INTRODUCTION
According to epidemiological data from the World Health Organization (WHO), diseases of the circulatory system, collectively known as cardiovascular diseases (CVDs) are the leading cause of death world-wide (http://www.who.int/mediacentre/factsheets/fs317/en), and a great number of resources have been committed to study the pathophysiology of CVDs with the goal of improving therapeutic interventions. The most common forms of CVD include atherosclerosis of various segments of the arterial tree (coronary, carotid, and cerebral arteries, aortic arch, abdominal artery), aneurysms of the thoracic and abdominal aorta, as well as cerebral arterial aneurysms, peripheral arterial disease (PAD), and venous insufficiency.
Although certain types of CVDs share some of the same pathogenic characteristics (e.g., neointima and atherosclerotic plaque formation as a result of chronic inflammation in atherosclerosis, medial degeneration in aortic aneurysms), they usually develop at distinct anatomic locations. As the most prevalent CVD risk factors including hypertension, diabetes, smoking, alcohol, lack of physical activity, obesity, high blood cholesterol and triglycerides, and stress are all systemic, regional genetic differences in the vascular bed are likely to be critical for these topographic disease patterns, as they cannot be attributed to hemodynamic differences alone. This view is underscored by aortic homograft transplant experiments in canines, in which atherosclerosis- susceptible segments of the abdominal aorta were transplanted into the atherosclerosis-resistent thoracic aorta and vice versa[1]. Examination of transplanted vessel segments subsequent to keeping the animals on an atherogenic diet revealed that the abdominal segments readily developed atherosclerotic lesions in their thoracic host environment, whereas the thoracic aortic segments continued to be free from lesion formation in the abdominal aorta. These results are consistent with data from a large-scale clinical study involving approximately 12000 patients that were treated surgically for atherosclerotic occlusive disease with a follow-up of over 25 years[2]. In this study the arterial bed was divided into four anatomical categories for analysis including the coronary arterial bed, the major branches of the aortic arch, the visceral branches of the abdominal aorta and the terminal abdominal aorta with its major branches. The data showed that the response to systemic risk factors is distinctly different in each of the four categories[2].
A search for potential determinants underlying these inherent differences in the response to systemic pathogenic triggers will have to consider the circumstance that vascular smooth muscle cells (VSMCs) residing in distinct segments of the arterial tree originate from different sources depending on their anatomic location. Lineage-mapping involving chick-quail chimera identified at least seven different sources including proepicardium, secondary heart field, neural crest, mesangioblasts, somites, splanchnic mesoderm and mesothelium that contribute to defined arterial segments, in addition to various types of progenitor cells residing primarily in either the media or adventitia with a more universal distribution[3]. Overall, this fate map reveals a segmented pattern of the arterial tree that seems to reflect the metameric patterning of the vertebrate embryo. As segment identities, and therefore diversities, are specified by members of the phylogenetically highly conserved family of Hox transcriptional regulators, these genes have to be viewed as prime candidates for determining different positional identities in the vascular bed that reflect regional differences in CVD susceptibility.
HOX-SPECIFIED POSITIONAL IDENTITIES IN THE CIRCULATORY SYSTEM
The conserved Hox gene family constitutes a genetic system of unique properties that is utilized initially during embryonic patterning for specifying positional identities along the anterior-posterior (A-P) axis[4,5]. The mouse and human genome harbor 39 Hox genes that are organized into four separate clusters designated Hoxa, -b, -c, and -d. Alignment of these transcriptionally unipolar clusters based on sequence similarities reveals 13 paralogous groups of Hox genes that are activated sequentially in distinct A-P embryonic domains such that genes of groups 1 and 2 are expressed first in the most anterior regions, whereas group 13 Hox genes are activated last by following the A-P morphogenetic progression. This modus of activation generates unique domains of combinatorial Hox activities at any given location of the embryo that has been referred to as the Hox code in analogy to the postal zip code for specifying positional identities[6]. Data obtained by large-scale gene expression profiling of adult fibroblasts derived from different anatomic regions in humans suggest that this embryonically established topographic Hox code is, at least to some degree, retained in the adult[7], where it is believed to be critical for maintaining positional identities by regulating local differentiation and signaling events.
