Published online May 26, 2014. doi: 10.4331/wjbc.v5.i2.204
Revised: January 15, 2014
Accepted: February 18, 2014
Published online: May 26, 2014
Processing time: 148 Days and 11 Hours
Safe trafficking of iron across the cell membrane is a delicate process that requires specific protein carriers. While many proteins involved in iron uptake by cells are known, only one cellular iron export protein has been identified in mammals: ferroportin (SLC40A1). Ceruloplasmin is a multicopper enzyme endowed with ferroxidase activity that is found as a soluble isoform in plasma or as a membrane-associated isoform in specific cell types. According to the currently accepted view, ferrous iron transported out of the cell by ferroportin would be safely oxidized by ceruloplasmin to facilitate loading on transferrin. Therefore, the ceruloplasmin-ferroportin system represents the main pathway for cellular iron egress and it is responsible for physiological regulation of cellular iron levels. The most recent findings regarding the structural and functional features of ceruloplasmin and ferroportin and their relationship will be described in this review.
Core tip: The ceruloplasmin-ferroportin system represents the main pathway for cellular iron egress in vertebrates and it is responsible for physiological regulation of cellular iron levels. This review focuses on the structural and functional features of the two proteins, with special emphasis on their coordinate regulation at the transcriptional and post-transcriptional levels.
- Citation: Musci G, Polticelli F, Bonaccorsi di Patti MC. Ceruloplasmin-ferroportin system of iron traffic in vertebrates. World J Biol Chem 2014; 5(2): 204-215
- URL: https://www.wjgnet.com/1949-8454/full/v5/i2/204.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v5.i2.204
The importance of iron for all eukaryotes, and particularly for humans, is well established. Iron is fundamental for the transport, storage and activation of oxygen, for electron transport and for many other important metabolic processes. It is therefore not surprising that any genetic defect leading to iron imbalance can have severe consequences on our health. The loss of regulation of iron metabolism can lead to development of iron overload as seen in hereditary hemochromatosis, a common inherited disorder which may lead to progressive organ dysfunction. Conversely, iron deficiency is typical of many pathological states, such as the anemia of chronic disease or anemia associated with inflammation. In the last fifteen years, several new genes and proteins involved in iron disorders in animal models and in humans have been identified, which has greatly improved our understanding of the molecular mechanisms of iron absorption, the regulation of iron transport and general iron homeostasis in mammals[1-3].
Serum transferrin and the almost ubiquitously expressed transferrin receptor-1 (TfR1) represent the most important system for distribution and delivery of iron to the different organs of the body. Iron delivery to the bloodstream for transferrin-dependent transport is mediated by enterocytes, which release iron absorbed from the diet, and mostly by macrophages, which recycle iron from damaged and senescent erythrocytes. These specialized cells export iron through the recently identified protein ferroportin (SLC40A1, initially also named Ireg-1 or MTP-1), the only known mammalian iron exporter[4-6]. A group of enzymes that convert Fe2+ to Fe3+ collaborates with ferroportin, facilitating iron loading onto transferrin, which binds only Fe3+. These enzymes belong to the family of the blue multicopper oxidases and possess ferroxidase activity; members of this family include ceruloplasmin, hephaestin and zyklopen in mammals.
In this review the most recent findings regarding the structural and functional features of ceruloplasmin and ferroportin and their relationships will be described. A list of the most relevant papers in the field is presented in Table 1.
| Topic | Ref. |
| Fpn identification and structure | [4,6,8,9,12,14,16] |
| Cp structure and function | [29,34,35,39,43,45,46,49,50,52,82] |
| Cp/Fpn connection | [40,42,52,53] |
| Transcriptional regulation of Cp/Fpn | [56-58,61,62,64,68,71] |
| Post-transcriptional regulation of Fpn | [21,24,73] |
| Aceruloplasminemia | [75-78,81,85] |
| Fpn disease | [10,88,89,91,92,94] |
Human ferroportin (Fpn) is constituted by 571 amino acids, the corresponding SLC40A1 gene is located on chromosome 2 (2q32), it spans about 20 kb and has 8 exons. Fpn has been identified in many organisms and its amino acid sequences can be easily retrieved from annotated genome projects. The protein is well conserved, with over 60% identity between distantly related proteins such as human and zebrafish Fpn, indicating a wide distribution and a critical role for Fpn. This assumption is supported by the finding that inactivation of the Fpn gene in mice is embryonically lethal[7].
Fpn is a polytopic membrane protein with a predicted 9-12 transmembrane topology. A model proposed by Liu et al[8] suggested that Fpn has 12 transmembrane domains. A number of studies have indicated that the N-terminus of Fpn is cytosolic[8-11]. On the other hand, the location of the C-terminus is unclear, with studies based on epitope-tagged proteins supporting the hypothesis of a cytosolic localization[8,12] and other studies claiming that the C-terminus is extracellular. In particular, Yeh et al[13] suggested that the presence of the epitope might affect the topology of Fpn. It should be noted, however, that epitope-tagged Fpn is fully functional with respect to transport activity and regulation.
Most questions regarding the structure and mechanism of action of Fpn could be answered by an experimentally determined three-dimensional structure of the protein. Unfortunately, such a structure will probably not be available in the near future due to the difficulties of obtaining crystals of membrane proteins. Therefore, functional studies of Fpn mostly rely on theoretical modeling to provide a framework for analysis of Fpn wild type and mutants.
Recently, two molecular models of human Fpn based on different approaches have been reported[9,14]. Both models predict that Fpn belongs to the major facilitator superfamily (MFS) of membrane transporters. Wallace and coworkers based their model on the topology proposed by Liu et al[8], and confirmed the intracellular localization of both N- and C-termini. They used the structure of the glycerol-3-phosphate transporter from E. coli as template for building a three-dimensional model of Fpn. Using the model, they showed that all reported loss-of-function Fpn mutations localize at the membrane/cytoplasm interface, while gain-of-function mutations are largely associated with the inner channel running down the axis of Fpn (see below for details on Fpn mutations and “ferroportin disease”). They concluded that the phenotypic variability of “ferroportin disease” likely arises from the different functional consequences of the various mutations.
On the other hand, using sensitive profile-profile alignment methods, Le Gac et al[14] provided an alignment of Fpn with MFS proteins. Along with the crystal structure of the E. coli EmrD antiporter, this alignment served as a basis for the homology modeling of the three-dimensional structure of Fpn. The authors focused their attention on key functional amino acids and disease-causing mutations, and showed that their model of Fpn could be used to identify critical amino acids. In particular, they proved the involvement of a specific tryptophan residue in both the iron export function and the mechanism of inhibition by hepcidin.
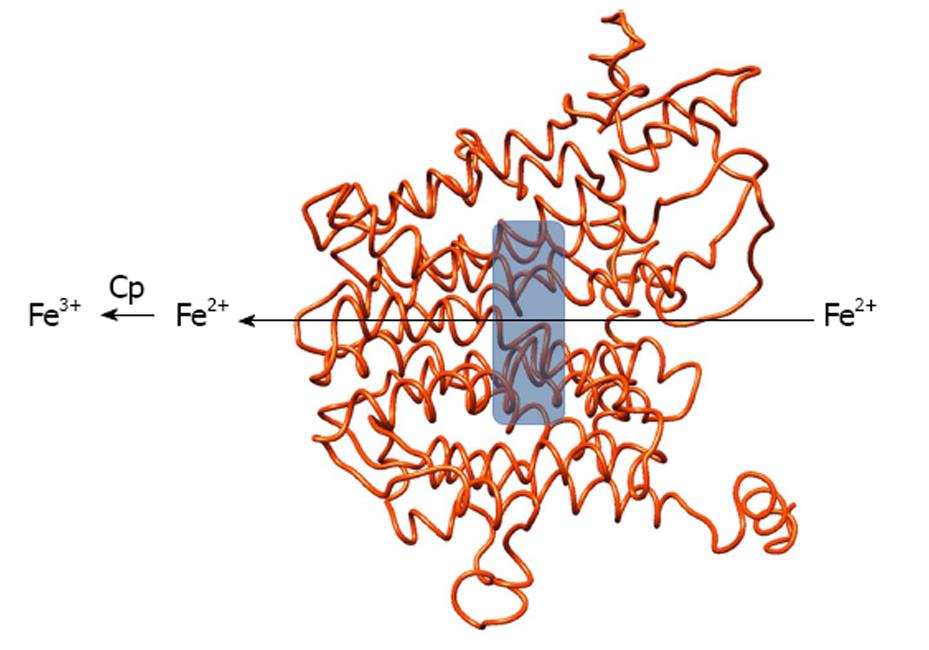
Neither model gives any clue about the localization of iron binding site(s) inside Fpn. We are currently building a different structural model of human Fpn using two MFS E. coli proteins (manuscript in preparation). A preliminary analysis shows that the model allows to postulate the presence of a potential iron binding site in the central cavity of the protein, whose relevance can be tested through measurement of the iron export ability of wild type and mutated Fpn. A depiction of our preliminary Fpn model and of the iron binding site is shown in Figure 1.
The multimeric structure of Fpn is still the subject of much debate, with reports demonstrating that the protein is dimeric[10,12,15] while other studies have suggested that it is a monomer[11,16-19]. Most of the studies addressing the oligomeric state of Fpn have relied on the use of recombinant Fpn tagged with different epitopes. The techniques employed are mainly (but not only) co-immunoprecipitation, gel-filtration chromatography and cross-linking. Evaluation of the effect of co-transfection of wild type and mutant Fpn on iron export function and subcellular localization has also been taken into consideration[10,15,16,18,19]. Conflicting results on the multimeric structure of Fpn obtained by the methods outlined above can have many explanations: the efficiency of co-immunoprecipitation can depend on the tags (and antibodies) or the experimental conditions imposed on the cell lysates. For instance, different groups have reported that it is possible to co-immunoprecipitate Fpn-GFP and Fpn-flag while co-precipitation of Fpn-flag and Fpn-myc was less reproducible. Also, high expression levels of recombinant Fpn could be in part responsible for reported discrepancies. Some negative results obtained with different cross-linkers might be explained by the chemical features of the reagent (i.e., group reactivity and spacer arm length), which can be suboptimal. Similarly, negative results obtained by fusion of Fpn to fluorescent/luminescent protein tags to exploit FRET or BRET do not necessarily imply the lack of Fpn dimers because these techniques are highly dependent on close spatial proximity of the probes. The most convincing evidence that Fpn is dimeric comes from cross-linking of endogenous Fpn in rat glioma C6 cells and bone marrow-derived macrophages, which resulted in doubling of the molecular mass of the protein[12]. This experimental set-up circumvents the possibility of artifacts due to the presence of the tags and/or overexpression of Fpn. In any case, the strength of the interaction between monomers appears to be quite low because differently tagged Fpn expressed separately and mixed after detergent-extraction from the lipid bilayer do not co-immunoprecipitate[10,12]. Multimerization of Fpn is particularly attractive to explain the dominant inheritance of “ferroportin disease” (see below).
Fpn is the receptor for hepcidin, a peptide of 25 amino acids forming a bent β-hairpin stabilized by four disulfide bonds. Inflammatory states and/or increased iron stores trigger the hepatic synthesis of the peptide[20]. Binding of hepcidin to Fpn leads to the internalization and degradation of Fpn, resulting in impaired iron export[21].
Conflicting reports have been published on the molecular mechanism of hepcidin-induced Fpn degradation. In particular, there is no agreement on the possible phosphorylation by JAK2 kinase of two tyrosine residues on Fpn in hepcidin-triggered internalization of the protein[22,23]. On the other hand, Fpn is certainly ubiquitinated on lysine residues before degradation[23,24]. The hepcidin binding site has been identified on the extracellular loop of Fpn containing cysteine in position 326[25]. Cells expressing the C326S mutant Fpn export iron normally but do not bind the peptide and export iron even in the presence of hepcidin[26]. Modeling of the hepcidin-Fpn interaction suggested that Cys326 is involved in a thiol-dependent interaction with hepcidin, perhaps involving the disulfide framework of hepcidin, while Phe324 and Tyr333 may form crucial contacts with two phenylalanine residues on the hepcidin moiety[27].
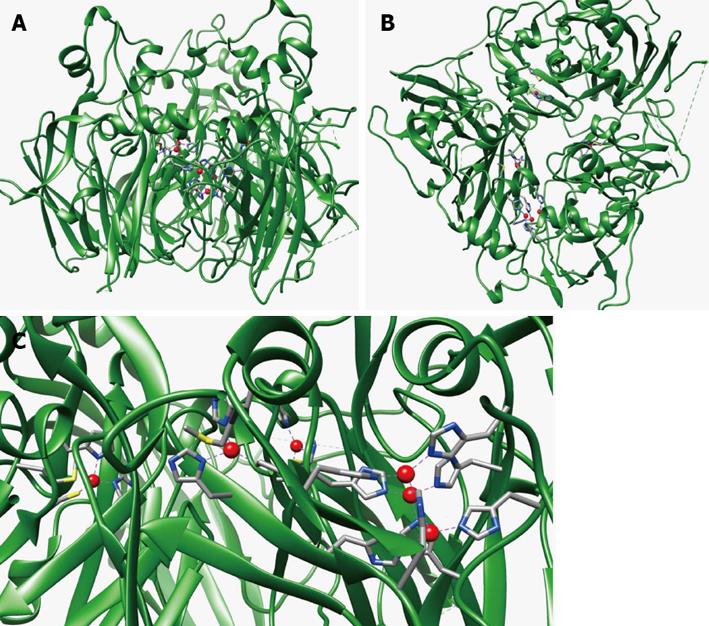
Ceruloplasmin (Cp) is an enzyme, ubiquitous among vertebrates, that belongs to the family of the multicopper oxidases. Members of this family posse multiple copper sites that can be classified, on the basis of their spectroscopic properties, in type 1, type 2 and type 3 sites[28]. Human Cp is constituted by 1046 amino acids; the Cp gene maps on chromosome 3 (3q23-q24), it spans about 65 kb and it is organized in 20 exons. Determination of the three-dimensional structure of Cp[29,30] has shown that this enzyme is made up of six domains arranged in a ternary symmetry. Domains 1 and 2, 3 and 4, and 5 and 6 interact with each other through extensive, highly packed hydrophobic interfaces, while polar interactions and loosely packed interfaces are observed between domains 2 and 3 and 4 and 5. Three of the six domains (domains 2, 4 and 6) bind a type 1 blue copper coordinated by nitrogen and sulphur ligands, supplied by histidine and cysteine residues arranged in tetrahedral geometry with an axial methionine ligand, which is absent in the type 1 site of domain 2.
Three more copper ions are coordinated by eight histidine ligands at the interface between domain 1 and 6. The latter copper ions represent the trinuclear cluster formed by two antiferromagnetically coupled type 3 and one type 2 copper ions. The oxidation of substrates is coupled to the reduction of oxygen to water in a mechanism involving electron transfer from the type 1 copper sites, the primary sites of substrate oxidation, to the trinuclear cluster, where oxygen binds and is reduced in a controlled way, i.e., without release of potentially toxic intermediates (O2-, H2O2). While electron entrance at type 1 copper sites in domains 4 and 6 is established, the role of the blue copper ion in domain 2 is less clear. In fact, there is no experimental evidence from crystallographic data that reducing substrates can bind in domain 2. Moreover, site-directed mutagenesis at this copper site failed to modify either the spectroscopic or catalytic properties of the protein[31]. Thus, the blue copper ion in domain 2 could be an “evolutionary relic” or, alternatively, it could serve for still unknown other functions. Figure 2 reports the structure of human Cp and the localization of its copper sites.
Beside copper, other metals have been proposed to bind to Cp. In particular, refined crystallographic data showed an extra metal-binding site in domain 1, likely filled with a calcium ion. The finding of a calcium binding site is consistent with a previous study from our laboratory showing that human and sheep Cp bind divalent ions, and that this could be exploited in a one-step purification protocol based on the affinity of the protein for calcium ions[32].
Cp is mainly synthesized by hepatocytes, where the P-type ATPase ATP7B incorporates copper into apo-Cp during transit through the trans-Golgi network[33], and secreted into the plasma where it is found at micromolar concentration. The molecular mechanism of copper loading of Cp by ATP7B is still unknown. Inspection of the structure of Cp shows that large solvent exposed loops connect the six domains of Cp. Despite a low degree of sequence homology, all these loops start with a C-X-R/K motif, with the cysteine residue stabilizing the loop by forming a disulfide bridge. Our recent work indicates that the basic residues of the five loops connecting the six domains of Cp, and the disulfide bridges that stabilize the loops, are required for proper copper loading by ATP7B[34].
A GPI-anchored form of Cp was initially identified on the plasma membrane of astrocytes[35] and leptomeningeal cells[36] in the CNS, in Sertoli cells[37] and in the retina[38]. Synthesis of this isoform is via alternative splicing of exons 19 and 20 where the last 5 amino acids are replaced by 30 alternative residues leading to addition of the GPI anchor[39]. More recently Cp-GPI has been detected also in macrophages[40], immune cells and hepatocytes[41] and in many other tissues[42], indicating a wider than anticipated distribution of this isoform.
Despite the knowledge of the details of the three-dimensional structure, the true biological function of Cp has been the subject of much debate mainly because Cp is a rather promiscuous enzyme, as regards the multitude of substrates it can act on and the possibility that copper bound to sites other than the active site can give rise to accessory activities. In fact, several functions have been attributed to Cp, ranging from copper transport to ferrous iron and biological amines oxidation, as well as antioxidant activity via prevention of the formation of free radicals in serum[43]. Conversely, pro-oxidant activity leading to LDL oxidation has also been attributed to Cp due to the presence of a seventh copper atom that is bound to a site unrelated to the active site[44]. However, among various substrates, the enzyme displays the highest affinity for ferrous ions and a role for Cp in iron metabolism had been proposed as early as in 1966[45]. The study of the ferroxidase activity of Cp evidenced two Km values which differ by approximately two orders of magnitude (Km1 0.6 µmol/L and Km2 50 µmol/L) and binding of Fe2+ in the vicinity of type 1 copper sites has been demonstrated by X-ray diffraction studies, soaking crystals of Cp with Fe2+[46]. Cp is thought to promote iron release from cells, facilitating loading of the metal onto transferrin, which only binds Fe3+. An important point regarding the ferroxidase activity of Cp is that Fe2+ readily oxidizes, at physiological pH, even in the absence of a protein catalyst. However, spontaneous oxidation of Fe2+ is potentially dangerous as it triggers the formation of oxygen radicals via Fenton chemistry. Thus ferroxidation by Cp would prevent iron-induced oxidative stress.
An increasing body of evidence supports earlier work[47,48] and points to an essential role for Cp in iron metabolism (and specifically in iron efflux from cells) via its ferroxidase activity. Stimulation of iron release from macrophages by Cp in the presence of apotransferrin and hypoxia has been demonstrated[49]. Targeted Cp gene disruption in mouse evidenced a striking impairment in the movement of iron out of reticuloendothelial cells and hepatocytes[50]. Moreover, increased deposition of iron in several regions of the CNS was noted in Cp-/- mice[51], and Cp-GPI was found to be required for iron efflux from astrocytes[52]. In addition, individuals carrying a defective gene coding for Cp, thus suffering from aceruloplasminemia, show normal copper homeostasis but present a severely impaired iron metabolism.
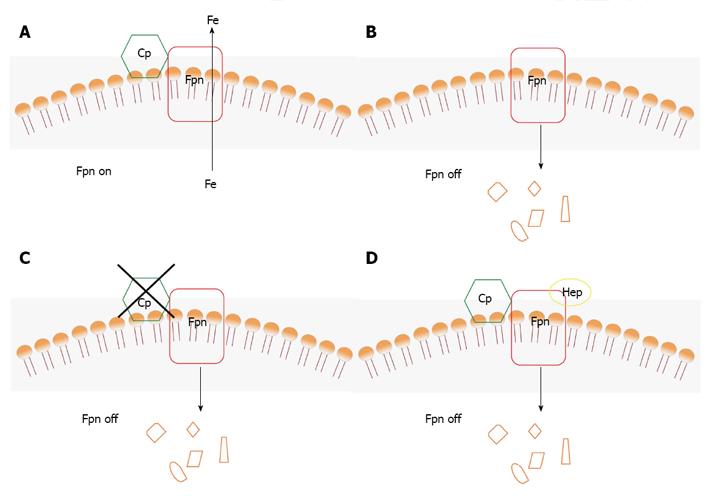
The essential role of the ferroxidase activity of Cp in iron release from cells was attributed to facilitation of loading of the metal onto transferrin, which only binds Fe3+. However, a new molecular connection between Cp and Fpn has been established by the finding that ferroxidase activity is required to stabilize Fpn at the cell surface in cells expressing Cp-GPI[40]. Thus, Cp can be considered as a second determinant of Fpn stability after hepcidin (Figure 3). As described in detail below, ferroxidase active Cp stabilizes Fpn at the plasma membrane supporting iron export (Figure 3A); on the other hand, absence of Cp or presence of an inactive Cp lead to degradation of Fpn in specific cell types (Figures 3B, C); hepcidin induces internalization and degradation of Fpn also if Cp is present (Figure 3D), unless hepcidin levels are very low. It is worth noting that removal of Fpn from the plasma membrane appears to be the only means to ‘turn off’ iron export from the cell because no inhibitor of Fpn is known.
The starting point was the observation that loss of Cp-GPI either by gene silencing or by incubation of rat C6 glioma cells and bone marrow macrophages with the copper chelator BCS led to disappearance of Fpn from the cell surface. Fpn was rapidly internalized and degraded in the absence of Cp-GPI. Addition of exogenous Cp or of the yeast ferroxidase Fet3p or of an iron chelator such as BPS or DFO, restored Fpn at the cell surface in cells silenced for Cp-GPI. The activity of the ferroxidase or the presence of the iron chelator were essential to lower the concentration of extracellular Fe2+ establishing an iron gradient and promoting removal of the metal from Fpn. In the absence of Cp-GPI, radioactive 59Fe remained associated with Fpn and the protein was found to be ubiquitinated on Lys253. It can be hypothesized that a conformational state of Fpn with bound iron is recognized by a specific ubiquitin ligase, triggering degradation of the transporter. The requirement for a ferroxidase to maintain iron transport appears specific to cells that express Cp-GPI, because transfected Fpn is stable in many cell lines that do not express this isoform of Cp. In this respect, this new function of Cp is particularly relevant for brain iron metabolism because any factor affecting the ferroxidase activity of Cp-GPI cannot be compensated by circulating plasma Cp, which is unable to cross the blood-brain barrier. Iron uptake by endothelial cells of the blood-brain barrier takes place through the Tf-TfR1 system, how the metal is then moved out of these cells and taken up by CNS cells is still unclear. Recent data indicate that iron efflux from brain microvasculature endothelial cells is mediated by Fpn and requires the action of a ferroxidase, which can be either endogenous hephaestin or extracellular Cp[53]. These findings highlight once again the importance of ferroxidases for correct cellular iron management. Astrocytes are in close contact with the abluminal surface of capillary endothelial cells and therefore are ideally positioned to control the transport of metabolites between the blood and the neuropil. Since astrocytes are able to take up and release iron, they have been proposed to be largely responsible for distributing iron in the brain[54]. Therefore, Fpn and Cp-GPI would represent the central system for release of iron from astrocytes to meet the requirements of neurons and other brain cells.
A physical interaction between Cp and Fpn has not been evidenced despite many efforts; however, it has been reported that Cp is able to partially prevent hepcidin-induced internalization of Fpn when cells are treated with 0.15 μmol/L hepcidin[42]. This finding could be taken as an indication that Cp can compete with hepcidin for binding to Fpn, suggesting that probably such interaction exists but it is transient and/or too weak to be detected. A direct consequence of this hypothesis is that the Cp-binding site on Fpn would partially overlap with the hepcidin-binding site. An alternative explanation would be that Cp interacts with hepcidin, making the peptide unavailable for binding to Fpn.
The Cp-Fpn functional connection is strengthened also by the finding that expression of the two proteins can be coordinately regulated in specific cell types.
Cp was recognized to be an acute phase protein many years ago, and it is known to be induced in response to pro-inflammatory stimuli, such as IL-1β[55-57], INF-γ[58] and IL-6[59]. Recent data demonstrate that IL-6 mediates induction of Cp via the transcription factor FOXO1[60]. Metal-dependent regulation of Cp has not been conclusively assessed, although indirect effects of iron deficiency mediated by hypoxia-inducible factor-1 (HIF-1) have been reported[61].
Expression of Fpn is regulated by different stimuli: iron and transition metals, heme, hypoxia and inflammation among others. Many studies have highlighted a tissue-specific regulation of expression of Fpn and point to Fpn regulation by systemic rather than local signals of iron status. Actually, two layers of regulation are active to control Fpn: one at the level of mRNA (transcriptional and post-transcriptional) and one at the level of the protein (hepcidin-dependent and hepcidin-independent internalization and degradation). Moreover, any factor affecting hepcidin synthesis in turn will affect Fpn protein levels.
The Fpn promoter contains different response elements sensitive to hypoxia, heme/oxidative stress and metals. The presence of HIF-Responsive-Elements was evidenced using Fpn reporter constructs and HIF2α was demonstrated to be a direct activator of Fpn transcription[62]. It is worth noting that HIF2α expression has recently been shown to depend on IRP1[63], strengthening the link between iron and hypoxia. Metal-Responsive-Element induction of Fpn mediated by the transcription factor MTF-1 in response to zinc was recently demonstrated[64]. Antioxidant-Responsive-Elements enable up-regulation of Fpn transcription in response to heme via activation of the redox-sensitive transcription factor Nrf2 in mouse and human macrophages[65,66]. Other studies indicated that heme-induced Fpn transcription required the release of iron from heme[67]. Ultimately, these results link transcriptional control of Fpn synthesis directly and indirectly to iron levels: i.e., iron is crucial for HIF2α stability and IRP1-mediated expression, iron mediates oxidative stress and activation of Nrf2.
Fpn is down-regulated by pro-inflammatory cytokines in reticuloendothelial cells, as demonstrated by the finding that treatment with IFN-γ and LPS reduced Fpn mRNA and iron release from monocytes[68,69]. Fpn mRNA and protein levels were also found to decrease significantly in astrocytes treated with LPS but not with IL-6 or TNF-α[70]. Interestingly, we have found that in rat C6 glioma cells Cp and Fpn are up-regulated by IL-1β, suggesting that the response of Fpn to cytokines might be tissue-specific[57]. The expression of Cp and Fpn in response to IL-1β requires the activation of MAP kinase pathways as a consequence of IL-1β receptor stimulation. Moreover, we have observed that IL-1β regulates the expression of Cp and Fpn genes through (1) p38 MAPK-mediated activation of C/EBP transcription factor; (2) ERK1/2-, JNK1- and partially p38 MAPK-dependent activation of AP-1; and (3) activation of NF-κB partially mediated by p38 MAPK[71]. A similar pathway was found to activate Fpn expression in response to the isoflavone genistein[72]. In this case, p38 MAPK activation was found to be triggered by activation of the estrogen receptor β.
At the post-transcriptional level, Fpn expression is regulated by iron-responsive sequences both at the 5’ UTR and at the 3’ UTR. Repression of Fpn mRNA translation in conditions of iron deficiency was shown to be mediated by the well-characterized IRE/IRP system, due to the presence of an IRE sequence at the 5’ UTR. Also the 3’ UTR of Fpn plays a role in post-transcriptional regulation of expression through a recently discovered miRNA-dependent mechanism. microRNAs are small non-coding RNAs that bind the 3’ UTR of target mRNAs driving translational repression or mRNA degradation. In particular, it has been demonstrated that miR-485-3p is induced during iron deficiency and it targets the 3’ UTR of Fpn to reduce iron export in several cell lines and primary macrophages[73]. In duodenal and erythroid precursor cells alternative splicing produces an isoform of Fpn lacking the 5’ IRE indicating that these cells can evade IRE/IRP-dependent translational repression[74] becoming sensitive to systemic rather than local (intracellular) cues. It would be interesting to evaluate whether miR-485-3p is expressed in these cell types and this isoform of Fpn is subject to miRNA-mediated control.
The importance of the ceruloplasmin-ferroportin system is highlighted by the fact that mutations in the Cp and Fpn genes lead to severe consequences. Impairment of the Cp-Fpn system is common to aceruloplasminemia and “ferroportin disease”, two genetic diseases that share a common phenotype of iron overload.
Aceruloplasminemia is a rare autosomal disease caused by mutations in the Cp gene[75,76]. Approximately forty mutations of the Cp gene have been so far described, including frameshift, nonsense and missense mutations[77,78]. Heterozygous individuals have partial Cp deficiency with normal iron metabolism and no clinical symptoms, with some exceptions. Homozygotes present iron overload mainly in the brain, but also in liver, pancreas and retina. Patients develop retinal degeneration, diabetes mellitus and neurological symptoms, which include ataxia, involuntary movements and dementia. Onset of clinical manifestations usually occurs in adulthood. Laboratory findings include absence of serum Cp ferroxidase activity (although low levels of Cp protein were reported in some cases), low transferrin saturation, high serum ferritin and moderate anemia; magnetic resonance imaging of the brain shows iron deposits in the basal ganglia, striatum, thalamus and dentate nucleus. These features place aceruloplasminemia in the group of disorders known as NBIA (neurodegeneration with brain iron accumulation), clearly distinguishing it from hereditary hemochromatosis (serum iron is high and the brain is usually not affected) and from disorders of copper metabolism, Menkes and Wilson disease, that are also characterized by low/absent serum Cp ferroxidase activity because of impaired functioning of copper ATPases ATP7A and ATP7B, respectively[33].
Iron-mediated oxidative stress has been shown to contribute to tissue injury and neuronal cell death in aceruloplasminemia. In particular, it has been suggested that astrocytes, which are the most affected cell type, accumulate iron and die from iron toxicity, while neuronal loss would be secondary to loss of metabolic support provided by astrocytes[79,80].
The ferroxidase activity of Cp-GPI plays a critical role in the targeting of Fpn to the plasma membrane in astrocytes and bone marrow-derived macrophages[40]. Thus, brain iron overload and low serum iron levels observed in aceruloplasminemia patients can be explained by impaired iron export from these cell types due to lack of active Cp. On the other hand, the origin of iron overload in liver and pancreas, which is observed in aceruloplasminemia patients has still to be clarified.
Actually, the situation is even more complicated. In fact, while it is obvious that frameshift and nonsense mutations produce a truncated non-functional Cp, in vitro characterization of missense mutants yielded some unexpected findings. The first mutants to be studied invariably lacked ferroxidase activity either due to retention in the endoplasmic reticulum (P177R) or to production as apo-Cp lacking copper (D58H, G631R Q692K and G969S), due to structural or folding defects[81-84]. Indeed, residue Pro177 is found in a hydrophobic pocket, while residues Gly631, Gln692 and Gly969 are close to type 1 copper sites, suggesting that substitutions in these positions can affect folding and copper binding. Residue Asp58 is located on the protein surface and it has been suggested that substitution with histidine could cause aberrant incorporation of copper. However, another set of mutants (I9F, Q146E, F198S, W264S, A331D, G606E, G876A) that we characterized based on their ability to stabilize Fpn on the plasma membrane of rat C6 glioma cells silenced for endogenous Cp-GPI, revealed that they were partly or fully functional[85]. Also other studies showed that some mutants (Y356H, G876A) appeared to partly retain ferroxidase activity, but were less efficient than wild type Cp in protecting Fpn from hepcidin[42]. In these cases, inspection of the structure of Cp suggests that the position of the mutations is such that the protein can retain ferroxidase activity.
A quite different scenario was apparent for mutant R701W, which has been found in a very young heterozygous patient with severe extrapyramidal movement coordination deficit[86]. Both isoforms of Cp R701W (secreted and GPI-anchored) were inactive due to lack of copper, and dominant over wild type Cp in glioma cells. Moreover, they induced dispersal of the Golgi apparatus and “functional silencing” of ATP7B[85]. Of note, Cp R701W could load copper in appropriate conditions, in particular when Ccc2p, the yeast homologue of ATP7B, was co-expressed. The resulting holo-Cp R701W was fully functional with respect to stabilization of Fpn[85]. It was reported that Cp R701W expressed in HeLa cells retained some oxidase activity but it was unable to stabilize Fpn at the cell surface[42], raising the possibility that a threshold level of activity might be required to observe this stabilizing effect. Further investigations have demonstrated that Cp R701W caused massive production of reactive oxygen (ROS) species in the cell. Scavenging ROS production with different antioxidants, such as N-acetyl-cysteine, glutathione and zinc, restored Golgi morphology and rescued Fpn on the cell membrane[87]. Whether ROS are produced directly by Cp R701W or by other cellular systems such as NOX, remains to be established. Residue Arg701 is found in the surface-exposed loop connecting domains 4 and 5 of Cp and it is difficult to understand why replacement with tryptophan should cause such a dramatic phenotype.
Hemochromatosis is the most common genetic iron overload disease, it is inherited recessively and it is caused by defects of genes (HFE, TfR2, HJV, HAMP) that ultimately lead to inefficient synthesis of hepcidin. Fpn missense mutations are responsible for a different form of hemochromatosis which exhibits autosomal dominant inheritance with rather heterogeneous phenotypes, the so-called “ferroportin disease”[88]. Decreased function of Fpn appears to be limiting for macrophage iron export but not for intestinal iron export, due to the very different amounts of the metal mobilized by enterocytes (1-2 mg/d) compared to reticuloendothelial cells (20-30 mg/d). Fpn missense mutants can give rise to two different phenotypes: iron overload in macrophages and low serum transferrin saturation due to mutants that are transport incompetent or are not correctly targeted to the plasma membrane (loss-of-function mutants); hepatocyte iron overload and high serum transferrin saturation due to mutants that are unable to respond to hepcidin (gain-of-function mutants)[89,90]. Most of the mutations identified so far appear to lead to loss-of-function of Fpn, affecting plasma membrane localization of the protein and (less commonly) iron export function.
Many studies on the molecular features of the Fpn mutants have attempted to correlate mutation with phenotype. However, such analyses are complicated by difficulties in establishing a satisfactory experimental model. In most cases, recombinant Fpn mutants have been overexpressed in HEK293T or polarized MDCK cells. Subcellular localization is determined by employing Fpn-GFP fusions, Fpn function is investigated by analyzing hepcidin-induced internalization and by assessing intracellular iron levels. Conflicting results have been reported for some Fpn mutants, possibly due to the different experimental systems and conditions employed. For example, expression of Fpn in polarized MDCK cells resulted primarily in plasma membrane localization for all 16 mutants examined[11], compared to nonpolarized HeLa or HEK293T cells where some intracellular staining was apparent but could be eliminated by treatment with cycloheximide. Discrepancies in hepcidin resistance can probably be attributed to differences in hepcidin concentration and time of incubation, such that partial resistance at low (0.4-0.7 µmol/L) hepcidin concentration[9,10,91,92] can become sensitivity at high (2 µmol/L) hepcidin concentration[11]. Also, if a mutant is found to be predominantly intracellular, impaired iron export or hepcidin-resistance would simply reflect unavailability of Fpn at the plasma membrane and not a true property of the mutant protein.
Resistance to hepcidin can derive from different mechanisms: mutation of residues belonging to the hepcidin-binding site (C326Y/S and S338R) or impairment of the mechanism of internalization of Fpn (Y64N, N144H/D/T)[26]. Mutation of other residues (G204S, Y501C, H507R) has been reported to result in hepcidin resistance[93-95], suggesting that the hepcidin-binding site is probably formed by residues belonging to more that one extracellular loop of Fpn.
Other mutations impact the iron transport function of Fpn for as yet unidentified reasons (I152F). In summary, it is evident that the difficulties of working in vitro with Fpn make it tricky to unequivocally link patient phenotype to molecular defects of Fpn. This is further complicated by phenotypic heterogeneity among patients carrying the same Fpn mutation[93], suggesting that modifier genes might influence the penetrance of the disease.
Less than fifteen years have passed from the initial discovery of Fpn and a huge amount of information has been gained on this elusive protein. However, many questions still require an answer regarding our understanding of the structure and function of Fpn and the full implications of the connection between Fpn and Cp. Fpn is predicted to belong to the MFS transporters that function with an alternate “inward open-outward open” mechanism, involving extensive conformational changes to translocate their substrate across the membrane. The molecular details of how Fpn works are still a mystery, it is also unknown if transport of iron is coupled to other ions (either as symport or antiport). Why does Cp stabilize Fpn only in specific cell types is not clear.
Future studies should be aimed at addressing these and many other questions, in order to gain a better understanding of how Fpn and Cp collaborate for correct iron handling by cells.
P- Reviewers: Ahmad N, Dovat S S- Editor: Ma YJ L- Editor: A E- Editor: Lu YJ
| 1. | Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010;142:24-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 1523] [Article Influence: 101.5] [Reference Citation Analysis (0)] |
| 2. | Pantopoulos K, Porwal SK, Tartakoff A, Devireddy L. Mechanisms of mammalian iron homeostasis. Biochemistry. 2012;51:5705-5724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 439] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 3. | Ganz T. Systemic iron homeostasis. Physiol Rev. 2013;93:1721-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 773] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 4. | Abboud S, Haile DJ. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem. 2000;275:19906-19912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 943] [Cited by in RCA: 929] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 5. | Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt SJ, Moynihan J, Paw BH, Drejer A, Barut B, Zapata A. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403:776-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1226] [Cited by in RCA: 1212] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 6. | McKie AT, Marciani P, Rolfs A, Brennan K, Wehr K, Barrow D, Miret S, Bomford A, Peters TJ, Farzaneh F. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell. 2000;5:299-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 1029] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 7. | Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1:191-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Liu XB, Yang F, Haile DJ. Functional consequences of ferroportin 1 mutations. Blood Cells Mol Dis. 2005;35:33-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 105] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Wallace DF, Harris JM, Subramaniam VN. Functional analysis and theoretical modeling of ferroportin reveals clustering of mutations according to phenotype. Am J Physiol Cell Physiol. 2010;298:C75-C84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | De Domenico I, Ward DM, Nemeth E, Vaughn MB, Musci G, Ganz T, Kaplan J. The molecular basis of ferroportin-linked hemochromatosis. Proc Natl Acad Sci USA. 2005;102:8955-8960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 163] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 11. | Rice AE, Mendez MJ, Hokanson CA, Rees DC, Björkman PJ. Investigation of the biophysical and cell biological properties of ferroportin, a multipass integral membrane protein iron exporter. J Mol Biol. 2009;386:717-732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | De Domenico I, Ward DM, Musci G, Kaplan J. Evidence for the multimeric structure of ferroportin. Blood. 2007;109:2205-2209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Yeh KY, Yeh M, Glass J. Interactions between ferroportin and hephaestin in rat enterocytes are reduced after iron ingestion. Gastroenterology. 2011;141:292-299, 299.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Le Gac G, Ka C, Joubrel R, Gourlaouen I, Lehn P, Mornon JP, Férec C, Callebaut I. Structure-function analysis of the human ferroportin iron exporter (SLC40A1): effect of hemochromatosis type 4 disease mutations and identification of critical residues. Hum Mutat. 2013;34:1371-1380. [PubMed] [DOI] [Full Text] |
| 15. | McGregor JA, Shayeghi M, Vulpe CD, Anderson GJ, Pietrangelo A, Simpson RJ, McKie AT. Impaired iron transport activity of ferroportin 1 in hereditary iron overload. J Membr Biol. 2005;206:3-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Gonçalves AS, Muzeau F, Blaybel R, Hetet G, Driss F, Delaby C, Canonne-Hergaux F, Beaumont C. Wild-type and mutant ferroportins do not form oligomers in transfected cells. Biochem J. 2006;396:265-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Pignatti E, Mascheroni L, Sabelli M, Barelli S, Biffo S, Pietrangelo A. Ferroportin is a monomer in vivo in mice. Blood Cells Mol Dis. 2006;36:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Schimanski LM, Drakesmith H, Talbott C, Horne K, James JR, Davis SJ, Sweetland E, Bastin J, Cowley D, Townsend AR. Ferroportin: lack of evidence for multimers. Blood Cells Mol Dis. 2008;40:360-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Song G, Jiang Q, Xu T, Liu YL, Xu ZG, Guo ZY. A convenient luminescence assay of ferroportin internalization to study its interaction with hepcidin. FEBS J. 2013;280:1773-1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Ganz T, Nemeth E. Iron imports. IV. Hepcidin and regulation of body iron metabolism. Am J Physiol Gastrointest Liver Physiol. 2006;290:G199-G203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 219] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 21. | Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090-2093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3282] [Cited by in RCA: 3538] [Article Influence: 168.5] [Reference Citation Analysis (0)] |
| 22. | De Domenico I, Lo E, Ward DM, Kaplan J. Hepcidin-induced internalization of ferroportin requires binding and cooperative interaction with Jak2. Proc Natl Acad Sci USA. 2009;106:3800-3805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Qiao B, Sugianto P, Fung E, Del-Castillo-Rueda A, Moran-Jimenez MJ, Ganz T, Nemeth E. Hepcidin-induced endocytosis of ferroportin is dependent on ferroportin ubiquitination. Cell Metab. 2012;15:918-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 256] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 24. | De Domenico I, Ward DM, Langelier C, Vaughn MB, Nemeth E, Sundquist WI, Ganz T, Musci G, Kaplan J. The molecular mechanism of hepcidin-mediated ferroportin down-regulation. Mol Biol Cell. 2007;18:2569-2578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 325] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 25. | De Domenico I, Nemeth E, Nelson JM, Phillips JD, Ajioka RS, Kay MS, Kushner JP, Ganz T, Ward DM, Kaplan J. The hepcidin-binding site on ferroportin is evolutionarily conserved. Cell Metab. 2008;8:146-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Fernandes A, Preza GC, Phung Y, De Domenico I, Kaplan J, Ganz T, Nemeth E. The molecular basis of hepcidin-resistant hereditary hemochromatosis. Blood. 2009;114:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 27. | Preza GC, Ruchala P, Pinon R, Ramos E, Qiao B, Peralta MA, Sharma S, Waring A, Ganz T, Nemeth E. Minihepcidins are rationally designed small peptides that mimic hepcidin activity in mice and may be useful for the treatment of iron overload. J Clin Invest. 2011;121:4880-4888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 180] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 28. | Solomon EI, Sundaram UM, Machonkin TE. Multicopper Oxidases and Oxygenases. Chem Rev. 1996;96:2563-2606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2913] [Cited by in RCA: 2729] [Article Influence: 94.1] [Reference Citation Analysis (0)] |
| 29. | Zaitseva I, Zaitsev V, Card G, Moshkov K, Bax B, Ralph A, Lindley P. The X-ray structure of human serum ceruloplasmin at 3.1Å: nature of the copper centers. J Biol Inorg Chem. 1996;1:15-23. [RCA] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 259] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 30. | Bento I, Peixoto C, Zaitsev VN, Lindley PF. Ceruloplasmin revisited: structural and functional roles of various metal cation-binding sites. Acta Crystallogr D Biol Crystallogr. 2007;63:240-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Bielli P, Bellenchi GC, Calabrese L. Site-directed mutagenesis of human ceruloplasmin: . production of a proteolytically stable protein and structure-activity relationships of type 1 sites. J Biol Chem. 2001;276:2678-2685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Musci G, Bonaccorsi di Patti MC, Petruzzelli R, Giartosio A, Calabrese L. Divalent cation binding to ceruloplasmin. Biometals. 1996;9:66-72. [PubMed] |
| 33. | Lutsenko S, Barnes NL, Bartee MY, Dmitriev OY. Function and regulation of human copper-transporting ATPases. Physiol Rev. 2007;87:1011-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 606] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 34. | Maio N, Polticelli F, De Francesco G, Rizzo G, Bonaccorsi di Patti MC, Musci G. Role of external loops of human ceruloplasmin in copper loading by ATP7B and Ccc2p. J Biol Chem. 2010;285:20507-20513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Patel BN, David S. A novel glycosylphosphatidylinositol-anchored form of ceruloplasmin is expressed by mammalian astrocytes. J Biol Chem. 1997;272:20185-20190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 235] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 36. | Mittal B, Doroudchi MM, Jeong SY, Patel BN, David S. Expression of a membrane-bound form of the ferroxidase ceruloplasmin by leptomeningeal cells. Glia. 2003;41:337-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Fortna RR, Watson HA, Nyquist SE. Glycosyl phosphatidylinositol-anchored ceruloplasmin is expressed by rat Sertoli cells and is concentrated in detergent-insoluble membrane fractions. Biol Reprod. 1999;61:1042-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Chen L, Dentchev T, Wong R, Hahn P, Wen R, Bennett J, Dunaief JL. Increased expression of ceruloplasmin in the retina following photic injury. Mol Vis. 2003;9:151-158. [PubMed] |
| 39. | Patel BN, Dunn RJ, David S. Alternative RNA splicing generates a glycosylphosphatidylinositol-anchored form of ceruloplasmin in mammalian brain. J Biol Chem. 2000;275:4305-4310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 156] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 40. | De Domenico I, Ward DM, di Patti MC, Jeong SY, David S, Musci G, Kaplan J. Ferroxidase activity is required for the stability of cell surface ferroportin in cells expressing GPI-ceruloplasmin. EMBO J. 2007;26:2823-2831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 265] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 41. | Marques L, Auriac A, Willemetz A, Banha J, Silva B, Canonne-Hergaux F, Costa L. Immune cells and hepatocytes express glycosylphosphatidylinositol-anchored ceruloplasmin at their cell surface. Blood Cells Mol Dis. 2012;48:110-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 42. | Kono S, Yoshida K, Tomosugi N, Terada T, Hamaya Y, Kanaoka S, Miyajima H. Biological effects of mutant ceruloplasmin on hepcidin-mediated internalization of ferroportin. Biochim Biophys Acta. 2010;1802:968-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 43. | Floris G, Medda R, Padiglia A, Musci G. The physiopathological significance of ceruloplasmin. A possible therapeutic approach. Biochem Pharmacol. 2000;60:1735-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 44. | Mukhopadhyay CK, Mazumder B, Lindley PF, Fox PL. Identification of the prooxidant site of human ceruloplasmin: a model for oxidative damage by copper bound to protein surfaces. Proc Natl Acad Sci USA. 1997;94:11546-11551. [PubMed] |
| 45. | Osaki S, Johnson DA, Frieden E. The possible significance of the ferrous oxidase activity of ceruloplasmin in normal human serum. J Biol Chem. 1966;241:2746-2751. [PubMed] |
| 46. | Lindley PF, Card G, Zaitseva I, Zaitsev V, Reinhammar B, Selin-Lindgren E, Yoshida K. An X-ray structural study of human ceruloplasmin in relation to ferroxidase activity. J Biol Inorg Chem. 1997;2:454-463. [RCA] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 93] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 47. | Ragan HA, Nacht S, Lee GR, Bishop CR, Cartwright GE. Effect of ceruloplasmin on plasma iron in copper-deficient swine. Am J Physiol. 1969;217:1320-1323. [PubMed] |
| 48. | Roeser HP, Lee GR, Nacht S, Cartwright GE. The role of ceruloplasmin in iron metabolism. J Clin Invest. 1970;49:2408-2417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 259] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 49. | Sarkar J, Seshadri V, Tripoulas NA, Ketterer ME, Fox PL. Role of ceruloplasmin in macrophage iron efflux during hypoxia. J Biol Chem. 2003;278:44018-44024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 50. | Harris ZL, Durley AP, Man TK, Gitlin JD. Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. Proc Natl Acad Sci USA. 1999;96:10812-10817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 415] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 51. | Patel BN, Dunn RJ, Jeong SY, Zhu Q, Julien JP, David S. Ceruloplasmin regulates iron levels in the CNS and prevents free radical injury. J Neurosci. 2002;22:6578-6586. [PubMed] |
| 52. | Jeong SY, David S. Glycosylphosphatidylinositol-anchored ceruloplasmin is required for iron efflux from cells in the central nervous system. J Biol Chem. 2003;278:27144-27148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 286] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 53. | McCarthy RC, Kosman DJ. Ferroportin and exocytoplasmic ferroxidase activity are required for brain microvascular endothelial cell iron efflux. J Biol Chem. 2013;288:17932-17940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 54. | Dringen R, Bishop GM, Koeppe M, Dang TN, Robinson SR. The pivotal role of astrocytes in the metabolism of iron in the brain. Neurochem Res. 2007;32:1884-1890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 160] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 55. | Barber EF, Cousins RJ. Interleukin-1--stimulated induction of ceruloplasmin synthesis in normal and copper-deficient rats. J Nutr. 1988;118:375-381. [PubMed] |
| 56. | Kuhlow CJ, Krady JK, Basu A, Levison SW. Astrocytic ceruloplasmin expression, which is induced by IL-1beta and by traumatic brain injury, increases in the absence of the IL-1 type 1 receptor. Glia. 2003;44:76-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 57. | di Patti MC, Persichini T, Mazzone V, Polticelli F, Colasanti M, Musci G. Interleukin-1beta up-regulates iron efflux in rat C6 glioma cells through modulation of ceruloplasmin and ferroportin-1 synthesis. Neurosci Lett. 2004;363:182-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Mazumder B, Mukhopadhyay CK, Prok A, Cathcart MK, Fox PL. Induction of ceruloplasmin synthesis by IFN-gamma in human monocytic cells. J Immunol. 1997;159:1938-1944. [PubMed] |
| 59. | Conley L, Geurs TL, Levin LA. Transcriptional regulation of ceruloplasmin by an IL-6 response element pathway. Brain Res Mol Brain Res. 2005;139:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 60. | Sidhu A, Miller PJ, Hollenbach AD. FOXO1 stimulates ceruloplasmin promoter activity in human hepatoma cells treated with IL-6. Biochem Biophys Res Commun. 2011;404:963-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 61. | Mukhopadhyay CK, Mazumder B, Fox PL. Role of hypoxia-inducible factor-1 in transcriptional activation of ceruloplasmin by iron deficiency. J Biol Chem. 2000;275:21048-21054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 214] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 62. | Taylor M, Qu A, Anderson ER, Matsubara T, Martin A, Gonzalez FJ, Shah YM. Hypoxia-inducible factor-2α mediates the adaptive increase of intestinal ferroportin during iron deficiency in mice. Gastroenterology. 2011;140:2044-2055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 221] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 63. | Wilkinson N, Pantopoulos K. IRP1 regulates erythropoiesis and systemic iron homeostasis by controlling HIF2α mRNA translation. Blood. 2013;122:1658-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 64. | Troadec MB, Ward DM, Lo E, Kaplan J, De Domenico I. Induction of FPN1 transcription by MTF-1 reveals a role for ferroportin in transition metal efflux. Blood. 2010;116:4657-4664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 65. | Marro S, Chiabrando D, Messana E, Stolte J, Turco E, Tolosano E, Muckenthaler MU. Heme controls ferroportin1 (FPN1) transcription involving Bach1, Nrf2 and a MARE/ARE sequence motif at position -7007 of the FPN1 promoter. Haematologica. 2010;95:1261-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 227] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 66. | Harada N, Kanayama M, Maruyama A, Yoshida A, Tazumi K, Hosoya T, Mimura J, Toki T, Maher JM, Yamamoto M. Nrf2 regulates ferroportin 1-mediated iron efflux and counteracts lipopolysaccharide-induced ferroportin 1 mRNA suppression in macrophages. Arch Biochem Biophys. 2011;508:101-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 176] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 67. | Delaby C, Pilard N, Puy H, Canonne-Hergaux F. Sequential regulation of ferroportin expression after erythrophagocytosis in murine macrophages: early mRNA induction by haem, followed by iron-dependent protein expression. Biochem J. 2008;411:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 68. | Yang F, Liu XB, Quinones M, Melby PC, Ghio A, Haile DJ. Regulation of reticuloendothelial iron transporter MTP1 (Slc11a3) by inflammation. J Biol Chem. 2002;277:39786-39791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 156] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 69. | Ludwiczek S, Aigner E, Theurl I, Weiss G. Cytokine-mediated regulation of iron transport in human monocytic cells. Blood. 2003;101:4148-4154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 310] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 70. | Urrutia P, Aguirre P, Esparza A, Tapia V, Mena NP, Arredondo M, González-Billault C, Núñez MT. Inflammation alters the expression of DMT1, FPN1 and hepcidin, and it causes iron accumulation in central nervous system cells. J Neurochem. 2013;126:541-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 295] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 71. | Persichini T, Maio N, di Patti MC, Rizzo G, Toscano S, Colasanti M, Musci G. Interleukin-1β induces ceruloplasmin and ferroportin-1 gene expression via MAP kinases and C/EBPβ, AP-1, and NF-κB activation. Neurosci Lett. 2010;484:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 72. | Persichini T, Maio N, di Patti MC, Rizzo G, Colasanti M, Musci G. Genistein up-regulates the iron efflux system in glial cells. Neurosci Lett. 2010;470:145-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 73. | Sangokoya C, Doss JF, Chi JT. Iron-responsive miR-485-3p regulates cellular iron homeostasis by targeting ferroportin. PLoS Genet. 2013;9:e1003408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 74. | Zhang DL, Hughes RM, Ollivierre-Wilson H, Ghosh MC, Rouault TA. A ferroportin transcript that lacks an iron-responsive element enables duodenal and erythroid precursor cells to evade translational repression. Cell Metab. 2009;9:461-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 208] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 75. | Harris ZL, Takahashi Y, Miyajima H, Serizawa M, MacGillivray RT, Gitlin JD. Aceruloplasminemia: molecular characterization of this disorder of iron metabolism. Proc Natl Acad Sci USA. 1995;92:2539-2543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 373] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 76. | Yoshida K, Furihata K, Takeda S, Nakamura A, Yamamoto K, Morita H, Hiyamuta S, Ikeda S, Shimizu N, Yanagisawa N. A mutation in the ceruloplasmin gene is associated with systemic hemosiderosis in humans. Nat Genet. 1995;9:267-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 302] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 77. | McNeill A, Pandolfo M, Kuhn J, Shang H, Miyajima H. The neurological presentation of ceruloplasmin gene mutations. Eur Neurol. 2008;60:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 78. | Kono S. Aceruloplasminemia. Curr Drug Targets. 2012;13:1190-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 79. | Jeong SY, David S. Age-related changes in iron homeostasis and cell death in the cerebellum of ceruloplasmin-deficient mice. J Neurosci. 2006;26:9810-9819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 117] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 80. | Oshiro S, Kawamura K, Zhang C, Sone T, Morioka MS, Kobayashi S, Nakajima K. Microglia and astroglia prevent oxidative stress-induced neuronal cell death: implications for aceruloplasminemia. Biochim Biophys Acta. 2008;1782:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 81. | Hellman NE, Kono S, Miyajima H, Gitlin JD. Biochemical analysis of a missense mutation in aceruloplasminemia. J Biol Chem. 2002;277:1375-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 82. | Hellman NE, Kono S, Mancini GM, Hoogeboom AJ, De Jong GJ, Gitlin JD. Mechanisms of copper incorporation into human ceruloplasmin. J Biol Chem. 2002;277:46632-46638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 122] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 83. | Kono S, Suzuki H, Takahashi K, Takahashi Y, Shirakawa K, Murakawa Y, Yamaguchi S, Miyajima H. Hepatic iron overload associated with a decreased serum ceruloplasmin level in a novel clinical type of aceruloplasminemia. Gastroenterology. 2006;131:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 84. | Hofmann WP, Welsch C, Takahashi Y, Miyajima H, Mihm U, Krick C, Zeuzem S, Sarrazin C. Identification and in silico characterization of a novel compound heterozygosity associated with hereditary aceruloplasminemia. Scand J Gastroenterol. 2007;42:1088-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 85. | di Patti MC, Maio N, Rizzo G, De Francesco G, Persichini T, Colasanti M, Polticelli F, Musci G. Dominant mutants of ceruloplasmin impair the copper loading machinery in aceruloplasminemia. J Biol Chem. 2009;284:4545-4554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 86. | Kuhn J, Miyajima H, Takahashi Y, Kunath B, Hartmann-Klosterkoetter U, Cooper-Mahkorn D, Schaefer M, Bewermeyer H. Extrapyramidal and cerebellar movement disorder in association with heterozygous ceruloplasmin gene mutation. J Neurol. 2005;252:111-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 87. | Persichini T, De Francesco G, Capone C, Cutone A, di Patti MC, Colasanti M, Musci G. Reactive oxygen species are involved in ferroportin degradation induced by ceruloplasmin mutant Arg701Trp. Neurochem Int. 2012;60:360-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 88. | Pietrangelo A. The ferroportin disease. Blood Cells Mol Dis. 2004;32:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 256] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 89. | Mayr R, Janecke AR, Schranz M, Griffiths WJ, Vogel W, Pietrangelo A, Zoller H. Ferroportin disease: a systematic meta-analysis of clinical and molecular findings. J Hepatol. 2010;53:941-949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 90. | Kasvosve I. Effect of ferroportin polymorphism on iron homeostasis and infection. Clin Chim Acta. 2013;416:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 91. | De Domenico I, McVey Ward D, Nemeth E, Ganz T, Corradini E, Ferrara F, Musci G, Pietrangelo A, Kaplan J. Molecular and clinical correlates in iron overload associated with mutations in ferroportin. Haematologica. 2006;91:1092-1095. [PubMed] |
| 92. | Drakesmith H, Schimanski LM, Ormerod E, Merryweather-Clarke AT, Viprakasit V, Edwards JP, Sweetland E, Bastin JM, Cowley D, Chinthammitr Y. Resistance to hepcidin is conferred by hemochromatosis-associated mutations of ferroportin. Blood. 2005;106:1092-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 188] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 93. | Détivaud L, Island ML, Jouanolle AM, Ropert M, Bardou-Jacquet E, Le Lan C, Mosser A, Leroyer P, Deugnier Y, David V. Ferroportin diseases: functional studies, a link between genetic and clinical phenotype. Hum Mutat. 2013;34:1529-1536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 94. | Létocart E, Le Gac G, Majore S, Ka C, Radio FC, Gourlaouen I, De Bernardo C, Férec C, Grammatico P. A novel missense mutation in SLC40A1 results in resistance to hepcidin and confirms the existence of two ferroportin-associated iron overload diseases. Br J Haematol. 2009;147:379-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 95. | Mayr R, Griffiths WJ, Hermann M, McFarlane I, Halsall DJ, Finkenstedt A, Douds A, Davies SE, Janecke AR, Vogel W. Identification of mutations in SLC40A1 that affect ferroportin function and phenotype of human ferroportin iron overload. Gastroenterology. 2011;140:2056-263, 2063.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 96. | Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605-1612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28715] [Cited by in RCA: 34444] [Article Influence: 1640.2] [Reference Citation Analysis (0)] |