example in Figure 2A). However, the current density of Vc neurons was significantly lower than that of SDH or Hip neurons (Figure 2B). Furthermore, it was found that spontaneous miniature excitatory post-synaptic currents (mEPSCs) could be detected in over 80% of SDH and Hip neurons recorded while less than 30% of Vc neurons tested showed mEPSC activity. Detailed analyses showed that the mean frequency and peak amplitude of mEPSCs recorded in cultured Vc neurons were around 0.98 Hz and 25 pA. Most mEPSCs recorded in Vc neurons had both AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) and NMDA (N-methyl-D-aspartate) receptor-mediated components with decay time constants around 4.8 ms and 94 ms, respectively. All of these parameters calculated from mEPSCs recorded in Vc neurons were similar to those found in SDH and Hip neurons[38-41].
Figure 2 Whole-cell responses mediated by glutamate.
A: Examples of current traces recorded in Vc neurons. The currents were evoked with glutamate at concentrations as indicated; B: Summary data (mean ± SEM) of peak current density evoked with 1000 μmol/L of glutamate respectively from Vc, SDH and Hip neurons. Values in brackets indicate number of neurons tested. bP < 0.01, independent t-test, indicate significant differences detected in Vc vs that in SDH or Hip neurons. Vc: Trigeminal subnucleus caudalis; SDH: Spinal dorsal horn; Hip: Hippocampus.
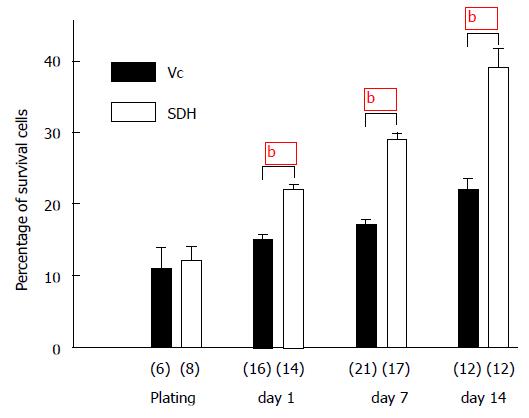
To understand the mechanisms which may underlie the differences observed in the development of Vc vs SDH or Hip neurons, cell viability in cultures were examined[38]. Embryonic Vc and SDH tissues containing similar proportions of live cells (11% in Vc and 12% in SDH) were initially plated, and live cells in both Vc and SDH cultures increased. However the increase in live cells in Vc was significantly less than that in SDH (Figure 3). At 14 d after plating, the percentage of live cells in Vc culture increased only to 22% whereas that in SDH increased to 39%. These data suggest the possibility that cell death occurs more in developing Vc than that in SDH. To confirm this possibility TUNEL (a terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling) was used to detect cell death in cultures of Vc and SDH. Cell death occurring in young and mature neurons was determined by TUNEL with co-labeling of Tuj1 and NeuN antibody, respectively. Cell death occurring in astroglial cells was determined by TUNEL with co-labeling of GFAP antibody. It was found that at 14 d after plating more mature neurons underwent cell death in Vc than SDH whereas cell death in young neurons or astroglial cells appeared similarly in these two regions[38].
Figure 3 Cell viability in in trigeminal subnucleus caudalis and spinal dorsal horn cultures.
Bars show the percentage values (mean ± SEM) of live cells in Vc and SDH cells detected during and after plating as indicated. bP < 0.01 (independent t-test) when compared with that in SDH cultures. Numbers in brackets indicate the number of dishes tested. Vc: Trigeminal subnucleus caudalis; SDH: Spinal dorsal horn; Hip: Hippocampus.
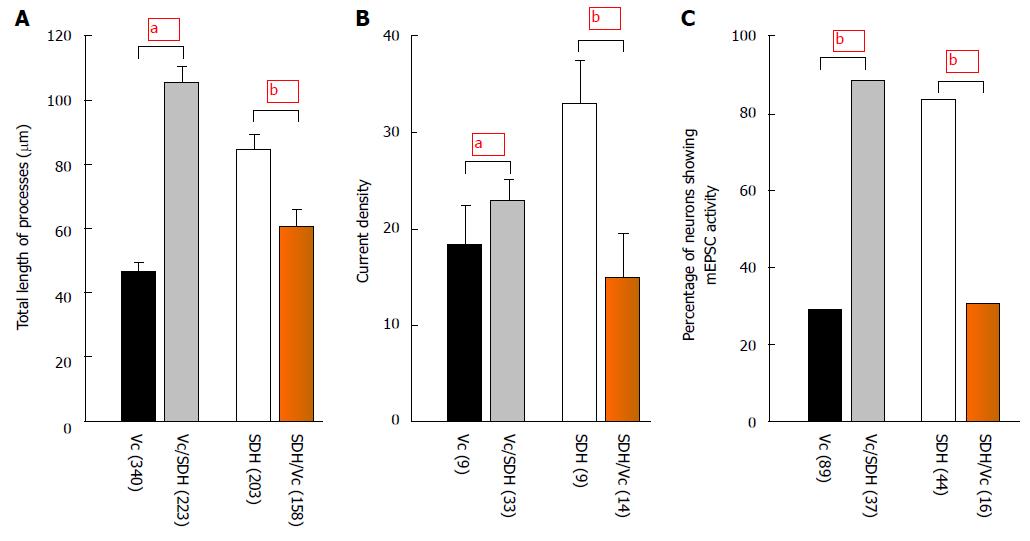
A particularly interesting finding in this study[38] was that the difference in cell survival in Vc vs SDH was still maintained in cultures either with neurobasal media supplemented with B27, β-FGF2 and L-glutamine [which minimizes the growth of glial cells (< 15%)[42]] or with MEM supplemented with 10% fetal bovine serum and insulin. Altering the concentration of β-FGF2 or insulin added into culture medium did not produce significant effects on the difference in cell survival between Vc and SDH. However, co-culture experiments where the tissue of one region was cultured in a tissue environment of another region showed that that the growth of Vc neurons co-cultured with SDH tissues was significantly promoted (Figure 4): (1) when compared with Vc neurons not co-cultured with SDH, the Vc neurons co-cultured with SDH had more and longer processes which merged and formed networks around 14 d after plating; and (2) the glutamate-mediated current density of the Vc neurons co-cultured with SDH increased and more neurons showed spontaneous mEPSC activity. In contrast, when compared with SDH neurons not co-cultured with Vc, the growth of SDH neurons co-cultured with Vc was significantly inhibited. The glutamate-mediated current density and the occurrence of spontaneous mEPSC activity reduced. These data strongly suggest that some factors which may inhibit or stimulate neuronal growth, are released in a region-specific manner, and thereby differentially regulate the development and function of Vc and SDH.
Figure 4 Effects of co-culture.
The bar graph shows summary data (mean ± SEM) of the total length of processes at day 7 after plating (A), current density (B) and occurrence of mEPSC activity (C) recorded at day 14 after plating. Vc/SDH: Vc cells co-cultured with SDH; SDH/Vc: SDH cells co-cultured with Vc; aP < 0.05, bP < 0.01 (independent t-test in A and B, Fisher’s exact test in C) when compared with those in co-cultures. Numbers in brackets indicate the number of neurons tested. Vc: Trigeminal subnucleus caudalis; SDH: Spinal dorsal horn. mEPSC: Miniature excitatory post-synaptic current.
Many factors may be involved in the Vc-specific regulation, e.g., growth factors including the epidermal growth factors[43], fibroblast growth factors (FGFs)[44-47], transforming growth factors[48], nerve growth factors[49], insulin[50,51], and members of the bcl-2 and caspase gene families[52]. However, the differences in neuronal growth observed in Vc vs SDH are similar, irrespective of the concentration of β-FGF2 or insulin supplemented[38], indicating their unlikely involvement in the differential growth regulation of Vc vs SDH. Since a group of non-protein ninhydrin-reacting small molecules (NF) isolated from human plasma (by column chromatography using Sephadex G-25 followed by G-15) has been shown to be involved in the regulation of neuronal growth[53-55], NF in Vc, SDH and Hip-conditioned media were investigated for their possible involvement[38]. Consistent with findings in human plasma, two ninhydrin components (NF1 and NF2) could be classified from media used for cell culture. To determine whether NF1 and NF2 isolated from conditioned medium used for Vc, SDH or Hip culture might produce different effects, cell viability in cortical cultures treated with the isolated NF1 or NF2 was also examined in this study[38]. When compared to cultured cortical cells without any treatment, no significant change in cell viability of cortical cells was observed following the application of either non-ninhydrin-reacting fractions isolated from conditioned culture media or NF1 and NF2 isolated from non-conditioned culture medium. However, application of NF1 isolated from Hip- or SDH-conditioned culture medium significantly enhanced cell viability in cortical cultures but application of NF1 isolated from Vc-conditioned medium produced no significant effect. Application of NF2 isolated from Vc-conditioned medium (Vc-NF2) significantly inhibited the growth and viability of cortical cells; only 20% of the cells remained alive in cortical cultures with a 24-h-treatment of Vc-NF2. However, NF2 isolated from either Hip- or SDH-conditioned medium produced no such effect. Thus, these findings strongly suggest that locally released ninhydrin-reacting small molecules may underlie the developmental differences between cultured Vc neurons and SDH neurons.