Initial evidence for the existence of a topographic Hox code in the circulatory system came from LacZ reporter gene studies in transgenic mice that revealed remarkable regionally restricted expression patterns for Hoxc10, Hoxc11, and Hoxa3 in subpopulations of VSMCs of the media, as well as in endothelial cells (ECs) within distinct segments of the vascular bed of young adult (6 wk), as well as 1 year old mice[8]. Apparently, this presumptive vascular Hox code is instrumental in maintaining vessel wall integrity and homeostasis as indicated by the region-specific vascular remodeling events upon its interruption. Specifically, this was demonstrated by inducing changes in the vascular Hoxc11 expression pattern that is normally restricted to the distal limb vasculature of adult mice (Figure 1). By utilizing an integrated tetracycline regulatory system and Transgelin (Tagln) promoter elements that drive expression universally in VSMCs of TetOn(Tagln-Hoxc11) transgenic mice upon doxycycline (dox) induction, these mice developed severe vessel wall defects (medial thinning, elastic laminae fragmentation, intimal lesion formation) in arterial segments where Hoxc11 is normally not expressed (carotid artery, aortic arch, thoracic aorta), whereas overexpression of Hoxc11 in its natural vascular domain of activity, including the lower femoral artery, resulted in a drastic increase in vessel diameter but without the structural defects observed upon ectopic expression[9]. Furthermore, human HOX transcriptome analysis of vascular ECs derived from different anatomic locations revealed specific HOX expression signatures that are believed to determine positional identities and regulate endothelial differentiation[10].
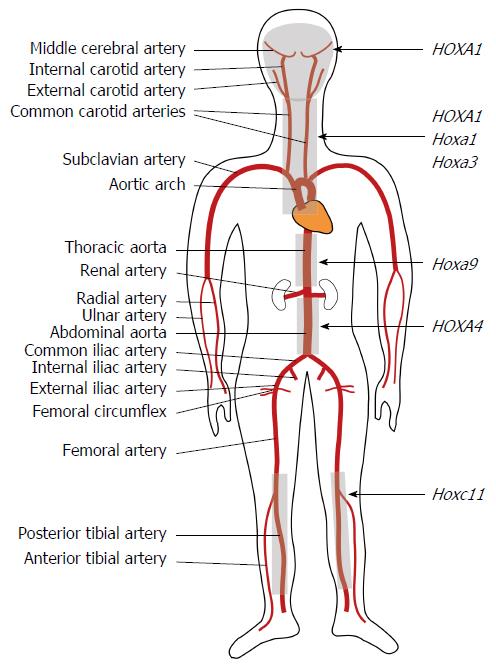
Figure 1 Map of Hox functional domains in the arterial tree.
The schematic shows a rough outline of the main human arterial segments. Localization of vascular defects associated either with mutated human HOX or mouse Hox alleles as indicated at the right were used to generate a cursory map of HOX/Hox activity domains (grey shading) assuming that the defects observed in the mouse map to roughly equivalent anatomic positions in humans. The demarcation of the HOXA4 activity domain is based on down-regulation of HOXA4 expression associated with AAA. The Hoxa9 domain in the thoracic aorta signifies an atherosclerosis-resistant segment.
Compared to gene expression profiling, an alternative approach to consider for mapping Hox activities in the circulatory system is to determine Hox functional domains by mutational analysis in mice and by linking human congenital vascular defects to mutant HOX alleles. The Hoxa3tm1Mrc mutant is perhaps the first case in which disruption of a Hox gene has been linked to severe cardiovascular defects in mice that include absence of the right carotid artery and stenosis of the aortic valves, in addition to abnormalities of the cardiac chambers, as well as other developmental defects[11]. In humans and mice, a homozygous HOXA1/Hoxa1 mutation was linked to a complex syndrome that includes malformations of the cerebral vasculature, the internal carotid arteries, and the cardiac outflow tract in addition to neuronal defects[12-14]. Mice homozygous for the Hoxc11tm1Mrc targeted allele developed greatly enlarged femoral arteries that were phenotypically very similar to the enlarged vessels seen upon induced overexpression of Hoxc11 in the distal hindlimb vasculature[9]. Furthermore, and of potential clinical relevance is the downregulation of HOXA4 expression associated with abdominal aortic aneurysms[15]. Interestingly, HOXA4 exhibits an A-P gradient in expression levels along the aorta (ibid) that might overlap with a gradient for Hoxa9 expression, for which highest levels were found in the thoracic aorta of mice[16] as discussed below.
