Published online Aug 26, 2014. doi: 10.4331/wjbc.v5.i3.377
Revised: April 2, 2014
Accepted: June 27, 2014
Published online: August 26, 2014
Processing time: 205 Days and 7.3 Hours
AIM: To determine if doxorubicin (Dox) alters hepatic proteome acetylation status and if acetylation status was associated with an apoptotic environment.
METHODS: Doxorubicin (20 mg/kg; Sigma, Saint Louis, MO; n = 8) or NaCl (0.9%; n = 7) was administered as an intraperitoneal injection to male F344 rats, 6-wk of age. Once animals were treated with Dox or saline, all animals were fasted until sacrifice 24 h later.
RESULTS: Dox treatment decreased proteome lysine acetylation likely due to a decrease in histone acetyltransferase activity. Proteome deacetylation may likely not be associated with a proapoptotic environment. Dox did not increase caspase-9, -8, or -3 activation nor poly (adenosine diphosphate-ribose) polymerase-1 cleavage. Dox did stimulate caspase-12 activation, however, it likely did not play a role in apoptosis induction.
CONCLUSION: Early effects of Dox involve hepatic proteome lysine deacetylation and caspase-12 activation under these experimental conditions.
Core tip: Doxorubicin (Dox) is an effective chemotherapeutic agent, but known to cause cardiotoxicity and hepatotoxicity. Cellular stress can alter proteome acetylation status in various experimental models, which has been associated with a proapoptotic environment. The effects of Dox on hepatic lysine acetylation status has not been studied. The study revealed five interesting findings that open the door for new areas of investigation: (1) Dox induces proteome lysine deacetylation; (2) lysine deacetylation is, at least in part, due to a decrease in histone acetyltransferase activity; (3) lysine deacetylation is likely not associated with an apoptotic environment; (4) Dox-induced hepatic injury is associated with caspase-12 activation; and (5) caspase-12 activation is not involved in apoptosis induction. These results may in the future translate to lysine acetylation homeostasis and/or caspase-12 as therapeutic targets.
- Citation: Dirks-Naylor AJ, Kouzi SA, Bero JD, Tran NT, Yang S, Mabolo R. Effects of acute doxorubicin treatment on hepatic proteome lysine acetylation status and the apoptotic environment. World J Biol Chem 2014; 5(3): 377-386
- URL: https://www.wjgnet.com/1949-8454/full/v5/i3/377.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v5.i3.377
Doxorubicin (Dox) is a highly effective therapeutic agent in treating various cancers, but proven to cause cardiotoxicity. Mechanisms of cardiotoxicity have not been distinctly defined, but published literature has suggested that the induction of oxidative stress, apoptosis and mitochondrial dysfunction are involved[1-3]. In addition to proven toxicity in cardiac tissue, hepatic tissue reveals molecular and morphological signs of toxicity[4-9]. Since oxidative stress has been shown to play a role in both cardiac and hepatic toxicity, the use of antioxidants as a preventative measure has been widely studied. However, supplementation with antioxidants in clinical trials have only shown limited protection[10]. Thus, additional research is essential to determine additional mechanisms involved in Dox-induced toxicities. Therefore, the aim of this study was to determine the role of proteome lysine acetylation dyshomeostasis and apoptosis in Dox-induced hepatic toxicity.
Lysine acetylation is a common posttranslational modification[11]. Lysine acetylation regulates function of histones, proteins affecting the ubiquitin-proteosome system, transcription factors, cytoskeletal components, energy generating enzymes, and oxidative stress defense proteins[11]. In various experimental models, cellular stress has been shown to alter proteome lysine acetylation status, causing deacetylation, and thereby affecting the apoptotic environment[12]. Moreover, normalization of the lysine acetylation status has been shown to prevent stimulation of apoptotic pathways[12]. The effects of Dox on proteome acetylation status is unknown, thus, we aimed to make this determination. We hypothesized that Dox-induced cellular injury and stress would lead to lysine deacetylation and may contribute to Dox-induced toxicity. Acetylation status is determined by activity of histone acetyltransferases (HATs) and histone deacetylases (HDACs)[11,13]. Thus, we also investigated potential mechanisms of deacetylation by assessing general HDAC activity and expression of sirtuins, one of several known classes of HDACs, as well as HAT activity.
Secondly, we aimed to determine if the proteome lysine acetylation status was associated with a proapoptotic environment, as shown in other experimental models. Apoptosis can be activated by a variety of specific signaling pathways (e.g., receptor-mediated, mitochondrial-mediated, endoplasmic reticulum (ER)-mediated, etc.), depending on the initiating stimulus, and is associated with a distinct apical caspase[14]. These apical caspases converge on caspase-3, which is considered the central executioner of apoptosis. Activation of caspase-3 is responsible for the demise of the cell and most of the characteristic morphology associated with apoptosis, such as DNA fragmentation, cell shrinkage, and membrane blebbing. Thus, we assessed the content and activation of apical caspases and the activity of caspase-3 to determine if the proteome acetylation status was associated with the apoptotic environment in this experimental model. Furthermore, the effects of Dox on apical caspases and their role in hepatic toxicity have not been previously studied.
The institutional Research Review Board approved all protocols and procedures. Male F344 rats, 6-weeks of age, were purchased from Charles River (Wilmington, MA). The rats were arbitrarily separated into groups and were housed in a light controlled and temperature (22 °C ± 2 °C) controlled facility. Doxorubicin (20 mg/kg; Sigma, Saint Louis, MO; n = 8) or NaCl (0.9%; n = 7) was injected intraperitoneal (IP). The dose of doxorubicin used in this study is equivalent to clinical doses used in humans that are pharmacologically scaled for use in rats[15,16]. Food and water was withheld from all animals during the experimental period (24 h). Doxorubicin administration can cause up to an approximate 70% decrease in both food and water intake within several hours of administration and typically persists for numerous days[17]. All other investigations examining the effects of Dox on the liver in laboratory animals did not control for differences in food and water intake, thus the Dox group was nutrient deficient while the control group received plenty of food and water. As a result, it was not possible to distinguish between the effects of Dox and the effects of anorexia. Anorexia has been shown to alter hepatic oxidative stress, autophagy, mitochondrial morphology, survival signaling, and many more processes[18-21]. Thus, to control for Dox-induced anorexia we fasted all animals. After exposure to ether, rats were sacrificed by dislocation of the cervical spine. Tissues were excised immediately following sacrifice, saline rinsed and frozen in liquid nitrogen. Tissues were stored at -80 °C until analysis.
Liver samples were homogenized (Power Gen 125, Fisher Scientific, Pittsburgh, PA) in ice-cold phosphate-buffered saline (2.68 mmol/L KCl , 1.75 mmol/L KH2PO4, 137 mmol/L NaCl, 10 mmol/L Na2HPO4, 5 mmol/L EDTA). Ten uL/mL of Halt Phosphatase Inhibitor Cocktail and 10 uL/mL of Halt Protease Inhibitor Cocktail (Pierce Biochemicals, Rockford, IL) was added to the buffer immediately before homogenization. The homogenate was centrifuged at 660 × g at 4 °C for 10 min. The supernatant was used for biochemical analysis. The Bicinchoninic Acid Protein Assay Kit (Sigma, Saint Louis, MO) was used to assess protein concentration. Samples were run in quadruplicate.
Protein (proteome) lysine acetylation and protein content of sirtuin 1 (Sirt1), sirtuin 3 (Sirt3), poly (ADP-ribose) polymerase-1 (PARP-1), and procaspase-1, -8, -9, -12 were determined by standard wet Western blot analysis. Proteins (50 ug) were separated on tris/glycine 4%-20% separating polyacrylamide PAGEr Gold Precast Gels (Lonza, Rockland, ME) under denaturing conditions and transferred to nitrocellulose membranes. Membranes were blocked in PBS blocking solution containing 5.0% powdered milk for one hour at room temperature. Membranes were incubated in primary antibody overnight at 4 °C (dilution of 1:1000; antibodies were purchased from Santa Cruz Biotechnology, INC, Santa Cruz, CA, sc-137254, sc-7150, sc-56036, sc-166320, sc-81663, sc-21747, sc-271014, sc-15404, sc-99143, sc-32268). Membranes were incubated with secondary HRP-linked antibody, with a dilution of 1:10000, for two hours shaking at room temperature. Bands of interest were imaged using SuperSignal West Pico Chemiluminescent Substrate (Pierce Biochemicals, Rockford, IL) and the Kodak IS4000R Imaging System (Carestream Health, Inc., New Haven, CT). To assess equal loading of protein, ponceau staining (Pierce Biochemicals, Rockford, IL) of the nitrocellulose membranes was used. Whole lane analysis for each sample was used to determine densitometry of Ponceau staining. Data are presented as arbitrary units of densitometry calculated by subtracting the background intensity from the mean intensity of each band. Arbitrary OD for each band was normalized to the densitometry of Ponceau staining of each lane to account for variances in loading. The utilization of Ponceau staining as a reproducible alternative to actin in assessing equal loading has been validated [22].
An HDAC Colorimetric Assay (BioVision, Milpitas, CA) was purchased to determine HDAC activity. The manufacturer’s instructions were followed. Samples were run in triplicate. Data are expressed as arbitrary OD/protein concentration as determined by the BCA Assay.
Histone acetyltransferse activity was measured using a HAT Colorimetric Assay Kit (BioVision, Milpitas, CA). The manufacturer’s instructions were followed. Samples were run in triplicate. Data are expressed as arbitrary OD/protein concentration as determined by the BCA Assay.
A Caspase-3 Colorimetric Assay Kit (BioVision, Milpitas, CA) was used to determine caspase-3 activity. The manufacturer’s instructions were followed. Samples were run in triplicate. Data are expressed as arbitrary OD/protein concentration as determined by the BCA Assay.
For statistical analysis, a Student’s t-test was used. P < 0.05 was deemed statistically significant. Data is presented as mean ± SEM. For all analysis the n = 8 for the Dox group and n = 7 for the control group.
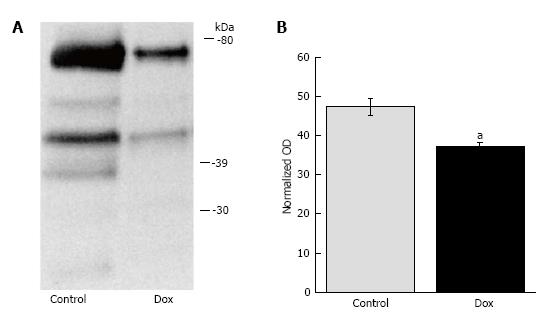
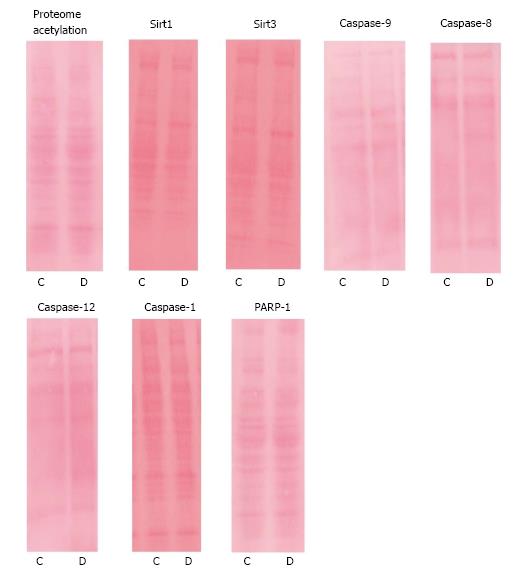
Since proteome lysine deacetylation has been associated with pro-apoptotic environment and cellular injury in alternative experimental models, we determined the effects of Dox on acetylation status in the liver. Dox promoted proteome lysine deacetylation of treated animals (42.88 ± 2.06 vs 32.03 ± 1.00, P = 0.002; control vs Dox, respectively; Figure 1). Proteins of molecular weight between 30-80 kDa appear to be most affected, as we did not observe protein bands detected by the lysine acetylation antibody above or below these molecular weights. These results suggest that early effects of acute Dox toxicity involve proteome lysine deacetylation.
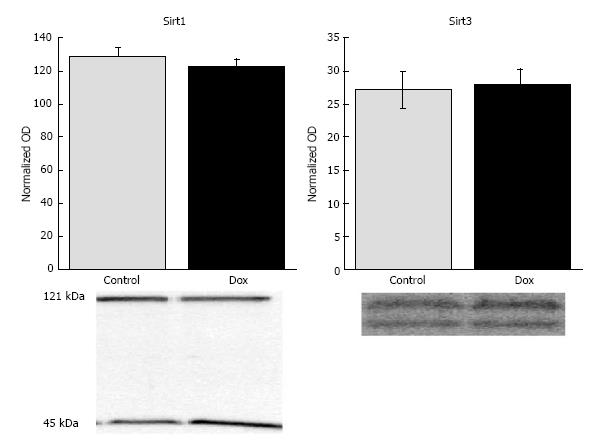
Sirtuins are a class of HDACs that have recently been under intense investigation, in part, due to their potential role in longevity[23]. To investigate the potential mechanism of Dox-induced deacetylation, we investigated the effects of Dox on the expression of Sirt1 and Sirt3, two of the most studied lysine deacetylases in the sirtuin family. Despite significant deacetylation, Sirt1 content was not affected by Dox treatment (128.57 ± 6.10 vs 122.89 ± 4.09, control vs Dox, respectively, P = 0.44) nor Sirt3 (27.14 ± 2.69 vs 28.00 ± 2.28, control vs Dox, respectively, P = 0.81; Figure 2).
Interestingly, a prominent band of approximately 45 kDa was detected in the liver with the Sirt1 antibody. Dox treatment increased the content of this species compared to control (139. 0 ± 4.25 vs 107.86 ± 4.06, respectively, P = 0.0001). It is possible that this species is a cleaved fragment of Sirt1. A recent study has shown, in chondrocytes, that Sirt1 can be cleaved into a 75 kDa fragment that plays a role in regulation of apoptosis[24]. The antibody used in this study does not target the 75 kDa fragment, however, the species detected may be the approximately 45 kDa remnant of the cleaved Sirt1.
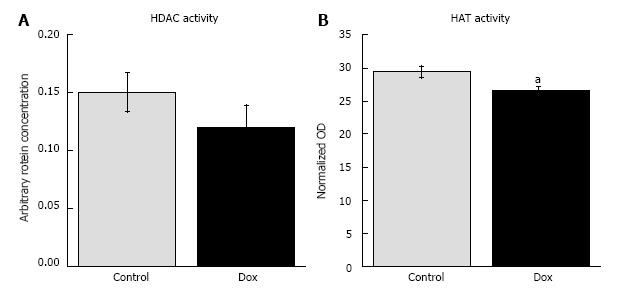
We also assessed general HDAC activity to determine if deacetylases might be responsible for the Dox-induced proteome lysine deacetylation. We did not observe a difference between Dox treated and control animals (0.120 ± 0.020 vs 0.151 ± 0.017, respectively, P = 0.29; Figure 3A). Thus, the data suggests that HDACs may not be responsible for the observed proteome lysine deacetylation in Dox treated animals.
Since the proteome deacetylation did not appear to be due to increased HDAC activity, we assessed HAT activity. Dox decreased hepatic HAT activity (0.209 ± 0.006 vs 0.189 ± 0.004, P = 0.017, Figure 3B). Thus, the data suggest that the Dox-induced proteome deacetylation is, at least in part, due to decreased HAT activity in livers of treated animals.
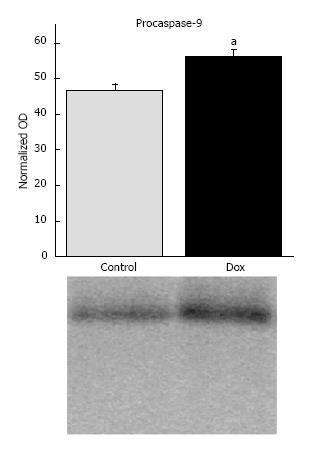
Caspase-9: To evaluate the influence of Dox treatment on the stimulation of apoptosis mediated by mitochondria, we determined the content of procaspase-9 and the presence of any cleavage products representing the active caspase. Dox treatment increased procaspase-9 content in the liver (46.86 ± 1.66 vs 56.22 ± 1.96, control vs Dox, respectively, P = 0.003; Figure 4). No cleavage products were detected. The results suggest that activation of the mitochondrial-mediated apoptotic pathway may not contribute to early mechanisms of hepatic toxicity.
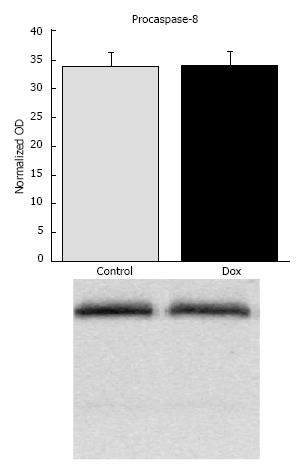
Caspase-8: To evaluate the influence of Dox treatment on the stimulation of receptor-mediated apoptosis, we determined the content of procaspase-8 and any potential cleavage products. Dox treatment did not affect content of procaspase-8 or its activation (33.81 ± 2.38 vs 33.99 ± 2.40, control vs Dox, respectively, P = 0.96; Figure 5). There was no statistical difference in the presence of lower molecular weight bands between treatment groups (data not shown). The results suggest that activation of caspase-8 by receptor-mediated mechanisms may not contribute to early mechanisms causing acute hepatic toxicity.
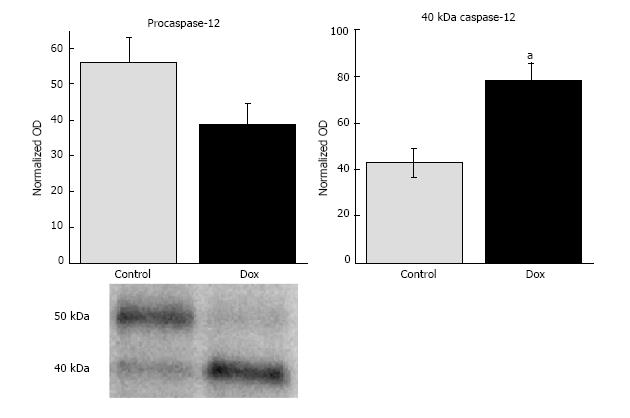
Caspase-12: Caspase-12 has been implicated in stimulating apoptosis in response to ER dysfunction and calcium dyshomeostasis[25-27], therefore, we assessed the content of procaspase-12 and any potential cleavage products. Hepatic procaspase-12 (50 kDa) content in Dox treated animals tended to be lower than those of control animals (38.66 ± 5.82 vs 56.0 ± 6.98, respectively) , however statistical significance was not reached (P = 0.075). The content of the cleavage product of caspase-12 (40 kDa) was significantly increased by Dox treatment (78.11 ± 7.73 vs 43.0 ± 5.91, Dox vs control, respectively, P = 0.004; Figure 6).
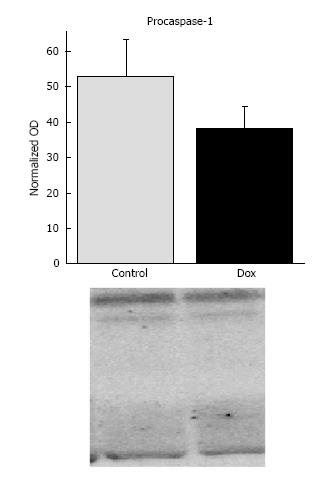
Caspase-1: Caspase-1 is an inflammatory caspase involved in the processing of proinflammatory cytokines. Aside from its implicated role in apoptosis, caspase-12 has been shown to play a role in the inflammatory process[28]. Caspase-12 is a dominant negative effector of caspase-1. Since we observed processing of caspase-12, we assessed expression and activation of caspase-1 to determine if processing of caspase-12 affected expression or activation of caspase-1. Dox treatment did not affect expression of procaspase-1 (52.9 ± 10.33 vs 38.2 ± 6.23, control vs Dox, respectively, P = 0.22). There was no statistical difference in the presence of lower molecular weight bands between treatment groups (data not shown), suggesting that Dox did not lead to its activation (Figure 7).
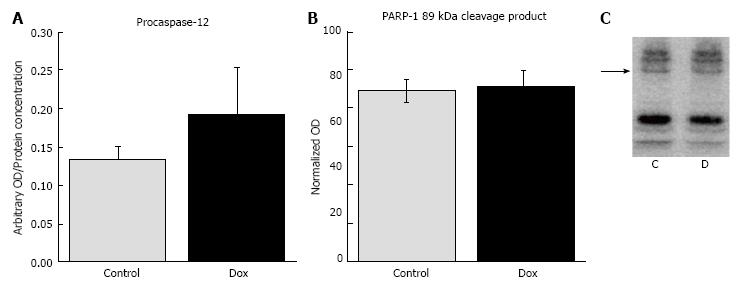
Markers of apoptosis: Caspase-3 is considered the central executioner of apoptosis and cleaves a plethora of substrates which lead to the demise of the cell and to the classical morphological characteristics of apoptosis, such as DNA fragmentation. Thus, we measured caspase-3 activity to assess activation of apoptosis. Dox did not affect the activity of caspase-3 activity in treated animals (0.193 ± 0.061) compared to control animals (0.134 ± 0.016, P = 0.45, Figure 8A). PARP-1 is an enzyme involved in DNA repair. Upon stimulation of apoptosis, PARP-1 is cleaved by various proteases, including caspase-3, in order to disengage the repair process. Thus, PARP-1 cleavage is a commonly used marker of apoptosis. In the current study, Dox treatment did not lead to the cleavage and inactivation of PARP-1. We analyzed PARP-1 and all cleavage products, but only included the data for the 89 kDa cleavage product since this is the product produced via cleavage by caspase-3 (88.86 ± 5.83 vs 91.1 ± 8.59, control vs Dox, respectively, P = 0.84, Figure 8B and C). The results suggest that apoptosis did not occur in response to acute Dox treatment, at least 24 h post injection under these experimental conditions. Figure 9 shows Ponceau staining of the samples used in all of the representative Western blots.
Recently, it has been shown that cellular stress can cause cellular deacetylation of nuclear and cytoplasmic proteins and that normalization of the acetylation status can protect cells from injury and death[12,29]. Furthermore, caloric restriction, an intervention shown to provoke protection against cellular insults and to increase longevity, increases acetylation status of the mitochondrial proteome in the liver, heart, and kidney[30]. However, the effects were tissue specific in that caloric restriction did not alter acetylation status of the mitochondrial proteome in the brain and caused deacetylation in brown adipose tissue[30]. Since proteome lysine deacetylation may be a marker of cellular stress in various tissues, we investigated the effects of Dox on proteome lysine acetylation status in the liver which was previously unknown. It was found that acute Dox treatment promoted lysine deacetylation which is consistent with the notion that an imbalance in acetylation status favoring deacetylation may signify cellular stress in the liver. Proteins most affected by Dox appear to be those with a molecular weight between 30-80 kDa.
Acetylation status is determined by the activities of HATs and HDACs. Sirt1 and Sirt3 are members of the class III HDACs, nicotinamide adenine dinucleotide (NAD+)-dependent enzymes, and are the most studied of the seven sirtuins[13]. Sirt1 primarily targets nuclear and some cytoplasmic proteins while Sirt3 targets mitochondrial proteins. Although acute Dox treatment induced proteome lysine deacetylation, Dox did not affect the protein content of Sirt1 or Sirt3 nor the overall HDAC activity. However, it did decrease HAT activity. These results suggest that proteome lysine deacetylation may not be due to a generalized increase in HDAC activity and may rather be due to a decrease in HAT activity.
No studies have previously reported the influence of Dox administration on protein levels of Sirt1 or Sirt3. However, a previous study reported that Dox administration increased the mRNA content of Sirt1 in the heart of treated mice[2]. The discrepancy could be due to tissue specific differences or differences in experimental design. A reduction in food intake and low nutrient availability increases the expression of Sirt1, as well as Sirt3[31-33]. To control for changes in food intake, we fasted both the control and treatment groups. Zhang et al did not control for Dox-induced changes in food intake and thus it cannot be determined if changes in Sirt1 expression are a direct effect of Dox or an indirect effect of anorexia.
Western analysis of Sirt1 revealed two prominent bands of 120 kDa and 45 kDa in the liver. The 120 kDa band corresponds to the expected molecular weight of Sirt1. It is unknown what the 45 kDa species is at this time. However, since this species was responsive to Dox treatment we searched the literature for possibilities. Three studies by the same group reported that Sirt1 can be cleaved under inflammatory conditions into a 75 kDa fragment in chondrocytes[24,34,35]. This fragment is void of deacetylation activity but shown to associate with cytochrome c on the mitochondrial membrane and to prevent apoptosis[24]. The cleavage of human Sirt1 occurs at amino acid residue 533 with the N-terminus being the 75 kDa fragment[34]. Our C-terminus Sirt1 antibody does not detect the N-terminus 75 kDa fragment, but may be detecting the C-terminus 45 kDa fragment. The content of this fragment increased with Dox treatment in the liver, possibly representing an increase in Sirt1 cleavage as a mechanism to prevent apoptosis in stressed hepatocytes. Future investigation is required to identify this Dox-responsive species.
To determine if proteome deacetylation was associated with a proapoptotic environment, as shown in other experimental models, we assessed the content and activation of several apical caspases associated with specific apoptotic stimuli. Activity of caspase-3 and cleavage of PARP-1 were also assessed. To our knowledge, this is the first investigation on the effects of acute Dox administration on the hepatic expression and activation of apical caspases. Our data suggests that acute Dox treatment is not likely associated with apoptosis induction. Dox administration did not stimulate the activation of caspase-9, caspase-8, caspase-3 or induce cleavage of PARP-1 in the liver under these experimental conditions. However, Dox treatment did increase the activation of caspase-12. Caspase-12 has been implicated in both apoptosis and inflammation. Early work suggested that in response to ER stress and calcium dyshomeostasis, caspase-12 can be activated by m-calpain and then induces apoptosis via cleavage of caspase-9 with consequent activation of caspase-3[25-27]. Our data suggests that caspase-12 may not be involved in apoptosis induction since we did not detect an increase in caspase-9 cleavage, caspase-3 activity or PARP-1 cleavage. In fact, more recent reports suggest that caspase-12 is incapable of cleaving caspase-9 or caspase-3. It was reported that the catalytic activity of caspase-12 was confined to autoprocessing and was unable to cleave other substrates[28,36]. Thus, caspase-12 may be playing a role in the inflammatory process rather than apoptosis. Caspase-12 has been shown to be an inhibitor of caspase-1. Caspase-12 binds procaspase-1 and prevents its activation and consequent cytokine processing[28]. Both procaspase-12 and the processed form of caspase-12 have been found to be a part of the caspase-1 inhibitory complex[28]. However, the role of caspase-12 cleavage in regulating the inflammatory response is unknown at this time, but has been suggested as a means for temporal limitation of the inhibitory effect of caspase-12[28]. The effects of Dox on the expression and activation of caspase-1 were assessed, but we did not observe an effect. Procaspase-12 has also been shown to have an inhibitory effect on NF-κB[37]. Thus, autoprocessing of caspase-12 may lead to decreased suppression of NF-κB and increased expression of anti-apoptotic proteins helping to protect the liver from Dox-induced apoptosis. This hypothesis is speculative and further studies are required to discern the role of caspase-12 in Dox-induced injury. In summary, lysine deacetylation likely is not associated with an apoptotic environment. However, a limitation is the lack of deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) to verify the lack of apoptosis induction. With this limitation, it cannot be conclusively ruled out that apoptosis independent of caspase activation or PARP-1 cleavage did not occur.
Although, our results suggest that Dox does not likely induce apoptosis, at least at this early time point under these experimental conditions, a previous study reported that Dox may induce apoptosis in the liver via the accumulation of p53, decreased expression of Bcl-xL, and cytochrome c release from mitochondria[5]. Cytochrome c release stimulates apoptosis via caspase-9 activation, however, they did not assess caspase-9 activity. Our results differ from Patel et al[5] in that we found that Dox does not increase caspase-9 activation. In the current study, we report that Dox increases the content of procaspase-9 with no evidence of cleavage products. We have also found that Dox decreases the activity of caspase-9 and mitophagy in hepatic tissue of these animals (data will be published elsewhere). We hypothesize that the decreased caspase-9 activity may be due to increased mitophagy, by the elimination of damaged mitochondria susceptible to cytochrome c release[38] and thus may be a protective response to an initial insult induced by Dox. Our results may contrast from Patel et al. due to differences in experimental design as there were several relevant disparities [5]. First, Patel et al[5] used an extremely high dose of Dox (60 mg/kg) which was three times the dose used in the current study. Secondly, our animals were fasted to control for the anorexic effect of Dox, while Patel et al[5] did not account for this variable. Therefore, the Dox animals were severely deficient in nutrients while the control animals were not. Thirdly, Patel et al[5] assessed Dox induced hepatic damage 48 h after treatment, while we examined toxicity at 24 h post treatment. It is possible that hepatic apoptosis may occur at a later time point due to the indirect effects of progressing peripheral organ damage, such as progression of heart failure. Animals treated with Dox usually show signs of heart failure within days of treatment which varies depending on the dose administered. Heart failure is known to cause liver damage[39]. Alternatively, complete fasting (compared to a 50%-70% reduction) of the animals had an unintended protective effect against Dox-induced hepatic apoptosis. Recently, it was shown that fasting prior to Dox treatment, but then fed ad libitum upon treatment, protected against cardiac toxicity by means of autophagy induction[40]. The absence of an ad-libitum fed Dox group and control group does not allow the determination of the effects of fasting on the variables assessed in our study. Yet, the objective was simply to eliminate this external variable. More studies are necessary to determine if fasting extends similar protection against hepatotoxicity.
Early effects of acute Dox treatment include altered proteome acetylation homeostasis favoring lysine deacetylation. The mechanism of proteome lysine deacetylation likely involves decreased activity of HATs, rather than increased activity of HDACs. In our experimental model, lysine deacetylation does not appear to be associated with an apoptotic environment. Acute Dox treatment did not increase cleavage of caspase-9, cleavage of caspase-8, cleavage of PARP-1, or caspase-3 activity. Dox treatment did stimulate caspase-12 activation, however, its activation does not appear to play a role in apoptosis induction. Thus, early mechanisms of hepatic toxicity may not involve induction of apoptosis, at least under these experimental conditions. The liver may be able to engage an early protective response against an initial Dox-induced insult. Further damage to the liver, which may include apoptosis, may occur at later time points as damage to peripheral organs progresses and may be an indirect effect of Dox. Alternatively, fasting of the animals in our experimental design may have provoked a protective response which prevented apoptosis. Further studies are required to discern the role of proteome lysine deacetylation, as well as the role of caspase-12, in Dox-induced hepatic toxicity. It is not clear whether lysine deacetylation and caspase-12 activation contribute to toxicity or play a role in a protective response. Clarification will elucidate the potential of acetylation homeostasis and caspase-12 as therapeutic targets against Dox-induced toxicity.
Doxorubicin-induced toxicity is a major cause of morbidity and mortality in cancer survivors. Thus, mechanisms of toxicity is essential to elucidate in order to prevent doxorubicin-induced tissue damage in vital organs.
Proteome lysine deacetylation has been shown to be associated with cellular injury in various experimental models. Normalization of the acetylation status has been shown to prevent cell death. However, the role of altered proteome lysine acetylation status in doxorubicin-induced toxicity has not been previously studied.
Doxorubicin treatment has been found to alter the hepatic proteome acetylation status. However, the modulation of the acetylation status was not associated with apoptosis, as shown in other experimental models. The results lay the groundwork for future studies to determine the role of proteome lysine deacetylation in doxorubicin-induced toxicity. Furthermore, caspase-12 was identified as having a role in doxorubicin-induced hepatic toxicity, which may be either pathological or protective.
Proteome lysine acetylation and caspase-12 may be potential therapeutic targets to prevent doxorubicin-induced toxicity.
Acetylation is a common posttranslational modification in which an acetyl group is covalently attached to a protein. Apoptosis is cellular suicide or programmed cell death.
The authors describe the evaluation of the effect of doxorubicin on proteome acetylation status and found that decreased proteome lysine acetylation was associated with decreased activity of histone deacetylases. Overall, the paper is well written.
P- Reviewer: Hancock WW, Perego P, Zeng LF S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Pointon AV, Walker TM, Phillips KM, Luo J, Riley J, Zhang SD, Parry JD, Lyon JJ, Marczylo EL, Gant TW. Doxorubicin in vivo rapidly alters expression and translation of myocardial electron transport chain genes, leads to ATP loss and caspase 3 activation. PLoS One. 2010;5:e12733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 2. | Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, Yeh ET. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18:1639-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1143] [Cited by in RCA: 1411] [Article Influence: 108.5] [Reference Citation Analysis (0)] |
| 3. | Childs AC, Phaneuf SL, Dirks AJ, Phillips T, Leeuwenburgh C. Doxorubicin treatment in vivo causes cytochrome C release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2: Bax ratio. Cancer Res. 2002;62:4592-4598. [PubMed] |
| 4. | Koçkar MC, Nazıroğlu M, Celik O, Tola HT, Bayram D, Koyu A. N-acetylcysteine modulates doxorubicin-induced oxidative stress and antioxidant vitamin concentrations in liver of rats. Cell Biochem Funct. 2010;28:673-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Patel N, Joseph C, Corcoran GB, Ray SD. Silymarin modulates doxorubicin-induced oxidative stress, Bcl-xL and p53 expression while preventing apoptotic and necrotic cell death in the liver. Toxicol Appl Pharmacol. 2010;245:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 6. | el-Missiry MA, Othman AI, Amer MA, Abd el-Aziz MA. Attenuation of the acute adriamycin-induced cardiac and hepatic oxidative toxicity by N-(2-mercaptopropionyl) glycine in rats. Free Radic Res. 2001;35:575-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Kalender Y, Yel M, Kalender S. Doxorubicin hepatotoxicity and hepatic free radical metabolism in rats. The effects of vitamin E and catechin. Toxicology. 2005;209:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 161] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 8. | Henninger C, Huelsenbeck J, Huelsenbeck S, Grösch S, Schad A, Lackner KJ, Kaina B, Fritz G. The lipid lowering drug lovastatin protects against doxorubicin-induced hepatotoxicity. Toxicol Appl Pharmacol. 2012;261:66-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Yagmurca M, Bas O, Mollaoglu H, Sahin O, Nacar A, Karaman O, Songur A. Protective effects of erdosteine on doxorubicin-induced hepatotoxicity in rats. Arch Med Res. 2007;38:380-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Ladas EJ, Jacobson JS, Kennedy DD, Teel K, Fleischauer A, Kelly KM. Antioxidants and cancer therapy: a systematic review. J Clin Oncol. 2004;22:517-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 184] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 11. | Kim SC, Sprung R, Chen Y, Xu Y, Ball H, Pei J, Cheng T, Kho Y, Xiao H, Xiao L. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell. 2006;23:607-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1168] [Cited by in RCA: 1219] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 12. | Li Y, Alam HB. Creating a pro-survival and anti-inflammatory phenotype by modulation of acetylation in models of hemorrhagic and septic shock. Adv Exp Med Biol. 2012;710:107-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Suzuki T. ‘Explorative Study on Isoform-Selective Histone Deacetylase Inhibitors’. Chem Pharm Bull (Tokyo). 2009;57:897-906. [RCA] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Chowdhury I, Tharakan B, Bhat GK. Caspases - an update. Comp Biochem Physiol B Biochem Mol Biol. 2008;151:10-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 264] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 15. | Freireich EJ, Gehan EA, Rall DP, Schmidt LH, Skipper HE. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep. 1966;50:219-244. [PubMed] |
| 16. | Glass B, Kloess M, Bentz M, Schlimok G, Berdel WE, Feller A, Trümper L, Loeffler M, Pfreundschuh M, Schmitz N. Dose-escalated CHOP plus etoposide (MegaCHOEP) followed by repeated stem cell transplantation for primary treatment of aggressive high-risk non-Hodgkin lymphoma. Blood. 2006;107:3058-3064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Gilliam LA, Ferreira LF, Bruton JD, Moylan JS, Westerblad H, St Clair DK, Reid MB. Doxorubicin acts through tumor necrosis factor receptor subtype 1 to cause dysfunction of murine skeletal muscle. J Appl Physiol (1985). 2009;107:1935-1942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Sorensen M, Sanz A, Gómez J, Pamplona R, Portero-Otín M, Gredilla R, Barja G. Effects of fasting on oxidative stress in rat liver mitochondria. Free Radic Res. 2006;40:339-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Marczuk-Krynicka D, T Hryniewiecki, J Piatek, and J Paluszak. ‘The Effect of Brief Food Withdrawal on the Level of Free Radicals and Other Parameters of Oxidative Status in the Liver’. Med Sci Monit. 2003;9:131-135. |
| 20. | Zhang YK, Wu KC, Klaassen CD. Genetic activation of Nrf2 protects against fasting-induced oxidative stress in livers of mice. PLoS One. 2013;8:e59122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J. Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc Natl Acad Sci USA. 2011;108:10190-10195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 751] [Cited by in RCA: 910] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 22. | Romero-Calvo I, Ocón B, Martínez-Moya P, Suárez MD, Zarzuelo A, Martínez-Augustin O, de Medina FS. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal Biochem. 2010;401:318-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 622] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 23. | Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012;13:225-238. [PubMed] |
| 24. | Oppenheimer H, Gabay O, Meir H, Haze A, Kandel L, Liebergall M, Gagarina V, Lee EJ, Dvir-Ginzberg M. 75-kd sirtuin 1 blocks tumor necrosis factor α-mediated apoptosis in human osteoarthritic chondrocytes. Arthritis Rheum. 2012;64:718-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Nakagawa T, Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol. 2000;150:887-894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 893] [Cited by in RCA: 902] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 26. | Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2547] [Cited by in RCA: 2611] [Article Influence: 104.4] [Reference Citation Analysis (0)] |
| 27. | Morishima N, Nakanishi K, Takenouchi H, Shibata T, Yasuhiko Y. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J Biol Chem. 2002;277:34287-34294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 702] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 28. | Roy S, Sharom JR, Houde C, Loisel TP, Vaillancourt JP, Shao W, Saleh M, Nicholson DW. Confinement of caspase-12 proteolytic activity to autoprocessing. Proc Natl Acad Sci USA. 2008;105:4133-4138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Li Y, Alam HB. Modulation of acetylation: creating a pro-survival and anti-inflammatory phenotype in lethal hemorrhagic and septic shock. J Biomed Biotechnol. 2011;2011:523481. [PubMed] |
| 30. | Schwer B, Eckersdorff M, Li Y, Silva JC, Fermin D, Kurtev MV, Giallourakis C, Comb MJ, Alt FW, Lombard DB. Calorie restriction alters mitochondrial protein acetylation. Aging Cell. 2009;8:604-606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 212] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 31. | Noriega LG, Feige JN, Canto C, Yamamoto H, Yu J, Herman MA, Mataki C, Kahn BB, Auwerx J. CREB and ChREBP oppositely regulate SIRT1 expression in response to energy availability. EMBO Rep. 2011;12:1069-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 32. | Palacios OM, Carmona JJ, Michan S, Chen KY, Manabe Y, Ward JL, 3rd , L . J. Goodyear, and Q. Tong, ‘Diet and Exercise Signals Regulate Sirt3 and Activate Ampk and Pgc-1alpha in Skeletal Muscle’. Aging (Albany NY). 2009;1:771-83. |
| 33. | Hayashida S, Arimoto A, Kuramoto Y, Kozako T, Honda S, Shimeno H, Soeda S. Fasting promotes the expression of SIRT1, an NAD+ -dependent protein deacetylase, via activation of PPARalpha in mice. Mol Cell Biochem. 2010;339:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 34. | Dvir-Ginzberg M, Gagarina V, Lee EJ, Booth R, Gabay O, Hall DJ. Tumor necrosis factor α-mediated cleavage and inactivation of SirT1 in human osteoarthritic chondrocytes. Arthritis Rheum. 2011;63:2363-2373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 35. | Gabay O, Oppenhiemer H, Meir H, Zaal K, Sanchez C, Dvir-Ginzberg M. Increased apoptotic chondrocytes in articular cartilage from adult heterozygous SirT1 mice. Ann Rheum Dis. 2012;71:613-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 36. | Lee HJ, Lee SH, Park SH, Sharoar MG, Shin SY, Lee JS, Cho B, Park IS. Purification of catalytically active caspase-12 and its biochemical characterization. Arch Biochem Biophys. 2010;502:68-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 37. | Labbé K, Miu J, Yeretssian G, Serghides L, Tam M, Finney CA, Erdman LK, Goulet ML, Kain KC, Stevenson MM. Caspase-12 dampens the immune response to malaria independently of the inflammasome by targeting NF-kappaB signaling. J Immunol. 2010;185:5495-5502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Yang Y, Xing D, Zhou F, Chen Q. Mitochondrial autophagy protects against heat shock-induced apoptosis through reducing cytosolic cytochrome c release and downstream caspase-3 activation. Biochem Biophys Res Commun. 2010;395:190-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Alvarez AM, Mukherjee D. Liver abnormalities in cardiac diseases and heart failure. Int J Angiol. 2011;20:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 40. | Kawaguchi T, Takemura G, Kanamori H, Takeyama T, Watanabe T, Morishita K, Ogino A, Tsujimoto A, Goto K, Maruyama R. Prior starvation mitigates acute doxorubicin cardiotoxicity through restoration of autophagy in affected cardiomyocytes. Cardiovasc Res. 2012;96:456-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |