Published online Nov 26, 2013. doi: 10.4331/wjbc.v4.i4.141
Revised: September 29, 2013
Accepted: October 17, 2013
Published online: November 26, 2013
Processing time: 210 Days and 19.9 Hours
AIM: To evaluate changes in neurotransmission induced by a psychoactive beverage ayahuasca in the hippocampus and amygdala of naive rats.
METHODS: The level of monoamines, their main metabolites and amino acid neurotransmitters concentrations were quantified using high performance liquid chromatography (HPLC). Four groups of rats were employed: saline-treated and rats receiving 250, 500 and 800 mg/kg of ayahuasca infusion (gavage). Animals were killed 40 min after drug ingestion and the structures stored at -80 °C until HPLC assay. The data from all groups were compared using Analysis of variance and Scheffé as post test and P < 0.05 was accepted as significant.
RESULTS: The results showed decreased concentrations of glycine (GLY) (0.13 ± 0.03 vs 0.29 ± 0.07, P < 0.001) and γ-aminobutyric acid (GABA) (1.07 ± 0.14 vs 1.73 ± 0.25, P < 0.001) in the amygdala of rats that received 500 of ayahuasca. Animals that ingested 800 mg/kg of ayahuasca also showed a reduction of GLY level (0.11 ± 0.01 vs 0.29 ± 0.07, P < 0.001) and GABA (0.98 ± 0.06 vs 1.73 ± 0.25, P < 0.001). In the hippocampus, increased GABA levels were found in rats that received all ayahuasca doses: 250 mg/kg (1.29 ± 0.19 vs 0.84 ± 0.21, P < 0.05); 500 mg/kg (2.23 ± 038 vs 084 ± 0.21, P < 0.05) and 800 mg/kg (1.98 ± 0.92 vs 0.84 ± 0.21, P < 0.05). In addition, an increased utilization rate of all monoamines was found in the amygdala after ayahuasca administration in doses: 250 mg/kg (noradrenaline: 0.16 ± 0.02 vs 0.36 ± 0.06, P < 0.01; dopamine: 0.39 ± 0.012 vs 2.39 ± 0.84, P < 0.001; serotonin: 1.02 ± 0.22 vs 4.04 ± 0.91, P < 0.001), 500 mg/kg (noradrenaline: 0.08 ± 0.02 vs 0.36 ± 0.06, P < 0.001; dopamine: 0.33 ± 0.19 vs 2.39 ± 0.84, P < 0.001; serotonin: 0.59 ± 0.08 vs 4.04 ± 0.91, P < 0.001) and 800 mg/kg (noradrenaline: 0.16 ± 0.04 vs 0.36 ± 0.06, P < 0.001; dopamine: 0.84 ± 0.65 vs 2.39 ± 0.84, P < 0.05; serotonin: 0.36 ± 0.02 vs 4.04 ± 0.91, P < 0.001).
CONCLUSION: Our data suggest increased release of inhibitory amino acids by the hippocampus and an increased utilization rate of monoamines by the amygdala after different doses of ayahuasca ingestion.
Core tip: Several studies have indicated that the main component of ayahuasca, N,N-dimethyltryptamine (DMT), is structurally similar to serotonin (5-hydroxytryptamine or 5-HT) and also has similarities with lysergic acid and mescaline, normally employed in drug addiction. This infusion contained DMT as a principal ingredient in a psychoactive beverage, used by more than 70 different indigenous groups spread throughout Brazil, Colombia, Peru, Venezuela and Ecuador. In human beings, it is also present in the brain as an endogenous substance and is found in blood, urine and cerebrospinal fluid. After oral administration of ayahuasca at different doses to naïve rats, we found that ayahuasca ingestion could modify neurotransmitter release in limbic brain structures.
