Published online Aug 26, 2014. doi: 10.4331/wjbc.v5.i3.387
Revised: May 7, 2014
Accepted: July 12, 2014
Published online: August 26, 2014
Processing time: 189 Days and 3.4 Hours
AIM: To determine the regulation of human hepcidin (HAMP) and mouse hepcidin (hepcidin-1 and hepcidin-2) gene expression in the liver by apoptosis using in vivo and in vitro experimental models.
METHODS: For the induction of the extrinsic apoptotic pathway, HepG2 cells were treated with various concentrations of CH11, an activating antibody for human Fas receptor, for 12 h. Male C57BL/6NCR and C57BL/6J strains of mice were injected intraperitoneally with sublethal doses of an activating antibody for mouse Fas receptor, Jo2. The mice were anesthetized and sacrificed 1 or 6 h after the injection. The level of apoptosis was quantified by caspase-3 activity assay. Liver injury was assessed by measuring the levels of ALT/AST enzymes in the serum. The acute phase reaction in the liver was examined by determining the expression levels of IL-6 and SAA3 genes by SYBR green quantitative real-time PCR (qPCR). The phosphorylation of transcription factors, Stat3, Smad4 and NF-κB was determined by western blotting. Hepcidin gene expression was determined by Taqman qPCR. The binding of transcription factors to hepcidin-1 promoter was studied using chromatin immunoprecipitation (ChIP) assays.
RESULTS: The treatment of HepG2 cells with CH11 induced apoptosis, as shown by the significant activation of caspase-3 (P < 0.001), but did not cause any significant changes in HAMP expression. Short-term (1 h) Jo2 treatment (0.2 μg/g b.w.) neither induced apoptosis and acute phase reaction nor altered mRNA expression of mouse hepcidin-1 in the livers of C57BL/6NCR mice. In contrast, 6 h after Jo2 injection, the livers of C57BL/6NCR mice exhibited a significant level of apoptosis (P < 0.001) and an increase in SAA3 (P < 0.023) and IL-6 (P < 0.005) expression in the liver. However, mRNA expression of hepcidin-1 in the liver was not significantly altered. Despite the Jo2-induced phosphorylation of Stat3, no occupancy of hepcidin-1 promoter by Stat3 was observed, as shown by ChIP assays. Compared to C57BL/6NCR mice, Jo2 treatment (0.2 μg/g b.w.) of C57BL/6J strain mice for 6 h induced a more prominent activation of apoptosis, liver injury and acute phase reaction. Similar to C57BL/6NCR mice, the level of liver hepcidin-1 mRNA expression in the livers of C57BL/6J mice injected with a sublethal dose of Jo2 (0.2 μg/g b.w.) remained unchanged. The injection of C57BL/6J mice with a higher dose of Jo2 (0.32 μg/g b.w.) did not also alter hepatic hepcidin expression.
CONCLUSION: Our findings suggest that human or mouse hepcidin gene expression is not regulated by apoptosis induced via Fas receptor activation in the liver.
Core tip: Apoptosis and Fas signaling participate in the pathogenesis of various liver diseases. Iron also contributes to liver injury. Changes in the expression of hepcidin, a key iron regulatory hormone, has been reported in various liver diseases. Recently, apoptosis has been implicated in the regulation of hepcidin. This study investigates effector caspase activation and apoptosis induced by Fas receptor signaling and its relationship to hepatic hepcidin expression.
- Citation: Lu S, Zmijewski E, Gollan J, Harrison-Findik DD. Apoptosis induced by Fas signaling does not alter hepatic hepcidin expression. World J Biol Chem 2014; 5(3): 387-397
- URL: https://www.wjgnet.com/1949-8454/full/v5/i3/387.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v5.i3.387
Apoptosis is involved in the pathogenesis of various liver diseases[1]. Hepatocyte apoptosis can be activated via the extrinsic apoptotic pathway through the binding of ligands to death receptors such as Fas, TNF receptor 1 and TRAIL receptor 2. Upon ligand binding, the receptor will trimerize and the C-terminal death domain will recruit Fas-associated protein with death domain to form death-inducing signaling complex (DISC), which subsequently recruits procaspase-8 and induces its self-cleavage and activation. Activated caspase-8 can directly cleave and activate caspase-3, the executioner caspase, which is responsible for the cleavage of target proteins to execute apoptosis. Caspase-3 activation is frequently used as a marker for apoptosis. Flice-Inhibitory Protein Long form (FLIPL) blocks apoptosis by inhibiting the recruitment and autoproteolytic cleavage of procaspase-8. In addition, in hepatocytes, the signal from death receptor can be amplified through the mitochondrial (intrinsic) apoptotic pathway. Activated caspase-8 can cleave Bcl-2 family protein, Bid. Truncated Bid (tBid) activates proapoptotic Bcl-2 family proteins, and induces permeabilization of the mitochondrial outer membrane and the leakage of the mitochondrial content including cytochrome c. Cytochrome c forms a complex with apoptotic peptidase activating factor 1, recruits and activates caspase-9, which subsequently cleaves caspase-3 and executes apoptosis.