HOX-REGULATED GENETIC PATHWAYS IN THE VASCULATURE - POTENTIAL CVD RELEVANCE
The vascular functions of Hox genes have perhaps been studied most extensively in cells of endothelial lineage within the context of angiogenesis as observed in normal physiological scenarios (e.g., wound healing) and under pathological conditions (e.g., tumorigenesis). This work has yielded useful insight into Hox-dependent pathways regulating EC phenotypic properties. Accordingly, all HOX genes of paralogous group 3 were found to stimulate angiogenesis albeit through different pathways[17]. HOXD3 promotes expression of integrinα5 and β3 subunit genes in response to FGF2[18-20]; heterodimers of these subunits with other subunits generate fibronectin receptors α5β1 and ανβ3 that facilitate adhesion and migration through fibronectin-rich granulation tissue during wound healing[17]. Consistent with this is the HOXD3-regulated increase in expression of the urokinase plasminogen activator (uPA) gene[18]. The human HOXD3 paralogs HOXA3 and HOXB3 also promote angiogenesis although through different pathways that involve increased EC migration and expression of Mmp14 and uPAR (HOXA3) and enhanced expression of the angiogenic ligand Ephrin A1 (HOXB3)[17]. Increased EC migration in tissue culture was also observed for HOXA9 through direct transcriptional activation of the Ephrin receptor B4 (EPHB4) gene[21]. HOXD10, on the other hand, was found to be inactive during angiogenesis but expressed in quiescent vascular endothelium, and pro-angiogenic factors (β4 and α3 integrins, MMP14, uPAR and cyclin D1) were downregulated in HOXD10-transfected ECs[22]. Likewise, HOXA5 is reportedly inactive in angiogenic endothelium but expressed in quiescent ECs where VEGFR2, ephrin A1, HIF1α, and COX2 were downregulated upon transfection with HOXA5[23].
As most of these Hox expression data and functional correlations have been obtained in cell culture systems, some of these results may not reflect the vascular patterns and functions observed in vivo. For example, Hoxa3 expression is induced together with Hoxd3 during cutaneous healing of excisional wounds in mouse[24]. However, the same gene was found to be abundantly expressed in VSMCs of major blood vessels in addition to ECs but in a topographically restricted manner[8].
As pointed out above, induction of ectopic Hoxc11 expression in VSMCs of TetOn (Tagln-Hoxc11) transgenic mice on an FVB/NTac background resulted in severe vascular defects. Consequently, these mice offer an opportunity to start defining Hoxc11-regulated pathways of pathological vessel wall remodeling. Preliminary results showed induction of the apoptotic marker CASP3, as well as matrix protein metalloproteinase MMP2 and MMP9 expression in medial VSMCs of the thoracic aorta following doxycycline (dox)-induced HOXC11 expression[9]. Both MMP2 and MMP9 are involved in extracellular matrix (ECM) breakdown and restructuring of the vessel wall, as well as breakdown of the basement membrane and cell migration[25,26], i.e., events that are consistent with the formation of the type of lesion formation observed in the thoracic aorta of these mice. Interestingly, in the lower femoral artery, where Hoxc11 overexpression resulted in an enlargement of an otherwise structurally normal vessel, only MMP2 was induced upon dox-treatment. Accordingly, the differential histological changes observed in response to Hoxc11 ectopic vs overexpression were mirrored by distinct differences in molecular responses, in this case MMP9 expression, that is usually associated only with injury and inflammatory responses[25]. Furthermore, data from chromatin immune-precipitation (ChIP) and transient co-transfection assays suggested that Mmp2 and Mmp9 are transcriptionally regulated by HOXC11, thus linking Hoxc11 directly to the regulation of vascular remodeling pathways involving these MMPs.
In a separate study, a search for factors that contribute to the commonly greater susceptibility to atherosclerosis in the aortic arch vs the thoracic aorta found that members of Hox paralogous groups 6-10 exhibit higher expression levels in the former across various mammalian species including mouse, rat, and porcine[16]. Remarkably, essentially the same differential Hox expression profiles were observed in human embryonic stem cells that were differentiated in vitro either along the neuroectoderm pathway into aortic arch-like VSMCs or along the paraxial mesoderm pathway into thoracic aorta-like VSMCs. These data suggest that Hox-specified positional identities of VSMCs established during development are retained in adulthood. Apparently this positional diversity is reflected by differential responses to inflammatory stimuli that are critical for the progression of vascular diseases such as atherosclerosis. This was illustrated by the greater activity and binding to DNA of the pro-inflammatory transcription factor NF-κB in aortic arch vs thoracic aorta VSMCs. Moreover, this NF-κB pattern apparently is, at least in part, due to a reciprocal regulatory relationship with Hoxa9 such that the higher levels of HOXA9 expression in the thoracic aorta repress NF-κB transcriptional activity, whereas the high levels of NF-κB actitivity in the arch repress Hoxa9 expression[16,27].
As mentioned before, downregulation of already low HOXA4 expression levels in the abdominal aorta was associated with abdominal aortic aneurysms (AAAs)[15] that are much more prevalent than thoracic aortic aneurysms (TAAs), which themselves occur primarily in the ascending aorta, i.e., the proximal region of the aortic arch[28]. While there exist many differences in the etiology, histology, and genetics of the two diseases, it is perhaps noteworthy that both develop predominantly in vessel segments that are situated at the low ends of Hox expression gradients with a greater pro-inflammatory predisposition in both cases, even though the inflammatory component in TAAs is lower than in AAAs[28]. This raises the intriguing question whether high levels of Hoxa9 and Hoxa4 expression may protect against the development of both aneurysms and atherosclerosis in the descending aorta, aka thoracic aorta. In that case, the further reduced HOXA4 levels clinically associated with AAA are likely the consequence of inflammatory stimuli, a view supported by in vitro data showing reduced HOXA4 expression in cultured human VSMCs and ECs treated with inflammatory cytokine IFN-γ[15].
Recent in vitro silencing experiments of HOX activities (HOXB7, HOXC6, HOXC8) in multipotent stem cells isolated from the adventitia of the human thoracic aorta provided evidence for Hox-dependent differentiation into VSMCs involving epigenetic mechanisms[29]. This raises the interesting possibility that Hox genes may be critical regulators of vascular cell populations that drive phenotype switching between homeostatic/regenerative vs pathological remodeling in vascular tissues in a region-specific manner.
CONCLUSION
Their persistent topographic expression patterns in post-natal vascular tissues suggest that Hox genes play a critical role in maintaining vessel wall homeostasis in a region-specific manner. Consequently, the idea that Hox-linked genetic polymorphisms affecting either their functional quality or expression levels may underlie CVD susceptibility in discrete portions of the vascular tree is a valid proposition. Albeit limited, the currently available in vivo data derived from analyses of Hox knockout and conditional Hox gain-of-function mutant mice, as well as from clinical studies support that view. Initial efforts of defining the genetic regulatory networks of Hox-dependent CVD processes implicate genes of diverse functional categories (ECM remodeling, transmembrane signaling, cell cycle control, inflammatory response, transcriptional control, etc.), as potential targets. The finding of region-specific reciprocal interactions of a Hox gene with a pro-inflammatory factor (NF-κB) is intriguing and might be of universal relevance in the search for therapeutic approaches. Within this context, defining the epigenetic mechanisms controlling Hox activities in blood vessels will be critical as indicated by data that associate hypomethylation of CpG islands within the HOXC11/HOXC9 genomic interval with the ectopic activation of HOXC10 and -9 in atherosclerotic coronary arteries[30].
P- Reviewer: Chawla M, Lyerly Jr J, Su H S- Editor: Ji FF L- Editor: A E- Editor: Wang CH