- Citation: Castro-Neto EF, Cunha RHD, Silveira DXD, Yonamine M, Gouveia TLF, Cavalheiro EA, Amado D, Naffah-Mazzacoratti MDG. Changes in aminoacidergic and monoaminergic neurotransmission in the hippocampus and amygdala of rats after ayahuasca ingestion. World J Biol Chem 2013; 4(4): 141-147
- URL: https://www.wjgnet.com/1949-8454/full/v4/i4/141.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v4.i4.141
Ayahuasca is a Quechua term derived from the juxtaposition of the words: (Aya) “soul”, “dead spirit”, and (Waska) “rope”, “vine”, and thus is loosely translatable as “vine of the souls” or “vine of the dead”. Ayahuasca refers to the vine that is the principal ingredient of a psychoactive beverage used by more than 70 different indigenous groups spread throughout Brazil, Colombia, Peru, Venezuela, Ecuador[1] and North America[2]. The word ayahuasca is used to describe the spiritual force in the beverage, which usually also contains a combination of other plants, such as Psychotria viridis or Diplopterys cabrerana[3,4].
Several studies[5-8] have indicated that the main component of ayahuasca, N,N-dimethyltryptamine (DMT), is structurally similar to serotonin (5-hydroxytryptamine or 5-HT) and also has similarities with lysergic acid and mescaline. The beta-carbolines also present in ayahuasca include harmine (HRM), harmalina (HRL) and tetrahydroharmine (THH). DMT is a short-acting hallucinogenic tryptamine, which is present in several plants used as admixtures to the Banisteriopsis caapi (B. caapi) vine in ayahuasca preparations[8,9]. In human beings, it is also present in the brain as an endogenous substance and is found in blood, urine and cerebrospinal fluid[10]. DMT is psychoactive but is inactivated following oral administration, probably due to degradation by gastrointestinal and liver monoamine oxidase (MAO)[5,11,12]. However, when DMT is combined with inhibitors of MAO, such as the beta carbolines present in B. caapi, it becomes able to reach the systemic circulation and subsequently the central nervous system, thus producing its effects[5].
DMT acts as an agonist of serotonergic receptors and its association with MAO inhibition favors a still greater availability of 5-HT in the synaptic cleft. Moreover, THH inhibits reuptake of 5-HT as well as competing with DMT for the same receptors, 5-HT2 and 5-HT1A.
According to McKenna[7], a deficit of serotonin reuptake sites in the frontal cortex has been found to correlate with aggressive behavior in alcoholics. If THH is able to specifically reverse the deficit it might have clinical applications in the treatment of this disruptive behavior.
In this context, the present work was designed to analyze possible changes induced by ayahuasca to neurotransmission in the amygdala and hippocampus of rats, which received three different doses of this infusion by gavage.
For this purpose, monoamines (noradrenaline, NA; dopamine, DA; and serotonin, 5-HT) as well as their principal metabolites were quantified using high performance liquid chromatography (HPLC). The amino acid neurotransmitters glutamate, glycine (GLY), taurine (TAU) and gamma-aminobutyric acid (GABA) were also quantified in these structures. The main components of ayahuasca were measured by gas chromatography and the concentrations of DMT, HRL, HRM and THH were determined.
The infusion of ayahuasca was supplied by Professor Dr. Dartiu Xavier da Silveira, from the Psychiatry Department of Universidade Federal de São Paulo, and this infusion was prepared by the Núcleo Senhora Santana, Campo Grande, Brazil on May 22nd 2008, for Master José Roberto de Souza under the auspices of Centro Espírita Beneficente União do Vegetal, and in the care of C Otávio Castelo. The infusion was previously lyophilized and stored at -18 °C under vacuum. After this procedure, each 200 mL of infusion was converted in 40.8 g of powder, which was maintained under proper conditions.
The concentration of the main alkaloid ayahuasca components was determined in this work using a gas chromatography procedure, as previously reported[13]. Briefly, analyses were performed using an Agilent gas chromatograph equipped with a nitrogen-phosphorous detector (GC-NPD). Chromatographic separation was achieved on an HP ultra-2-fused-silica capillary column (25 m × 0.2 mm × 0.33 μm) film thickness using ultra-pure nitrogen as the carrier gas at a constant flow rate of 0.6 mL/min. Injections of 1 μL were made in split mode (ratio 1:20). The injector port and detector temperature were maintained at 200 and 250 °C respectively. The oven temperature was maintained at 150 °C for 1 min and programmed to rise at 10 °C/min to 250 °C before being held at this latter temperature for 7 min.