A role for apoptosis has been suggested in the regulation of hepcidin[2,3]. Hepcidin, an antimicrobial peptide synthesized primarily by the liver, is the central regulator of iron metabolism. It is synthesized as an 84 amino acid precursor peptide, which is then cleaved to its 25 amino acid biologically active circulatory form. Unlike humans, who have one copy of hepcidin gene (HAMP), mice have two hepcidin genes, hepcidin-1 and hepcidin-2. Similar to HAMP, hepcidin-1 is involved in the regulation of iron homeostasis but the function of hepcidin-2 is unknown. Hepcidin exerts its regulatory function by blocking the uptake and export of dietary iron from the intestine and the release of iron from macrophages. Hepcidin achieves this by binding to ferroportin, the only known iron exporter, and causing its internalization and degradation via the lysosomal pathway. The suppression of hepcidin expression in the liver therefore leads to systemic iron overload whereas its induction causes iron deficiency and anemia.
Weizer-Stern et al[4] have demonstrated that p53, a tumor suppressor and inducer of apoptosis, participates in the regulation of hepcidin. In their study, a putative p53 response element on hepcidin gene promoter has been identified and validated by chromatin immunoprecipitation assays. Over-expression of p53 in hepatoma cells has been shown to induce hepcidin gene transcription and conversely, the silencing of p53 resulted in down-regulation of hepcidin expression[4]. It is however unclear whether p53-mediated apoptosis is involved in the regulation of hepatic hepcidin expression[4]. On the other hand, Li et al[5] have suggested a role for Fas signaling in the regulation of hepcidin expression in tissue culture cells and female mouse livers. A lethal dose of anti-Fas activating antibody, Jo2 has been reported to exert an immediate stimulatory and a late suppression effect on hepcidin mRNA expression in the liver[5]. Although a relationship between FLIPL, IL-6, Stat3 and hepcidin expression has been shown, they did not however establish a direct correlation between apoptosis and hepcidin. Besides committing cell death, Fas induced DISC formation also participates in the activation of cell signaling pathways, including IL-6 and NF-κB[6]. Of note, hepcidin expression is regulated by various signaling pathways. As an acute phase protein, hepcidin is stimulated by endotoxin and inflammatory cytokine signaling[7-9]. The effect of IL-6 is mediated through the activation of Jak/Stat pathway and the binding of Stat3 to hepcidin gene promoter[10,11]. As an iron regulatory protein, hepcidin is also regulated by the signals from iron sensors, such as bone morphogenetic protein 6 (BMP6)[12-14]. The BMP receptor-specific Smad pathway (via the phosphorylation of transcription factors, Smad1/5/8) has been shown to be involved in the up-regulation of hepcidin transcription. BMP6 knockout mice exhibit iron overload and reduced hepcidin expression[15-17]. Similarly, mice lacking the expression of the common Smad protein, Smad4 exhibit iron overload and a dramatic decrease in the expression of hepcidin in the liver[18]. In addition, growth factors such as epidermal growth factor and hepatocyte growth factor suppress the expression of hepcidin by inhibiting the signaling of the BMP-Smad pathway[19].
The aim of this study is to investigate the causal relationship between Fas-signaling-induced effector caspase activation and apoptosis, and the regulation of human and mouse hepcidin gene transcription. These studies will help us to further understand the regulation and the role of hepcidin in liver diseases.
HepG2 human hepatoma cells were maintained in high glucose Dulbecco’s modified Eagle’s medium supplemented with glutamine and 10% fetal calf serum (Atlantic Biologicals). 1.3 × 106 or 3.9 × 106 cells were seeded in 25 cm2 or 75 cm2 flasks, respectively 24 h prior to experiments. Cells were exposed to an activating antibody, which is specific for human Fas (clone CH11, Millipore) at different concentrations for 12 h.
Animal experiments were approved by the Animal Ethics Committee at the University of Nebraska Medical Center. C57BL/6J (the Jackson Laboratory) and C57BL/6NCR (NIH) strain male mice were maintained on a standard chow diet. 6 wk to 8 wk old male mice were injected intraperitoneally (i.p.) with an activating antibody, which is specific for mouse Fas (clone Jo2, BD Biosciences), at 0.2 μg or 0.32 µg per gram of body weight (b.w.). As control for i.p. injections, which per se might cause an acute phase reaction, a group of mice were injected with similar volume of 0.9% NaCl. All mice were sacrificed 1 or 6 h following injections.
RNA isolation, cDNA synthesis and quantitative PCR (qPCR) were performed, as described previously[20]. The sequence of Taqman fluorescent probe [5’6-(FAM); 3’ (TAMRA-Q)] and primers, and SYBR green primers are shown in Tables 1 and 2.
| Gene | Forward primer (5’-3’) | Reverse primer (5’-3’) | Taqman probe |
| HAMP | TGCCCATGTTCCAGAGGC | CCGCAGCAGAAAATGCAGAT | AAGGAGGCGAGACACCCACTTCCC |
| Human Gapdh | TGAAGGTCGGAGTCAACGG | AGAGTTAAAAGCAGCCCTGGTG | TTTGGTCGTATTGGGCGCCTGG |
| Hepcidin-1 | TGCAGAAGAGAAGGAAGAGAGACA | CACACTGGGAATTGTTACAGCATT | CAACTTCCCCATCTGCATCTTCTGCTGT |
| Hepcidin-2 | GCGATCCCAATGCAGAAGAG | TGTTACAGCACTGACAGCAGAATC | AGGAAGAGAGACATCAACTTCCCCATCTGC |
| Mouse Gapdh | TCACTGGCATGGCCTTCC | GGCGGCACGTCAGATCC | TTCCTACCCCCAATGTGTCCGTCG |
| Gene | Forward primer (5’-3’) | Reverse primer (5’-3’) |
| Mouse SAA3 | GCCTGGGCTGCTAAAGTCAT | TGCTCCATGTCCCGTGAAC |
| Mouse IL-6 | CTGCAAGAGACTTCCATCCAG | AGTGGTATAGACAGGTCTGTTGG |
| Mouse Gapdh | GTGGAGATTGTTGCCATCAACGA | CCCATTCTCGGCCTTGACTGT |
Caspase-3 activity assays were performed with Ac-DEVD-AMC caspase-3 fluorogenic substrate (BD Biosciences) as described previously[21].
Blood collected from the right atrium of the anesthetized animal before sacrifice and allowed to coagulate at room temperature for 30 min was centrifuged at 1300 ×g for 10 min to remove blood cells. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) liver enzyme levels in mice sera were measured at Clinical Chemistry Laboratory at University of Nebraska Medical Center.
The isolation of nuclear lysates from the livers and western blotting were performed, as described previously[22]. Anti-phospo-Stat3, anti-Stat3, anti-phospho-Smad 1/5, anti-p65 and Gapdh primary antibodies, and secondary antibodies were obtained commercially (Santa Cruz, Cell Signaling).
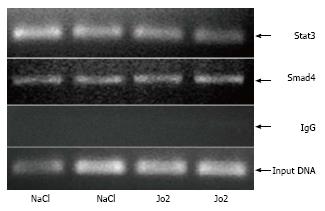
Chromatin immunoprecipitation (ChIP) assays were performed, as described previously[22,23]. Briefly, chromatin, isolated from formalin-fixed livers, was sheared by sonication and an aliquot was saved as total input DNA. Sheared chromatin was incubated with anti-Stat3, anti-Smad4 antibodies or control IgG and protein A-conjugated agarose beads (Santa Cruz, Cell signaling). Eluted DNA and total input DNA were subsequently analyzed by PCR using primers (sense: 5’- gccatactgaaggcactga -3’; antisense: 5’- gtgtggtggctgtctagg -3’) to amplify a 358bp region of the mouse hepcidin-1 promoter.
SPSS software was used for statistical analysis. The significance of difference between two groups was determined by Student’s t-test. A value of P < 0.05 was accepted as statistically significant.
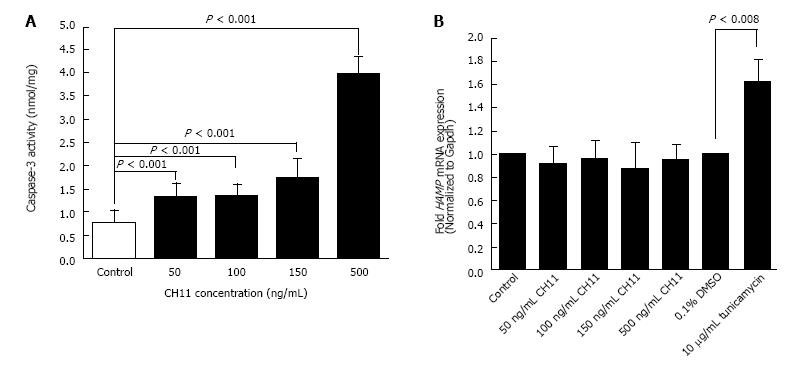
CH11 antibody treatment for 12 h induced apoptosis in HepG2 cells in a concentration dependent manner, as confirmed with caspase-3 activity assay. A significant induction of caspase-3 activity was observed at 50 ng/mL CH11 concentration, which increased four-fold with a 500 ng/mL concentration (Figure 1A). The level of HAMP mRNA expression in CH11-treated HepG2 cells was similar to that of control cells, as determined by qPCR (Figure 1B). HepG2 cells treated with 10 μg/mL of tunicamycin, a known inducer of hepcidin gene expression, for 8 h were used as the positive control (Figure 1B). These findings strongly suggest that the Fas-mediated apoptotic pathway does not alter HAMP transcription.