To obtain insights into whether Vc-NF2 may also be released in a region-specific manner in vivo, Vc, SDH or Hip slices were prepared from rats at day 1 after birth, and the media used for culturing the slices were sampled at 3-4 d after slice plating[38]. Like its effects on cortical cells in culture, NF1 from Hip or SDH slice-conditioned medium was found to enhance cell viability and increase the number of live cells in cortical cultures, but NF1 isolated from Vc slice-conditioned medium had no significant effect. NF2 isolated from Vc slice-conditioned medium inhibited cell growth and viability, whereas NF2 isolated from Hip or SDH slice-conditioned medium did not induce such changes. Thus, these findings have provided further evidence that Vc-NF2 and SDH-NF1 are important factors involved in region-specific regulation in the dorsal horn ex vivo. The finding of consistent effects on neuronal growth induced by Vc-NF2, SDH-NF1 or Hip-NF1 isolated from conditioned media either for embryonic or postnatal cultures excludes the possibility that the differences observed in neuronal development of Vc vs SDH or Hip might be induced by the different culture methods used.
It has been shown that different subtypes of neurons such as interneurons and projection neurons in SDH mature at different rates[56,57]. Thus, the differences documented in the neuronal development and functions of Vc vs SDH in animals of the same ages and under the same conditions[38] could conceivably be also related to differences in the developmental stage of the CNS regions. However, whether interneurons and projection neurons in Vc mature at different rates is still unknown. A rostrocaudal gradient of inhibitory factors which are diffusible and released from the ventral horn have been found in the developing spinal cord[58-60]. Although more detailed investigations are still needed, the findings that less trophic and more growth-inhibitory factors may be released locally from Vc in comparison to SDH or Hip indicate that these locally released factors may be involved also in the regulation of the maturation rate of neurons of different subtypes and/or in different CNS regions.
FUTURE RESEARCH DIRECTIONS
In the CNS, the functional specificities of neurons are predetermined at a very early stage of development. The region-specific control of proliferation, survival and differentiation of neural precursors plays a critical role. The ninhydrin-reacting small molecules in fraction NF1 or NF2 isolated from conditioned medium used for Vc, SDH or Hip culture induced significant changes in neuronal growth while those from unconditioned medium did not, confirming that specific molecules are released by neural tissues in culture[38]. These data have: (1) demonstrated that the involvement of locally released factors in the region-specific regulation of neuronal development in Vc and SDH which are CNS regions critically involved in pain signal transmission; (2) provided novel insights for understanding CNS regionalization and functional organization; and (3) raised several important questions for future investigations that are needed for the clarification of neuroregenerative processes and for the development of new therapeutic approaches for treating neurodegenerative diseases and pain.
To determine how the ninhydrin-reacting small molecules are released in Vc and SDH and from what types of cells
As noted above, neuronal growth in cultures with neurobasal medium with the addition of B27, β-FGF2 and L-glutamine, was significantly improved and glial cells were much reduced in Vc, SDH and Hip cultures when compared with those in cultures with MEM supplemented with 10% fetal bovine serum and insulin. Nonetheless, neurons in Vc cultures still showed significantly slower growth, lower glutamate receptor activity and more cells undergoing death when compared with those in SDH and Hip cultures. Thus, there is the possibility that the growth-trophic and growth-inhibitory molecules such as in SDH-NF1 and Vc-NF2 may be released from neurons as well as from glial cells. Studies are needed to determine how and from what types of cells these molecules are released in Vc and in SDH. The characterization of intrinsic and extrinsic signals underlying the regulation of the release of the growth-trophic factor SDH-NF1 and the growth-inhibitory factor Vc-NF2 will be key for research advances aiming to understand mechanisms underlying the region-specific regulation of neuronal development in Vc and SDH.
To determine how and what types of cells may be regulated by the locally released molecules in NF1 or NF2 in Vc and SDH
Different neuronal populations have been found to be differentially regulated during development[1,2,49,61,62]. The data obtained in experiments of TUNEL with co-labeling of GFAP, NeuN or Tuj1 antibody showed that at day 7 after plating, significantly more NeuN- and GFAP-stained Vc cells showed TUNEL when compared with those in SDH, but no such difference was noted between Tuj1-labeled neurons in Vc vs SDH[38]. At day 14 after plating, there were still more neurons showing NeuN co-labeling with TUNEL in Vc but no difference between Vc and SDH could be found in neurons showing Tuj1 co-labeling or in astroglial cells showing GFAP co-labeling. These findings therefore imply that molecules in Vc-NF2 may selectively target intracellular signaling pathway(s) developed in mature neurons and thereby causing neuronal death. The cellular types in Vc and SDH are heterogeneous. In Vc and SDH, glutamate and GABA are the principal excitatory and inhibitory transmitters, respectively. Genetically, whether neurons will develop to a glutamatergic or GABAergic cells depends upon the expression of homeobox genes Tlx3, Tlx1 and Lbx1[63-65]. Thus, characterization of the development of Vc and SDH cells with gene-related labeling needs to be conducted to clarify which neuronal and astrogial cell types in Vc, SDH or Hip may be regulated by locally released NF1 or NF2.
As noted above, many fewer Vc neurons in culture showed spontaneous EPSC activity and the glutamate-mediated whole-cell current density was significantly low, when compared with those in neurons in SDH or Hip cultures[38]. Co-cultures of Vc with SDH significantly increased the glutamate-mediated whole-cell current density and mEPSC occurrence in Vc whereas co-culture of SDH with Vc significantly reduced the glutamate-mediated whole-cell current density and mEPSC occurrence in SDH neurons. Although locally released Vc-NF2 and SDH-NF1 have been found to be important factors underlying the alteration of cell growth in Vc and SDH, it still needs to be determined if the glutamate-mediated whole-cell current density and mEPSC activity may also be directly modulated by bath applied Vc-NF2 or SDH-NF1.
Moreover, although experimental data have demonstrated that locally released Vc-NF2 and SDH-NF1 play important roles in the region-specific regulation of neuronal growth ex vivo[38], there is a need for studies in vivo comparing the rate of growth, expression of glutamate receptors and cell death of Vc vs SDH neurons. The characterization of the effects of locally released factors in vivo still represents an important future research direction. Neurons in Vc and SDH play crucial roles in pain and related sensorimotor functions. Studies addressing whether and how the locally released factors may affect nociceptive and sensorimotor functions in the trigeminal and spinal systems are particularly important for understanding pathophysiological conditions expressing pain and/or sensorimotor dysfunction.
To determine the chemical nature of SDH-NF1 and Vc-NF2
Two fractions called fractions V3A and V3[54,55] containing non-protein, ninhydrin-reacting small molecules can be isolated by using size exclusion chromatography on Sephadex G-25 followed by Sephadex G-15 from human plasma. Furthermore, the fraction V3A is found to have insulin-like activity, while fraction V3 is an inhibitor of insulin action[54,55]. Fraction V3A contains chiro-inositol and galactosamine, and fraction V3 contains myoinositol 1,2-cyclic phosphate and galactosamine. The myoinositol 1,2 cyclic phosphate group is essential for the inhibitory action of fraction V3[54,55]. V3A applied to hippocampal or cortical cultures significantly increases neuronal growth while V3 inhibits neuronal growth[53]. Non-protein, ninhydrin-reacting small molecule fractions (NF1 and NF2) can be isolated from culture media by using the same size exclusion chromatography as that used to isolate fractions V3 and V3A from human plasma. Interestingly, the elution profiles of NF1 and NF2 are very similar to those of fractions V3 and V3A from human plasma[38]. However, so far the chemical nature of the fractions NF1 and NF2 remains unknown. Thus, the identification of the chemical structures of molecules in Vc-NF2 and SDH-NF1 should be a major focus of future research in order to develop useful agents to selectively block the effect of Vc-NF2 or SDH-NF1. These agents should be very useful tools not only for investigating region-specific regulation in areas of the CNS such as Vc and SDH, but also for developing new approaches to diagnose and treat neurodegenerative diseases, pain and sensorimotor dysfunction.