A sample solution containing ayahuasca (0.5 mL), borate buffer (pH 9.0, 2.0 mL) and the internal standard diphenhydramine (100 μL of a solution 1.0 mg/mL) was loaded onto a C18 cartridge mounted on a vacuum manifold and conditioned with methanol (2 mL), deionized water (1.0 mL) and borate buffer (pH 9.0; 2.0 mL). The loaded cartridge was further washed with deionized water and with a solution of acetonitrile-water (1:9). After drying the cartridges under full vacuum for 7 min, the sample was eluted with methanol (2 mL). This solution (1 μL) was injected in the GC-NPD system and the retention time and concentration were obtained after comparison with stock standard solution[13].
At least 1 wk before the experiments, adult male Wistar rats, weighing 220-280 g, were randomly selected from the same pool and allocated to groups of five, housed under conditions of controlled temperature and humidity on a standard light/dark cycle of 12 h (lights off at 7:00 pm). Rat chow pellets and water were provided ad libitum. The experiments were performed with the approval of the Institutional Ethics Committee (DHEW Publication, NIH, 80-23), (number 1050/09) and all efforts were made to minimize animal suffering. Four groups of rats were employed: saline-treated (n = 5) and rats receiving 250 mg/kg (n = 8), 500 mg/kg (n = 8) and 800 mg/kg (n = 8) of lyophilized ayahuasca orally. The animals’ behavior after drug administration was analyzed by three different observers and the rats were killed 40 min after drug ingestion. The brain structures were separated and stored at -80 °C until assay. Another group of rats (n = 8) was employed to study their behavior after drug administration (500 mg/kg). Changes in the behavior were analyzed by three different observers during 60 min.
Monoamines and amino acids were identified and quantified using a HPLC system with electrochemical and fluorescence detectors, respectively.
Brain structures were removed, placed on an ice-chilled plate, weighed and stored at -80 °C until assay. Tissues were ultrasonically homogenized in a 0.1 mol/L solution of HClO4 containing 0.02% Na2S2O2 (15 μL of solution for each milligram of tissue), dihydroxybenzylamine (DHBA, 146.5 ng/mL), as the internal standard for monoamines, and homoserine (HSER, 10 μg/mL), as the internal standard for amino acids. The samples were centrifuged at 11000 g at 4 °C for 40 min, and then the supernatant was filtered and injected into an HPLC system. The monoamines NA, DA and 5-HT, as well as their metabolites, were quantified as previously described by our group[14]. In summary, a Shimaszu LC-10AD isocratic system was employed, with a 20 μL injection loop and a Spheri-5 RP-18 5 μm column (220 × 4.6 mm), using electrochemical detection at 0.75 V and a mobile phase composed of phosphate/citrate pH 2.64, 0.02 mol/L, 0.12 mmol/L ethylene diamine tetraacetic acid and 0.06% heptane sulphonic acid, in 10% methanol, at a flow rate of 1 mL/min. Concentrations of monoamines and metabolites were expressed as mean ± SD ng/mg wet tissue. Turnover rates of monoamines were calculated by the ratio between metabolites and monoamine concentrations.
To assay amino acids, the supernatant was filtered and submitted to an o-phthaldehyde (OPA) derivatization and then injected into the HPLC system. Amino acid derivatization was done by dissolving 27 mg of OPA in 1 mL of methanol, adding 5 μL of 2-mercaptoethanol and 9 mL of 0.1 mol/L sodium tetraborate (pH 9.3) solution. Before sample analysis, a solution was prepared with 1 mL of stock solution and 2 mL of sodium tetraborate 0.1 mol/L. The pre-column derivatization was completed by reacting 100 μL of this solution with 50 μL of sample or amino acid standard solution for 2 min before the injection[15]. An isocratic HPLC system was used with a fluorescence detector, a 20 μL sample injector and an RP-18 column (50 × 4.6 mm). The mobile phase consisted of sodium phosphate 0.05 mol/L (pH 5.95) with methanol 11.5%. The flow rate of this HPLC system was 3.5 mL/min and the detector was employed with an excitation of 348 nm and emission of 460 nm.