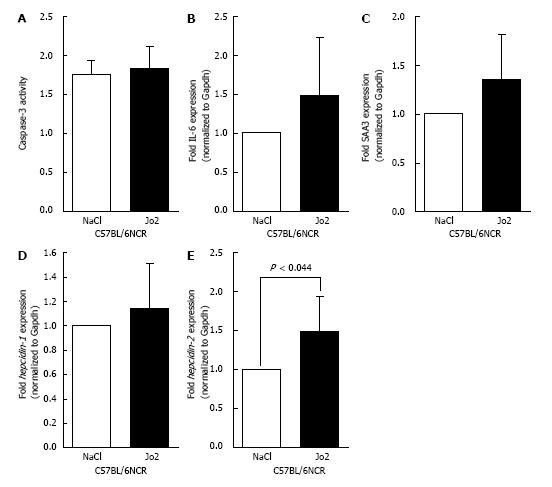
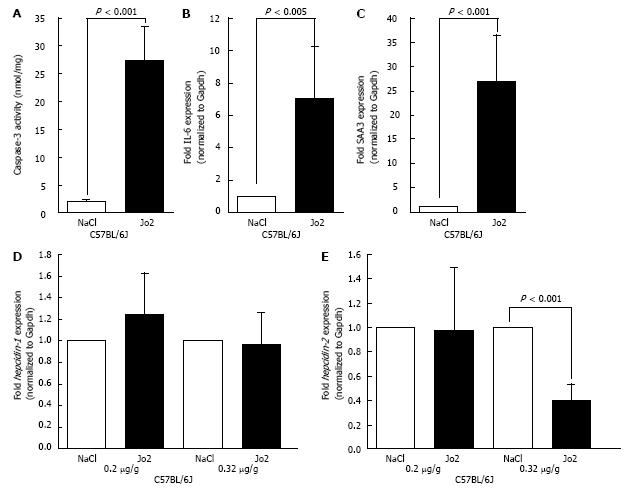
Since activation of human Fas did not alter human hepcidin gene expression, we performed experiments with activating antibody specific to mouse Fas, Jo2. C57BL/6NCR mice were injected with Jo2 antibody (0.2 μg/g b.w.) or NaCl (control), and sacrificed 1 h later. No significant increase in caspase-3 activity was observed in Jo2-injected mice compared to control mice (Figure 2A). The activation of acute phase reaction in the livers of these mice was evaluated by determining the levels of IL-6 and SAA3 mRNA expression by qPCR. Similar to caspase-3 activity, no significant changes were observed with the expression of these acute phase reaction genes (Figure 2B and C). The mRNA expression of hepcidin-1 in mice livers was also unaltered by Jo2 exposure, as confirmed by qPCR (Figure 2D). However, 1 h Jo2 treatment induced a small but significant increase in hepcidin-2 mRNA expression (Figure 2E).
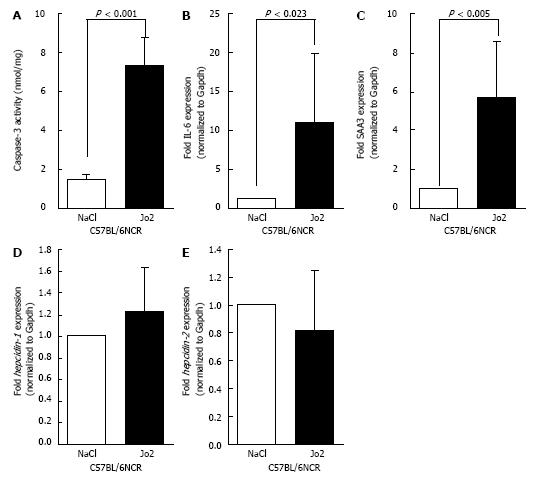
To further study the effect of Fas-mediated apoptosis, C57BL/6NCR mice were sacrificed 6 h after Jo2 or NaCl injections. A significant increase in caspase-3 activity was observed in the livers of Jo2-injected mice (Figure 3A). Similarly, the expression of acute phase reaction markers, IL-6 and SAA3 were also elevated in mice exposed to Jo2 for 6 h compared to control mice (Figures 3B and C). The levels of hepcidin-1 and hepcidin-2 mRNA expression in mice treated with Jo2 for 6 h were not significantly different from that in control mice (Figures 3D and E).
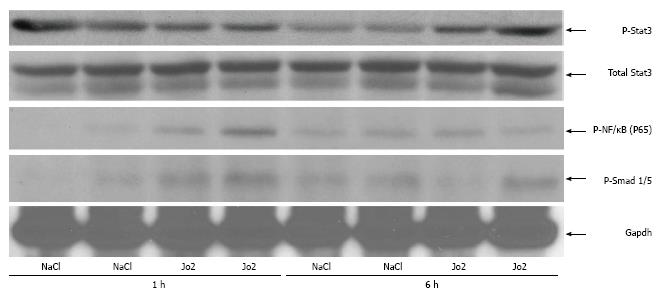
The cytokine, IL-6 is known to activate hepcidin transcription via the Jak/Stat3 signaling pathway[10,11].We therefore investigated the activation of Stat3 in mice injected with Jo2 antibody (0.2 μg/g b.w.) or NaCl and sacrificed 1 or 6 h later. Six hours, but not 1 h of Jo2 treatment, was sufficient to induce the phosphorylation of Stat3 in the livers of mice, compared to respective control mice (Figure 4). Despite the activation of Stat3, we did not observe any significant changes in Stat3 binding to hepcidin-1 promoter in Jo2-treated mice compared to control mice, as determined by ChIP assays (Figure 5).
NF-κB is one of the important transcription factors activated by Fas ligand binding. NF-κB activates the transcription of inflammatory cytokines including IL-6[1]. We therefore investigated the phosphorylation of the p65 subunit of NF-κB in mice treated with Jo2 for 1 or 6 h time periods. In contrast to Stat3, Jo2 induced a fast and transient activation of NF-κB. The phosphorylation of p65 in the liver was observed within 1 h after Jo2 injection and was absent at 6 h after Jo2 exposure (Figure 4).
Besides Jak/Stat3 pathway, hepcidin is also regulated by bone morphogenetic protein 6 (BMP6) and Smad pathway. This pathway has also been suggested to play a negative role in growth factor-induced regulation of hepcidin expression in the liver[19]. We therefore determined the activation of transcription factors, Smad1 and Smad5, which are activated downstream of BMP signaling pathway. Similar to NF-κB, Jo2 treatment induced an early and transient activation of Smad1/5 in the liver. The induction in Smad1/5 phosphorylation observed by 1 h Jo2 exposure was significantly weakened by 6 h after Jo2 injection (Figure 4). The binding of Smad4, the common mediator of Smad signaling, to mouse hepcidin-1 promoter was also examined by ChIP assays. No significant increase in Smad4 occupancy of hepcidin-1 promoter region harboring a Smad4 binding site was observed at 6 h after Jo2 injection, as compared to controls (Figure 5).
In order to investigate the effect of Jo2 further, we employed a substrain of C57BL/6 mice. Of note, C57BL/6NCR and C57/BL/6J strains exhibit substantial genetic differences[24]. Compared to that observed with C57BL/6NCR, C57BL/6J mice treated with Jo2 (0.2 μg/g b.w.) for 6 h exhibited a significantly higher elevation of caspase-3 activity (Figure 6A). Similar robust activation was also observed with the expression of acute phase marker genes, IL-6 and SAA3 (Figures 6B and C). However, despite stronger apoptosis and acute phase reactions, Jo2 treatment did not induce any significant changes in the expression of both hepcidin-1 and hepcidin-2 in the livers of C57BL/6J mice, as was the case with C57BL/6NCR mice (compare Figures 6D, E and3D, E). Furthermore, the treatment of C57BL/6J mice with an even higher concentration of Jo2 (0.32 μg/g b.w.) did not induce any changes in the mRNA level of hepcidin-1. (Figure 6D). However, the treatment with 0.32 µg/g of Jo2 induced a significant suppression of hepcidin-2 mRNA expression (Figure 6E).
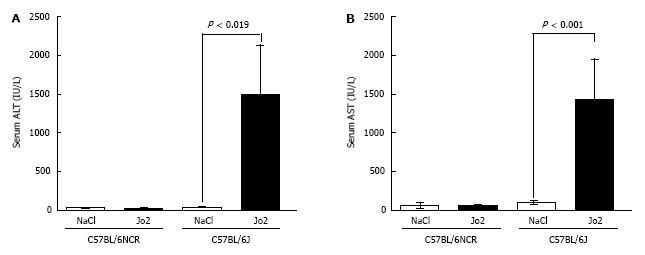
Jo2-induced apoptosis and acute phase reaction was stronger in the livers of C57BL/6J mice, compared to C57BL/6NCR mice. We therefore measured the serum levels of liver enzymes, ALT and AST, which is a commonly used diagnostic test to determine liver function and injury. Jo2 injected at a concentration of 0.2 μg/g did not cause a significant elevation of serum ALT or AST levels in C57BL6/NCR mice (Figure 7). However, the sera of C57BL/6J mice injected with 0.2 μg/g of Jo2, exhibited a dramatic increase in both ALT and AST levels, compared to controls (Figure 7). The injection of C57BL/6J mice with a higher dose of Jo2 (0.32 μg/g) induced further increase in serum ALT and AST levels (data not shown).
Apoptosis is one of the key factors which contribute to the pathogenesis of many liver diseases[1,25,26]. Apoptosis not only causes hepatocyte death directly but also induces inflammation and hepatic fibrosis[27,28]. The inhibition of caspase enzymes via known caspase inhibitors has been shown to effectively alleviate hepatocyte apoptosis and tissue damage in animal models of liver injury[29]. Due to its highly reactive nature and the liver serving as the major storage organ for it, iron is considered an important secondary risk factor in the progression of various liver diseases[30,31]. Therefore, it is of great importance to understand the interaction between apoptosis and iron metabolism. Since hepcidin is the central regulator of iron homeostasis and is primarily synthesized in the liver, this study investigated the effect of apoptosis on the regulation of hepcidin expression in the liver. Previously, Weizer-Stern et al[4] have elegantly demonstrated that p53, a tumor suppressor gene and an inducer of apoptosis, elevates human hepcidin gene transcription in HepG2 cells by binding to the corresponding response elements in hepcidin gene promoter. They have also reported that the overexpression of p53 blunts the stimulatory effect of IL-6 on hepcidin gene expression. Although indirect, these findings, for the first time, suggested a relationship between apoptosis and hepcidin and thereby the regulation of iron metabolism. However, due to various reasons, the in vivo relevance of this potential interaction is unclear. First, apoptosis signaling in cancer cell lines is frequently distorted and secondly, forced overexpression of p53 might have caused artificial effects. However, in a recent study, Li et al[5] have investigated the relationship between Fas -activated apoptosis signaling and expression of hepcidin gene expression. Fas activation decreased both mouse and human hepcidin mRNA expression in vitro. They have also shown that Balb/C3 female mice injected with lethal dose of Jo2 antibody (which killed mice within 4 h) exhibit a biphasic regulation of mouse hepcidin mRNA expression in the liver, namely an immediate elevation (within 0.5-1 h) followed by a suppression (within 4 h). They suggested that these changes in hepcidin expression correlates with the changes in FLIPL and IL-6 expression as well as the activation of the transcription factors, NF-κB, and Stat3. The knock-down or over-expression of FLIPL exerted a negative and a positive effect, respectively, on hepcidin expression. Based on their data, Li et al[5] have proposed a model suggesting that the stimulatory effect of Fas on hepcidin expression is achieved via IL-6 and Stat3, which themselves are activated by FLIPL and NF-κB. However, Li et al[5] did not confirm the presence (and the level) of apoptosis in the livers of Jo2-injected mice. Hence, it is unclear whether Fas-mediated apoptosis is directly involved in the regulation of hepcidin gene expression in the liver.
In our current study, we examined the effect of Fas signaling on hepatic hepcidin gene expression both in vivo and in vitro. In in vitro studies, the effect of CH11, an activating antibody specific for human Fas, was evaluated on hepcidin expression in HepG2 hepatoma cells. Even though CH11 induced apoptosis in a concentration dependent manner, as confirmed by the increased caspase-3 activity, the expression of human hepcidin gene was not significantly altered in these cells. Although we cannot exclude the possibility that Fas-mediated signaling in HepG2 hepatoma cells might be different than primary human hepatocytes, our findings strongly suggest that hepcidin gene expression in hepatocytes does not correlate with the significant induction of caspase activation. Of note, the liver is composed of various cell types and it is therefore feasible that not only hepatocytes, but other cells such as Kupffer cells, might be involved in the regulation of hepcidin gene by apoptosis. Hence, an in vivo experimental model whereby male C57BL/6 mice are injected with Jo2 antibody to specifically activate Fas-mediated apoptosis was employed to study hepcidin gene expression in whole liver. Male mice were chosen for these studies because unlike humans, female mice express higher levels of hepcidin compared to male mice[32]. Sublethal concentrations of Jo2 antibody were chosen for our experiments because based on the reports in the literature, this dose of Jo2 is more suitable for studies, which investigate the activation of Fas-mediated apoptosis in liver diseases[33]. Accordingly, we observed no lethality under our experimental conditions. Mice with short-term (1 h) Jo2 treatment did not display any significant changes both in the activity of caspase-3 enzyme and the expression of acute phase marker genes, IL-6 and SAA3, strongly suggesting the absence of apoptosis and acute phase responses in the livers of these mice. The macroscopic appearance of the livers from short-term Jo2-injected mice was also similar to those in control mice (data not shown). Further, the expression level of mouse hepcidin-1 was not affected in the livers of these mice. In contrast, longer treatment with Jo2 (6 h) significantly induced apoptosis and acute phase reaction. Concurrently, the livers of these mice displayed macroscopic differences such as a darker color suggesting the presence of hepatic hemorrhage and liver injury (data not shown). Interestingly, these changes did not correlate with the level of hepcidin-1 mRNA expression in the liver. Similar to short-term, the longer treatment of mice with Jo2 did not alter the level of hepcidin gene expression.
It is well known that hepcidin gene transcription is strongly stimulated by IL-6 and Stat3 pathway. Since, we have shown that long-term, but not short-term, Jo2 treatment can induce acute phase reactions in the liver, the phosphorylation status of Stat3 was investigated to confirm its activation. In accordance with our acute phase gene expression findings, we observed Stat3 phosphorylation following 6 h, but not 1 h of Jo2 treatment. Taken together, our findings show that the activation of IL-6/Stat3 axis by Fas is not sufficient to induce hepcidin-1 transcription and strongly suggest the presence of inhibitory mechanisms. This is also supported by our ChIP findings, which show that the occupancy of hepcidin-1 promoter by Stat3 is similar in the livers of both Jo2-treated and control mice despite the differences in the activation status of Stat3. In contrast to Stat3, the phosphorylation of NF-κB was observed with short-term, but not long-term, Jo2 treatment. NF-κB is involved in inflammatory cytokine production in the liver including IL-6[26]. It is therefore possible that Jo-2-mediated early phase activation of NF-κB subsequently facilitates the induction of IL-6 transcription and consequent activation of Stat3, which was observed in the livers of mice with longer (6 h) Jo2 treatment.
Hepcidin is also activated by BMP/Smad pathway and Smad4 knockout mice display reduced hepcidin expression[18]. However, 6 h Jo2 administration did not significantly alter the phosphorylation of Smad1/5 proteins, which are transcription factors known to be activated by BMP pathway. Growth factors have been shown to suppress the signaling of BMP/Smad pathway and its stimulatory effect on hepcidin gene expression in the liver[19]. Of note, liver injury is known to stimulate the expression of growth factors as part of the liver regeneration process. It is therefore feasible that Fas-induced liver injury might suppress Smad activation and thereby counteract the stimulation of hepcidin-1 transcription by Stat3. However, it should also be noted that despite significant differences in the level of liver injury, acute phase response and apoptosis, both C57BL/6J and C57BL/6NCR mice (under similar experimental conditions) did not display any significant changes in liver hepcidin-1 expression. This suggests that mechanisms other than growth factors and inhibitory Smads might play a role in this process. Jo2-induced liver damage accompanied by DNA damage and the activation of p53 might be involved since p53 has been shown to suppress the stimulatory effect of IL-6 on hepcidin gene expression[4]. Furthermore, we observed differential regulation of hepcidin-2 expression by Jo2 in the liver. Since the function of hepcidin-2 is unknown, the significance of this finding and its potential role in liver injury and disease will be addressed in future studies.
In conclusion, Fas ligand-induced signaling and apoptosis do not play a significant role in the regulation of human (HAMP) or mouse (hepcidin-1) hepatic hepcidin gene expression, as shown by in vitro and in vivo experimental systems using human or mouse Fas receptor-activating antibodies, CH11 or Jo2 , respectively. The induction of extrinsic apoptotic pathway via Fas receptor signaling, as confirmed by effector caspase activation, significantly induced the phosphorylation and activation of the transcription factor, Stat3 in the liver. Stat3 is well known to be involved in inflammation-mediated elevation of hepcidin expression. However, no significant binding of Stat3 to hepcidin gene promoter was observed, as confirmed by chromatin immunoprecipitation studies. Our findings strongly suggest that the activation of Stat3 by Fas signaling-mediated apoptosis is not sufficient to stimulate hepcidin transcription in the liver. Using different strains of mice, we were also able to confirm that the severity of Fas-induced apoptosis (acute phase reaction or tissue injury) does not correlate with its effect on hepcidin gene expression in the liver. Interestingly, Jo2 treatment induced changes in hepcidin-2 expression in a time-dependent manner but the function of this mouse gene is as yet unknown.
Apoptosis is widely observed and participates in the pathogenesis of various liver diseases. Iron, due to its highly reactive nature as a transitional metal, acts as a secondary risk factor in various liver diseases. Hepcidin, a small peptide primarily synthesized in the liver, is the central regulator of iron metabolism. Hepcidin maintains iron homeostasis by inhibiting iron absorption by the enterocytes in the duodenum and iron release by the macrophages of reticuloendothelial system. Hepcidin expression in the liver has been shown to be modulated by iron, inflammation and hypoxia but a direct role of apoptosis in hepcidin regulation has not been elucidated.
Iron plays a role in liver injury and the transcriptional regulation of hepcidin gene in liver diseases has been highlighted by recent studies from various laboratories. A better understanding of the regulation of hepcidin and thereby iron homeostasis in the pathogenesis of liver diseases might facilitate the development of novel diagnosis and treatment strategies.
P53, a tumor suppressor and inducer of apoptosis, has been reported to promote hepcidin gene transcription through direct binding to its promoter. An independent study has recently suggested the involvement of Fas signaling in the regulation of liver hepcidin expression. However, a causal relationship between Fas-mediated effector caspase activation and apoptosis, and the regulation of hepcidin gene transcription has not been demonstrated. This study addressed this question by quantifying the level of Fas-induced apoptosis by caspase-3 activity assays and correlating it to both human and mouse hepcidin gene expression. In addition, different strains of mice with significant variances in their response to Fas treatment were also employed. Collectively, the authors’ findings clearly demonstrate the lack of correlation between Fas-mediated apoptosis and hepatic hepcidin gene transcription.
The findings of this study, which strongly suggest that Fas-mediated apoptosis is not involved in the regulation of hepcidin expression, will further our understanding of the pathogenesis of liver diseases associated with increases in hepatic iron content. Furthermore, the authors’ findings, which demonstrate strain-specific responses to anti-Fas treatment, highlights the importance of choosing optimal mice strains for studies with Fas signaling in the liver.
Apoptosis, also known as programmed cell death, is characterized by the sequential activation of a series of caspases, which also serve as markers for apoptosis. The pathogenesis of many liver diseases involve apoptotic pathways. Hepcidin is a small antimicrobial peptide synthesized mainly by the hepatocytes. It regulates iron homeostasis by binding to and inducing the degradation of the only known iron exporter, ferroportin.
In this paper, Lu et al aimed to determine the regulation of human hepcidin (HAMP) and mouse hepcidin (hepcidin-1 and hepcidin-2) gene expression in the liver by apoptosis using in vivo and in vitro experimental models. The role of hepcidin in liver fibrosis, via apoptosis, has emerged in recent years. Given the goal of achieving an explanation about the role of hepcidin is a growing concern, the analysis is justified and the aim of the study is clinically relevant. It is a well-designed study and the conclusions are consistent with the results.
P- Reviewer: Ampuero J, Ditzel M, Yu HP S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Schattenberg JM, Galle PR, Schuchmann M. Apoptosis in liver disease. Liver Int. 2006;26:904-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Ganz T. Hepcidin and iron regulation, 10 years later. Blood. 2011;117:4425-4433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 671] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 3. | Ganz T, Nemeth E. Hepcidin and disorders of iron metabolism. Annu Rev Med. 2011;62:347-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 356] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 4. | Weizer-Stern O, Adamsky K, Margalit O, Ashur-Fabian O, Givol D, Amariglio N, Rechavi G. Hepcidin, a key regulator of iron metabolism, is transcriptionally activated by p53. Br J Haematol. 2007;138:253-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Li X, Xu F, Karoopongse E, Marcondes AM, Lee K, Kowdley KV, Miao CH, Trobridge GD, Campbell JS, Deeg HJ. Allogeneic transplantation, Fas signaling, and dysregulation of hepcidin. Biol Blood Marrow Transplant. 2013;19:1210-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Guicciardi ME, Gores GJ. Life and death by death receptors. FASEB J. 2009;23:1625-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 485] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 7. | Villarroel P, Le Blanc S, Arredondo M. Interleukin-6 and lipopolysaccharide modulate hepcidin mRNA expression by HepG2 cells. Biol Trace Elem Res. 2012;150:496-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101:2461-2463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 962] [Cited by in RCA: 995] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 9. | Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1638] [Cited by in RCA: 1666] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 10. | Verga Falzacappa MV, Vujic Spasic M, Kessler R, Stolte J, Hentze MW, Muckenthaler MU. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood. 2007;109:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 434] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 11. | Pietrangelo A, Dierssen U, Valli L, Garuti C, Rump A, Corradini E, Ernst M, Klein C, Trautwein C. STAT3 is required for IL-6-gp130-dependent activation of hepcidin in vivo. Gastroenterology. 2007;132:294-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 241] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 12. | Babitt JL, Huang FW, Wrighting DM, Xia Y, Sidis Y, Samad TA, Campagna JA, Chung RT, Schneyer AL, Woolf CJ. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet. 2006;38:531-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 823] [Cited by in RCA: 780] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 13. | Truksa J, Peng H, Lee P, Beutler E. Bone morphogenetic proteins 2, 4, and 9 stimulate murine hepcidin 1 expression independently of Hfe, transferrin receptor 2 (Tfr2), and IL-6. Proc Natl Acad Sci USA. 2006;103:10289-10293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 250] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 14. | Lin L, Valore EV, Nemeth E, Goodnough JB, Gabayan V, Ganz T. Iron transferrin regulates hepcidin synthesis in primary hepatocyte culture through hemojuvelin and BMP2/4. Blood. 2007;110:2182-2189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 201] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 15. | Shanmugam NK, Cherayil BJ. Serum-induced up-regulation of hepcidin expression involves the bone morphogenetic protein signaling pathway. Biochem Biophys Res Commun. 2013;441:383-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Andriopoulos B, Corradini E, Xia Y, Faasse SA, Chen S, Grgurevic L, Knutson MD, Pietrangelo A, Vukicevic S, Lin HY. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet. 2009;41:482-487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 633] [Cited by in RCA: 592] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 17. | Meynard D, Kautz L, Darnaud V, Canonne-Hergaux F, Coppin H, Roth MP. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat Genet. 2009;41:478-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 462] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 18. | Wang RH, Li C, Xu X, Zheng Y, Xiao C, Zerfas P, Cooperman S, Eckhaus M, Rouault T, Mishra L. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab. 2005;2:399-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 475] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 19. | Goodnough JB, Ramos E, Nemeth E, Ganz T. Inhibition of hepcidin transcription by growth factors. Hepatology. 2012;56:291-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 20. | Harrison-Findik DD, Klein E, Crist C, Evans J, Timchenko N, Gollan J. Iron-mediated regulation of liver hepcidin expression in rats and mice is abolished by alcohol. Hepatology. 2007;46:1979-1985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Harrison-Findik DD, Lu S, Zmijewski EM, Jones J, Zimmerman MC. Effect of alcohol exposure on hepatic superoxide generation and hepcidin expression. World J Biol Chem. 2013;4:119-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Gerjevic LN, Liu N, Lu S, Harrison-Findik DD. Alcohol Activates TGF-Beta but Inhibits BMP Receptor-Mediated Smad Signaling and Smad4 Binding to Hepcidin Promoter in the Liver. Int J Hepatol. 2012;2012:459278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Weinmann AS, Bartley SM, Zhang T, Zhang MQ, Farnham PJ. Use of chromatin immunoprecipitation to clone novel E2F target promoters. Mol Cell Biol. 2001;21:6820-6832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 319] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 24. | Mekada K, Abe K, Murakami A, Nakamura S, Nakata H, Moriwaki K, Obata Y, Yoshiki A. Genetic differences among C57BL/6 substrains. Exp Anim. 2009;58:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 267] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 25. | Rust C, Gores GJ. Apoptosis and liver disease. Am J Med. 2000;108:567-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 132] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Chakraborty JB, Oakley F, Walsh MJ. Mechanisms and biomarkers of apoptosis in liver disease and fibrosis. Int J Hepatol. 2012;2012:648915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 27. | Canbay A, Taimr P, Torok N, Higuchi H, Friedman S, Gores GJ. Apoptotic body engulfment by a human stellate cell line is profibrogenic. Lab Invest. 2003;83:655-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 311] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 28. | Canbay A, Feldstein A, Baskin-Bey E, Bronk SF, Gores GJ. The caspase inhibitor IDN-6556 attenuates hepatic injury and fibrosis in the bile duct ligated mouse. J Pharmacol Exp Ther. 2004;308:1191-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 166] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 29. | Natori S, Higuchi H, Contreras P, Gores GJ. The caspase inhibitor IDN-6556 prevents caspase activation and apoptosis in sinusoidal endothelial cells during liver preservation injury. Liver Transpl. 2003;9:278-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Fargion S, Valenti L, Fracanzani AL. Beyond hereditary hemochromatosis: new insights into the relationship between iron overload and chronic liver diseases. Dig Liver Dis. 2011;43:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Datz C, Felder TK, Niederseer D, Aigner E. Iron homeostasis in the metabolic syndrome. Eur J Clin Invest. 2013;43:215-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 32. | Vujic Spasic M, Kiss J, Herrmann T, Kessler R, Stolte J, Galy B, Rathkolb B, Wolf E, Stremmel W, Hentze MW. Physiologic systemic iron metabolism in mice deficient for duodenal Hfe. Blood. 2007;109:4511-4517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Haga S, Terui K, Zhang HQ, Enosawa S, Ogawa W, Inoue H, Okuyama T, Takeda K, Akira S, Ogino T. Stat3 protects against Fas-induced liver injury by redox-dependent and -independent mechanisms. J Clin Invest. 2003;112:989-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 86] [Article Influence: 3.9] [Reference Citation Analysis (0)] |