Standard concentrations of amino acids and monoamines were tested and the retention time was verified for each substance to certify that there were no peaks overlapping on sample delivery. The amino acids were expressed as mean ± SD (nmol/L per milligram) wet tissue.
The data from all groups were compared using Analysis of variance and Scheffé as post test and P < 0.05 was accepted as significant.
After ayahuasca administration, the behavior of the animals was evaluated qualitatively and compared with that of saline-treated rats. Ten min after infusion administration, all rats that received ayahuasca showed increased exploratory behavior, with increased sniffing and chewing. After this period, they exhibited hyperkinesia, oral and masticatory movements and blinking. The hyperkinesia evolved to loss of foot strength, enlarged base and semi-closed eyes, 30 min after ayahuasca administration. The number of fecal residues was similar in control and treated animals. This altered behavior was progressively normalized and 60 min after ayahuasca administration, all rats showed normal activity.
The original infusion of ayahuasca employed contained DMT = 0.59 mg/mL, THH = 0.99 mg/mL, HRL = 0.19 mg/mL and HRM = 5.09 mg/mL. Table 1 summarizes the concentration of each active principle administered to each group of rats.
| Ayahuasca lyophilized | 250 mg/kg | 500 mg/kg | 800 mg/kg |
| DMT | 6.02 | 12.04 | 19.26 |
| THH | 10.10 | 20.20 | 32.32 |
| HRL | 1.94 | 3.88 | 6.20 |
| HRM | 51.92 | 103.89 | 166.19 |
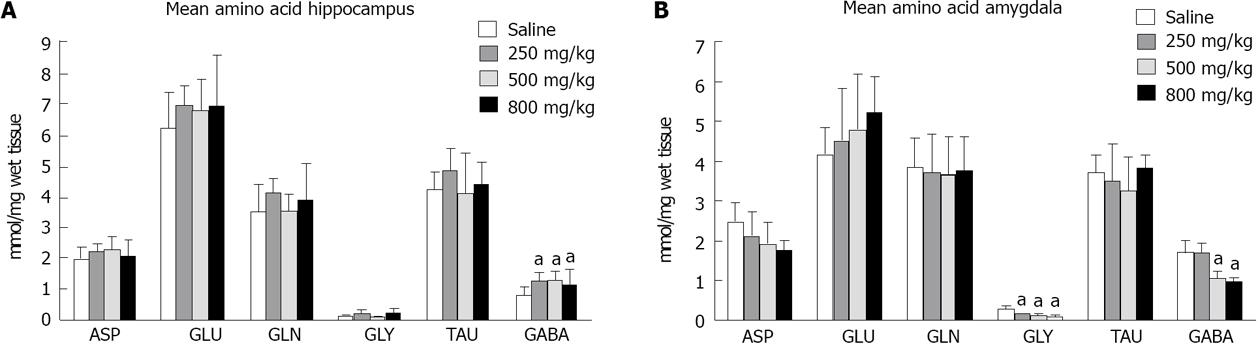
When the concentrations of amino acids were analyzed in the hippocampus, we found an increased level of GABA in the groups of rats that received 250, 500 and 800 mg/kg of ayahuasca, when compared with saline-treated animals (Figure 1A). In contrast, the data illustrated in Figure 1B show that the amygdala presented a decreased concentration of GLY in all drug-treated groups and decreased GABA only in those rats that received the highest ayahuasca concentrations (500 and 800 mg/kg).
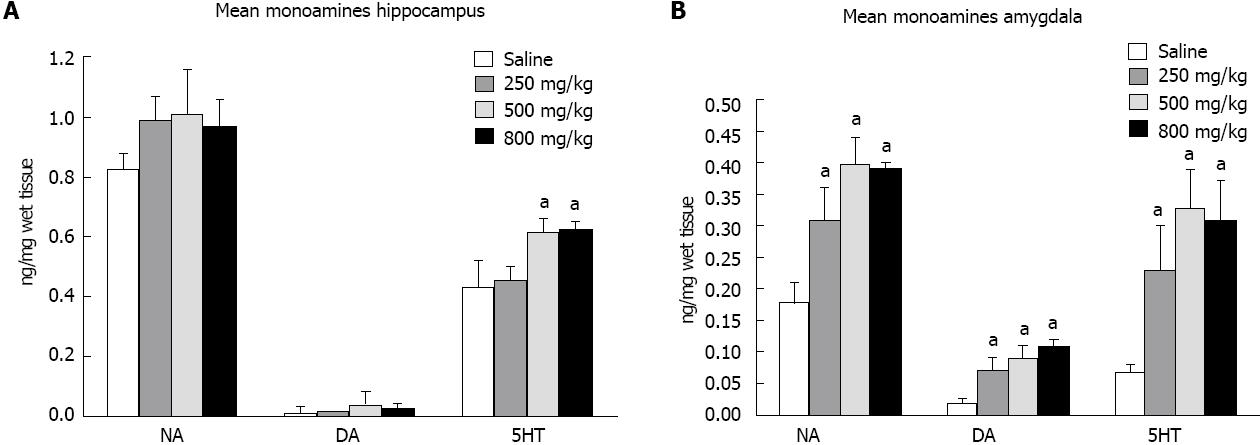
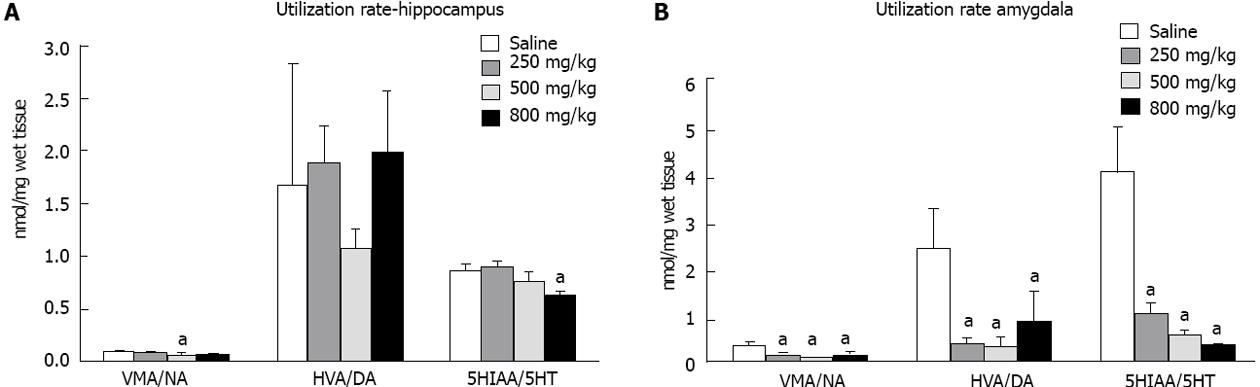
The hippocampus of ayahuasca-treated rats showed greater variation when 500 and 800 mg/kg of drug was administered. Animals that received these doses showed increased concentrations of 3,4-dihydroxyphenylacetic acid, 5-hydroxyindoleacetic acid and homovanillic acid (data not shown). In addition, the level of 5-HT was also increased in those rats that received 500 or 800 mg/kg of ayahuasca (Figure 2A). However, the comparison between drug-treated and saline-treated groups showed only a decreased utilization rate for 5-HT in the hippocampus of rats that received 800 mg/kg (Figure 3A).
The amygdala presented the biggest change with regard to monoamine levels. NA, DA and 5-HT all showed increased concentrations in all the studied groups. Analyzing the utilization rates of monoamines in the amygdala, we found that NA, DA and 5-HT were less utilized or less degraded, as shown in Figure 3B.
Ayahuasca infusions prepared in different South American countries contain different concentrations of psychotropic agents. According to McKenna et al[5], each milliliter of ayahuasca from Peru contains DMT (0.6 mg), HRM (4.67 mg), HRL (0.41 mg) and THH (1.6 mg). Meanwhile, the great majority of Brazilian ayahuasca contains, on average: DMT (0.6 mg/mL); HRM (1.2 mg/mL) HRL (0.2 mg/mL) and THH (1.07 mg/mL)[16]. In this context, our data are in accordance with those described by other authors since we found DMT = 0.59 mg/mL, THH = 0.99 mg/mL, HRL = 0.19 mg/mL and HRM = 5.09 mg/mL in the ayahuasca sample.
The present study aimed to investigate the effects of the ingestion of different concentrations of the ayahuasca infusion upon the levels of some neurotransmitters (monoamines and amino acids), as well as the utilization rate of monoamines in the hippocampus and amygdala of naive rats.
No changes were found in the glutamate concentrations, either in the hippocampus or the amygdala of treated animals, which indicates that this excitatory amino acid is not involved in ayahuasca-induced behavioral changes. However, a reduction in GLY levels in the amygdala was observed with the administration of 250, 500 and 800 mg/kg of infusion, and a reduction in GABA levels was seen after administration of 500 and 800 mg/kg, suggesting increased release of both inhibitory amino acids in this brain structure. In contrast, in the hippocampus, an increased concentration of GABA was found in all ayahuasca-treated groups, suggesting decreased release of this neurotransmitter, compatible with increased excitation in the hippocampus and increased inhibition in the amygdala of ayahuasca-treated rats.
In this context, we showed here, for the first time, that ayahuasca induces changes in the concentration of inhibitory amino acids in two limbic structures. The opposite effects found in the hippocampus and amygdala could support the activation and/or inhibition of several pathways involved in important processes, such as memory and learning and emotional behavior. Furthermore, the GABA ergic system has also been linked to an amnesic function in the hippocampal-dependent declarative memory[17] and a change in this pathway could represent modification of this function. In direct opposition to this idea, data from Doering-Silveira et al[18] showed that ayahuasca-user adolescents did not differ from control subjects when neuropsychological tests were applied to both groups. Thus, more studies are needed to elucidate these findings.
The determination of monoamine content showed that among the analyzed structures, the amygdala was most affected by ayahuasca treatment; all the concentrations used in this work increased the levels of NA, DA and 5-HT in this limbic structure. The utilization rates of these monoamines were also reduced, suggesting MAO inhibition or increased synthesis of these neurotransmitters, or both.
In the hippocampus, 5-HT levels were also increased in rats that received 500 and 800 mg/kg of ayahuasca. However, the comparison between drug-treated and saline-treated groups showed a decreased utilization rate for 5-HT in the hippocampus only when the higher dose of ayahuasca was employed.
These data show that, although with some variation, ayahuasca increases the concentration of monoamines in limbic structures, mainly in the amygdala. Furthermore, previous studies have shown an increased stimulation of 5-HT receptors by the individual ayahuasca components [5,6] showing an over stimulation of monoamine pathways in the brain.
According to Schwarz et al[19], HRM and HRL also stimulate DA release from striatal slices and these data, in association with MAOA inhibition, suggest that B. caapi could be tested in the treatment of Parkinson disease. In addition, Schmoldt et al[20] observed that MAO inhibition is able to reduce oral ethanol self-administration, due to high levels of DA and 5-HT in the synaptic cleft. In this context, ayahuasca could also be useful in the treatment of alcohol dependence. Recent data showed an important application in therapies for addiction[9,11,21], anxiety disorders[22] and Parkinson disease[19,23,24], and modulates REM and slow-wave sleep in healthy volunteers[25]. HRM is also related to inhibition of angiogenesis and suppression of tumor growth through activation of p53 in endothelial cells[26].
Taken together, these data show that ayahuasca components have different actions on different brain structures, involving changes in monoamines and amino acid concentrations.
Ayahuasca is an infusion containing psychoactive compounds, such as N,N-dimethyltryptamine (DMT), is structurally similar to serotonin (5-hydroxytryptamine) and also has similarities with lysergic acid and mescaline. This infusion also has beta-carbolines, such as harmine, harmalina and tetrahydroharmine. DMT is a short-acting hallucinogenic tryptamine, which is present in several plants used as admixtures to the Banisteriopsis caapi vine in ayahuasca preparations.
As ayahuasca use has been growing in the world, mainly in South and recently in North America, the knowledge of how it can modify normal neurotransmitters in limbic structures is important since this infusion could be employed in the treatment of other brain pathologies.
This work shows, for the first time, detailed changes in amino acids and monoamines, as well as their utilization rates in limbic structures of the central nervous system, after ayahuasca ingestion by naïve rats.
Clinical use of ayahuasca could be important as an additional drug in treatment of major depression and/or in other central pathologies.
Utilization rate of a monoamine is used to visualize the release of this neurotransmitter in specific regions of the CNS. It is described as the rate between its main metabolite and neurotransmitter concentration (metabolite concentration/monoamine concentration).
Ayahuasca is a psychoactive plant preparation which contains a serotonin analog dimethyltryptamine and monoamine oxidase beta-carbolines. However, in vivo effects on monoamine neurotransmitters and other amino acid transmitters have not been reported. In this manuscript, the authors observed the effect of orally administered ayahuasca on the content of monoamines (and their metabolites) and amino acid neurotransmitters in two important brain structures in the limbic system. Their results demonstrated that ayahuasca mainly increased monoamines in the amygdala, accompanied by a reduction of their metabolites.
P- Reviewers: Chang YC, Lei S S- Editor: Zhai HH L- Editor: Roemmele A E- Editor: Wu HL
| 1. | Grob CS, McKenna DJ, Callaway JC, Brito GS, Neves ES, Oberlaender G, Saide OL, Labigalini E, Tacla C, Miranda CT. Human psychopharmacology of hoasca, a plant hallucinogen used in ritual context in Brazil. J Nerv Ment Dis. 1996;184:86-94. [PubMed] |
| 2. | Harris R, Gurel L. A study of ayahuasca use in North America. J Psychoactive Drugs. 2012;44:209-215. [PubMed] |
| 3. | Ott J. Ayahuasca Analogues: Pangaean Entheogens. Kennewick, Washington: Natural Books Co 1999; . |
| 4. | dos Santos RG. Safety and side effects of ayahuasca in humans--an overview focusing on developmental toxicology. J Psychoactive Drugs. 2013;45:68-78. [PubMed] |
| 5. | McKenna DJ, Towers GH, Abbott F. Monoamine oxidase inhibitors in South American hallucinogenic plants: tryptamine and beta-carboline constituents of ayahuasca. J Ethnopharmacol. 1984;10:195-223. [PubMed] |
| 6. | Callaway JC. A proposed mechanism for the visions of dream sleep. Med Hypotheses. 1988;26:119-124. [PubMed] |
| 7. | McKenna DJ, Callaway JC, Grob CS. The scientific investigation of Ayahuasca: a review of past and current research. Heffter Rev Psychedel Res. 1998;1:65-77 Available from: http://www.erowid.org/chemicals/ayahuasca/ayahuasca_journal3.shtml. |
| 8. | Callaway JC, McKenna DJ, Grob CS, Brito GS, Raymon LP, Poland RE, Andrade EN, Andrade EO, Mash DC. Pharmacokinetics of Hoasca alkaloids in healthy humans. J Ethnopharmacol. 1999;65:243-256. [PubMed] |
| 9. | Brierley DI, Davidson C. Harmine augments electrically evoked dopamine efflux in the nucleus accumbens shell. J Psychopharmacol. 2013;27:98-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Su TP, Hayashi T, Vaupel DB. When the endogenous hallucinogenic trace amine N,N-dimethyltryptamine meets the sigma-1 receptor. Sci Signal. 2009;2:pe12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Liester MB, Prickett JI. Hypotheses regarding the mechanisms of ayahuasca in the treatment of addictions. J Psychoactive Drugs. 2012;44:200-208. [PubMed] |
| 12. | Brierley DI, Davidson C. Developments in harmine pharmacology--implications for ayahuasca use and drug-dependence treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:263-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Pires AP, De Oliveira CD, Moura S, Dörr FA, Silva WA, Yonamine M. Gas chromatographic analysis of dimethyltryptamine and beta-carboline alkaloids in ayahuasca, an Amazonian psychoactive plant beverage. Phytochem Anal. 2009;20:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Cavalheiro EA, Fernandes MJ, Turski L, Naffah-Mazzacoratti MG. Spontaneous recurrent seizures in rats: amino acid and monoamine determination in the hippocampus. Epilepsia. 1994;35:1-11. [PubMed] |
| 15. | Donzanti BA, Yamamoto BK. An improved and rapid HPLC-EC method for the isocratic separation of amino acid neurotransmitters from brain tissue and microdialysis perfusates. Life Sci. 1988;43:913-922. [PubMed] |
| 16. | Callaway JC, Raymon LP, Hearn WL, McKenna DJ, Grob CS, Brito GS, Mash DC. Quantitation of N,N-dimethyltryptamine and harmala alkaloids in human plasma after oral dosing with ayahuasca. J Anal Toxicol. 1996;20:492-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Rau V, Iyer SV, Oh I, Chandra D, Harrison N, Eger EI, Fanselow MS, Homanics GE, Sonner JM. Gamma-aminobutyric acid type A receptor alpha 4 subunit knockout mice are resistant to the amnestic effect of isoflurane. Anesth Analg. 2009;109:1816-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Doering-Silveira E, Lopez E, Grob CS, de Rios MD, Alonso LK, Tacla C, Shirakawa I, Bertolucci PH, Da Silveira DX. Ayahuasca in adolescence: a neuropsychological assessment. J Psychoactive Drugs. 2005;37:123-128. [PubMed] |
| 19. | Schwarz MJ, Houghton PJ, Rose S, Jenner P, Lees AD. Activities of extract and constituents of Banisteriopsis caapi relevant to parkinsonism. Pharmacol Biochem Behav. 2003;75:627-633. [PubMed] |
| 20. | Schmoldt A, Benthe HF, Haberland G. Digitoxin metabolism by rat liver microsomes. Biochem Pharmacol. 1975;24:1639-1641. |
| 21. | Thomas G, Lucas P, Capler NR, Tupper KW, Martin G. Ayahuasca-assisted therapy for addiction: results from a preliminary observational study in Canada. Curr Drug Abuse Rev. 2013;6:30-42. [PubMed] |
| 22. | Geller JL. Clinical guidelines for the use of involuntary outpatient treatment. Hosp Community Psychiatry. 1990;41:749-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Wang YH, Samoylenko V, Tekwani BL, Khan IA, Miller LS, Chaurasiya ND, Rahman MM, Tripathi LM, Khan SI, Joshi VC. Composition, standardization and chemical profiling of Banisteriopsis caapi, a plant for the treatment of neurodegenerative disorders relevant to Parkinson’s disease. J Ethnopharmacol. 2010;128:662-671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Samoylenko V, Rahman MM, Tekwani BL, Tripathi LM, Wang YH, Khan SI, Khan IA, Miller LS, Joshi VC, Muhammad I. Banisteriopsis caapi, a unique combination of MAO inhibitory and antioxidative constituents for the activities relevant to neurodegenerative disorders and Parkinson’s disease. J Ethnopharmacol. 2010;127:357-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Barbanoj MJ, Riba J, Clos S, Giménez S, Grasa E, Romero S. Daytime Ayahuasca administration modulates REM and slow-wave sleep in healthy volunteers. Psychopharmacology (Berl). 2008;196:315-326. [PubMed] |
| 26. | Dai F, Chen Y, Song Y, Huang L, Zhai D, Dong Y, Lai L, Zhang T, Li D, Pang X. A natural small molecule harmine inhibits angiogenesis and suppresses tumour growth through activation of p53 in endothelial cells. PLoS One. 2012;7:e52162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |