Published online Aug 26, 2013. doi: 10.4331/wjbc.v4.i3.71
Revised: July 3, 2013
Accepted: July 23, 2013
Published online: August 26, 2013
Processing time: 106 Days and 20 Hours
AIM: To test the growth-promoting activity of the polyamine spermidine bound to various polymeric compounds in supramolecular complexes.
METHODS: A thiazolyl blue cell viability assay was used to determine the growth-promoting potency of spermidine-supramolecular complexes in a human skin fibroblast cell line exposed to spermidine and different spermidine-supramolecular complexes that were obtained by combining spermidine and polyanionic polymers or cyclodextrin. Reconstituted human vaginal epithelium was exposed to a specific spermidine-supramolecular complex, i.e., spermidine-hyaluronan (HA) 50, and cell proliferation was determined by Ki-67 immunohistochemical detection. Transepithelial electrical resistance and histological analysis were also performed on reconstituted human vaginal epithelium to assess tissue integrity.
RESULTS: The effect of spermidine and spermidine-supramolecular complexes was first tested in skin fibroblasts. Spermidine displayed a reverse dose-related mode of activity with mmol/L growth inhibition, whereas 30% stimulation over basal levels was detected at μmol/L and nmol/L levels. Novel spermidine-supramolecular complexes that formed between spermidine and polyanionic polymers, such as HA, alginate, and polymaleate, were then tested at variable spermidine concentrations and a fixed polymer level (0.1% w/v). Spermidine-supramolecular complexes stimulated the cell growth rate throughout the entire concentration range with maximal potency (up to 80%) at sub-μmol/L levels. Similar results were obtained with spermidine-(α-cyclodextrin), another type of spermidine-supramolecular complex. Moreover, the increased expression of Ki-67 in the reconstituted human vaginal epithelium exposed to spermidine-HA 50 showed that the mode of action behind the spermidine-supramolecular complexes was increased cell proliferation. Functional and morphological assessments of reconstituted human vaginal epithelium integrity did not show significant alterations after exposure to spermidine-HA, thus supporting its safety.
CONCLUSION: Spermidine found in spermidine-supramolecular complexes displayed potentiated regenerative effects. Safety data on reconstituted human vaginal epithelium suggested that assessing spermidine-supramolecular complex efficacy in atrophic disorders is justified.
Core tip: Previous in vitro studies showed that spermidine may have different, or even opposite, effects on cell survival, leading to either proliferation or apoptosis depending on a variety of factors. We showed that the inclusion of spermidine in supramolecular complexes with various polymers optimized its use for regenerative purposes. Spermidine-supramolecular complexes stimulated cell proliferation but did not cause significant alterations to vaginal tissue integrity. These results suggest that growth pathways in senescent or damaged tissues may be activated by the controlled release of spermidine from spermidine-supramolecular complexes to provide a faster recovery.
-
Citation: Ghisalberti CA, Morisetti A, Bestetti A, Cairo G. Potent trophic activity of spermidine supramolecular complexes in
in vitro models. World J Biol Chem 2013; 4(3): 71-78 - URL: https://www.wjgnet.com/1949-8454/full/v4/i3/71.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v4.i3.71
Spermidine belongs to the class of biogenic, small, polycationic polyamines found ubiquitously in microbial, plant, and animal cells. These compounds interact with negatively charged molecules such as DNA, RNA or proteins and, thus, are involved in a wide array of functions. It has been shown that polyamines play a role in DNA and RNA stabilization, the modulation of mRNA translation, and cellular signaling[1-3]. Their large range of involvement in processes and cellular responses suggests that polyamines play a key role in the control of cell growth, survival, and proliferation.
In humans, spermidine is derived from three sources: cellular synthesis, food intake, and production by the gut microflora. In normal healthy cells, the levels of spermidine and other polyamines are tightly controlled by multiple mechanisms that balance synthesis, catabolism and transport. The first biosynthetic step is the production of L-ornithine from L-arginine by mitochondrial arginase. Ornithine is then decarboxylated by ornithine decarboxylase to produce putrescine, which is then transformed into spermidine by spermidine synthase. Spermidine may be further converted to spermine by spermine synthase[4].
Changes in polyamine levels have been associated with aging and disease, with levels declining continuously with age[5]. However, several lines of evidence have indicated the beneficial effects of polyamines; dietary spermidine supplementation showed protective effects against aging in human cells and laboratory animals. Moreover, epidemiological studies showed that prolonged life expectation was associated with higher polyamine food intake[6-11]. Experimental studies showed that polyamines are essential for eukaryotic cell growth and viability, as they were found to positively promote vital functions such as proliferation, cell reprogramming, autophagy, migration and differentiation[12-15].
However, not all of the data obtained in cultured cells are supportive of a positive role for spermidine on cell growth. For example, the role of polyamines in cell death, particularly apoptosis, is still controversial. In different cellular models, polyamine depletion inhibited mitochondrial and death receptor-dependent apoptosis pathways[16]. Conversely, another study indicated that polyamine-depletion in IEC-6 cells delayed the onset of apoptosis and conferred protection against DNA damaging agents, suggesting an involvement of the caspase activating signal cascade[17]. Examples of either activation or prevention of apoptosis due to polyamine depletion are known for several cell lines[18].
The dual activity of polyamines may depend on the fact that the local concentration of spermidine seems to be a critical factor, as was shown for its effect on microtubule assembly[19]. Another important factor is the working mechanism of polyamines, which seem to perform their different functions through the formation of supramolecular complexes with large anionic molecules such as DNA and RNA, as was shown for lymphocytes, in which it is estimated that 60% of spermidine is bound to RNA[20]. The formation of different spermidine-supramolecular complexes that release sustained, minute amounts of polyamines may influence the concentration, and hence the activity, of spermidine, e.g., by triggering reparative processes on senescent or damaged tissues. In view of a possible therapeutic use of spermidine to restore physiological status in tissues undergoing senescence-dependent alterations, finding optimal conditions (i.e., formation of spermidine-supramolecular complexes) for such use is critical. In particular, it is conceivable that spermidine could restore a healthy status in urogenital tissues and mucosae undergoing senescence, a common condition affecting a large segment of postmenopausal women. To investigate this issue, we tested the effects of spermidine and various spermidine-supramolecular complexes in fibroblasts and in reconstituted human vaginal epithelium.
Spermidine 3HCl (Sigma-Aldrich, Milan Italy); Hyaluronate high molecular weight, sodium salt (Bioiberica, Barcelona, Spain); Oligomeric hyaluronate (Tixupharma, Milan, Italy); Sodium alginate (Santialgine™ S1100, Cargill, United States); co-[methylvinylether/maleic acid]-polymer, sodium salt, (PMVE/MA, Gantrez™ S-97 BF, ISP, United States); α-Cyclodextrin (Cavamax® W6 Pharma, Wacker Chemie AG).
Spermidine-supramolecular complexes with polyanionic polymers were prepared by adding 0.1% w/v of the following polymers to a 1/10 serially diluted solution of spermidine: hyaluronate (HA), oligomeric hyaluronate (OHA), sodium alginate (Alg), and gantrez (Gz). The resulting spermidine-supramolecular complexes are illustrated in Table 1, expressed as the ratio (meq/meq) of cationic equivalents from spermidine and anionic equivalents from the anionic polymer. For the assay on reconstituted human vaginal epithelium tissue, a spermidine-supramolecular complex containing spermidine (5 μmol/L) and HA in a 1:50 meq:meq ratio, hereafter termed spermidine-HA50, was prepared.
| Spermidine concentration | Spermidine-HA | Spermidine-Alg | Spermidine-Gz |
| 0 nmol/L | HA 0.1% | Alg 0.1% | Gz 0.1% |
| 1 nmol/L | SMC 10-6:1 | SMC 10-6:3 | SMC 10-6:9 |
| 10 nmol/L | SMC 10-5:1 | SMC 10-5:3 | SMC 10-5:9 |
| 100 nmol/L | SMC 10-4:1 | SMC 10-4:3 | SMC 10-4:9 |
| 1 μmol/L | SMC 10-3:1 | SMC 10-3:3 | SMC 10-3:9 |
| 10 μmol/L | SMC 10-2:1 | SMC 10-2:3 | SMC 10-2:9 |
| 100 μmol/L | SMC 10-1:1 | SMC 10-1:3 | SMC 10-1:9 |
| 1 mmol/L | SMC 1:1 | SMC 1:3 | SMC 1:9 |
The spermidine-supramolecular complex as an inclusion complex was prepared by dissolving 1.944 g of α-cyclodextrin (aCD) in 15 mL of water; this solution was added to 1 mmol of spermidine 3HCl, and then 0.2 mL of 1 mol/L NaOH was added under slow stirring. This stock solution was serially diluted to reach the required concentrations.
The human skin fibroblast cell line ATCC-CRL-2703 was grown under standard culture conditions in DMEM. Spermidine and the different spermidine-supramolecular complexes were added to wells containing semi-confluent cells. Cells were exposed to test products for 24 and 48 h (medium was replaced after 24 h). At the end of the incubation period, cells were washed with 200 μL of PBS, and cell viability was evaluated using thiazolyl blue (MTT) as an indicator of mitochondrial function. Briefly, 200 μL of MTT-medium was added to each culture well and left for 4 h at 37 °C and 5% CO2. The MTT-medium was then removed, and 200 μL of MTT solvent solution (10% Triton X-100 plus 0.1 mol/L HCl in anhydrous isopropanol) was added. The plate was shaken on a rotary plate for 20-30 min to ensure that all of the formazan crystals were dissolved. Absorbance was measured at 570 nm on a microplate reader, and the background absorbance at 690 nm was subtracted. For each concentration, six replicates were carried out. The results are expressed as percentage (%) cell proliferation compared to an untreated control cell culture.
Reconstituted human vaginal epithelium, obtained from SkinEthic™ (Lyon, France), was derived from the immortalized human cell line A431 grown for 5 d on polycarbonate filters. Reconstituted human vaginal epithelium has a strict morphological similarity to the normal vaginal epithelium. Reconstituted human vaginal epithelia were placed in 6- or 12-well plates containing 0.75-1.00 mL of maintenance medium and incubated at 37 °C in 5% CO2 overnight. The reconstituted human vaginal epithelia (duplicate samples per treatment) were incubated for 24 h at 37 °C with 50 μL of a 1 mg/mL aqueous solution of spermidine-HA 50 and then fixed in 10% formol-saline for immunohistochemical processing. To evaluate the expression of Ki-67, a known marker of cell proliferation, the Ki-67 antibody (Invitrogen, Eugene, OR, United States) was applied overnight at a 1:100 dilution, and detection was obtained using a commercial kit (Superpicture Polymer Detection Kit, Invitrogen) containing horseradish peroxidase and diaminobenzidine (DAB) as chromogenic agents.
Transepithelial electrical resistance, which is the discriminating parameter in the EU-validated rat skin electrical resistance test to measure the integrity of stratum corneum and its barrier function, was evaluated in reconstituted human vaginal epithelia. The higher the transepithelial electrical resistance value, the lower the loss of intercellular water content. Transepithelial electrical resistance of reconstituted human vaginal epithelia was measured in triplicate before and after exposure to spermidine-HA50, prepared as described above, or to physiological saline for 24 h at 37 °C. Briefly, 5 mL of spermidine-HA50 or physiological saline was applied to the tissue sample in a plate containing 5 mL of physiological saline; transepithelial electrical resistance was evaluated in the range of 0-20 kΩ relative to the tissue surface by a Millicell ERS instrument (Millipore, MA, United States). Three measurements were made before and after treatment; the latter measurement was performed after washout of the compound. The value (Ω) of the void support was subtracted from the values obtained for tissues; a further correction was made regarding tissue surface (0.5 cm2) as follows: mean value (Ω) of 3 determinations – value of void support = value (Ω) × 0.5 (tissue surface). At the end of the procedure, the tissue was rinsed and fixed in 10% formol saline, processed through paraffin wax, cut and stained with hematoxylin-eosin for histopathological examination (× 20 magnification, DFC-320 Leica camera). Tissue damage was scored from 0 to 4, in order of increasing severity, by a blinded examiner.
The data were subjected to statistical analysis using a t-test for independent samples. For spermidine and spermidine+cyclodextrin groups, each experimental time point was compared to untreated cells, and the results were considered significant for P < 0.05. For the spermidine + HA, spermidine + OHA, spermidine + Alg and spermidine + Gz groups, each experimental time point was compared to cells treated with the respective vehicle (HA, OHA, Alg, Gz), and the results were considered significant for P < 0.05.
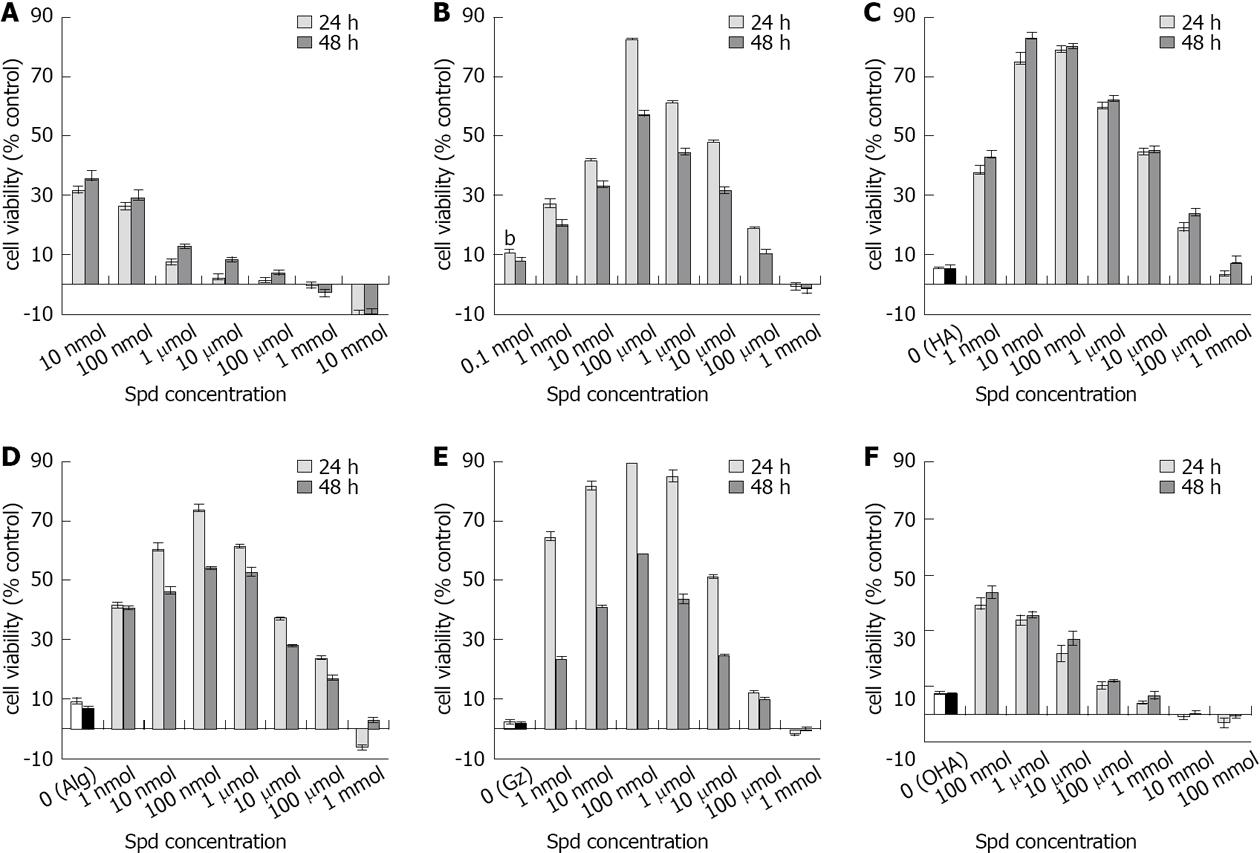
To evaluate the effect of spermidine on cell growth, human fibroblasts were exposed to spermidine either as a free salt or in various spermidine-supramolecular complexes (Table 1). The exposure of fibroblasts to spermidine alone for 24 or 48 h increased the cell number compared to untreated cells by approximately 30% at 10 and 100 nmol/L. However, higher concentrations resulted in the progressive reduction of the stimulatory effect, with the highest doses showing an almost linear, dose-dependent inhibitory effect (Figure 1A).
Conversely, the inclusion of spermidine in spermidine-supramolecular complexes produced a completely different bell-shaped response pattern (Figure 1B-E). Spermidine in complex with α-cyclodextrin (Spermidine-aCD) that was serially diluted to provide concentrations ranging from 1 to 0.1 nmol/L exhibited excellent stimulatory activity, with a peak at 100 nmol/L showing 80% stimulation over untreated cells and a decrease in stimulation at higher concentrations (Figure 1B).
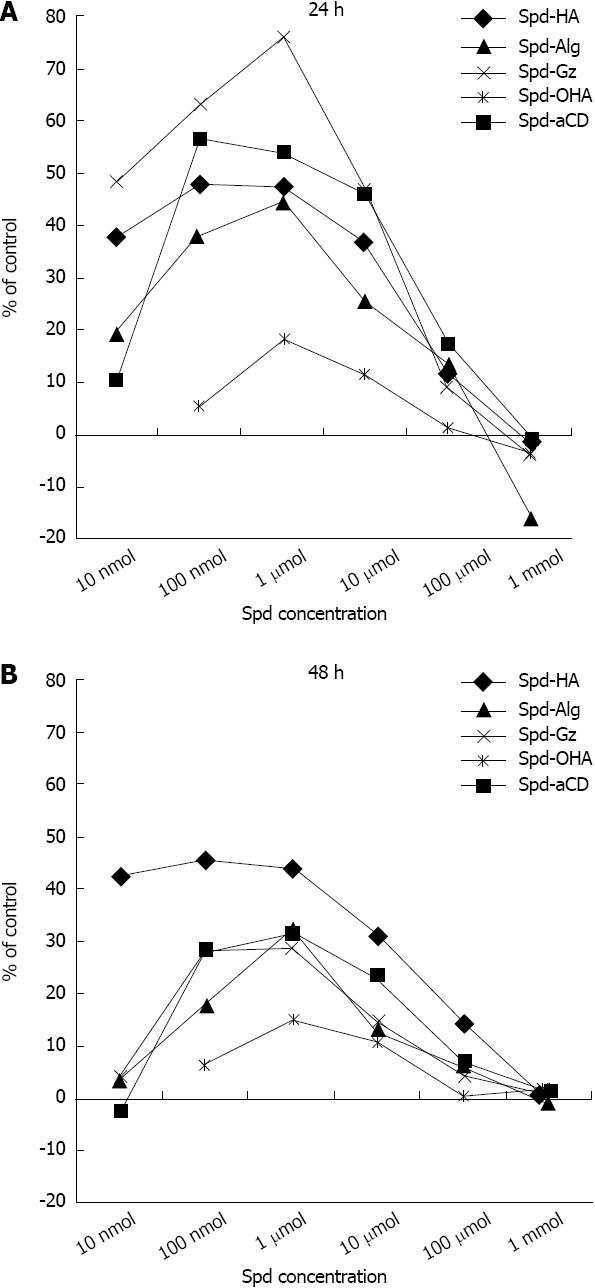
Exposure to spermidine-supramolecular complexes formed with different polyanionic polymers such as HA and Alg also resulted in positive responses. Because Alg and HA themselves may display regenerative properties, the effect of spermidine-supramolecular complexes was compared to that of the polymers alone. Spermidine-HA increased fibroblast cell viability by approximately 80% within the 10-100 nmol/L range (Figure 1C). Spermidine-Alg produced a similar, although flatter, bell-shaped curve (Figure 1D). Maximal activity was observed at 10 to 1 μmol/L. It is noteworthy that Alg alone showed better stimulatory properties than HA, a well-known and widely applied regenerative biopolymer. Spermidine-Gz, a complex between spermidine and a synthetic polymer, also showed a strong effect; interestingly, spermidine-Gz activity at 24 h was roughly two-fold higher than at 48 h (Figure 1E). The rich anionic density of the polymethylvinylether-co-maleic anhydride, polymer that constitutes Gz and an expected slower release of entrapped spermidine may account for the higher effect of spermidine-Gz at 24 h. Importantly, exposure of fibroblasts to OHA, a salt formed by depolymerized HA and spermidine, showed a pattern similar to that obtained with spermidine (compare Figure 1A and F), i.e., greater stimulation than depolymerized HA alone at low concentrations, but no effect at doses above 100 μmol/L. This reinforces the idea that the association of spermidine with polymers in supramolecular complexes provides a better stimulating effect over a wide range of concentrations. To better extrapolate the effect of the association between spermidine and spermidine-supramolecular complexes, the data were processed by subtracting the contribution of both spermidine at corresponding concentrations and the relative polyanion for each spermidine-supramolecular complex. The results reported in Figure 2 illustrate the efficacy profile of the association by itself.
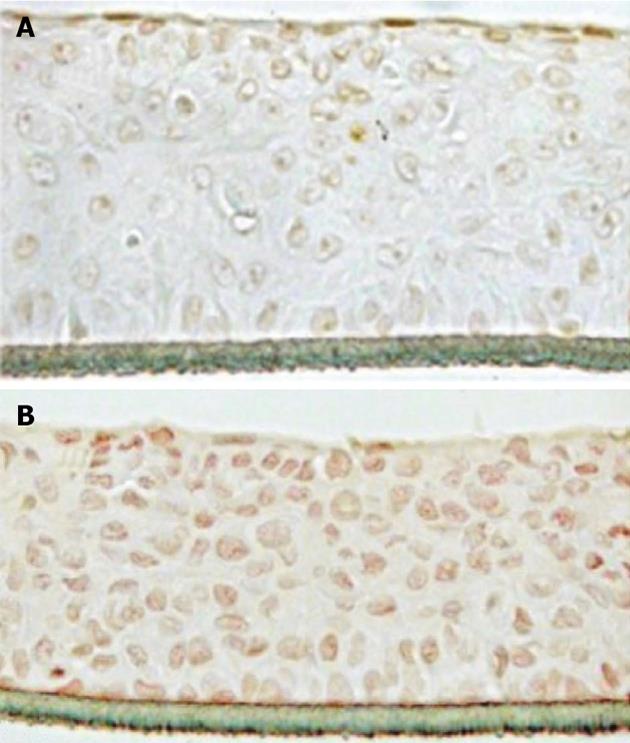
We next investigated the molecular pathways behind the growth stimulating effect of spermidine-supramolecular complexes. To this purpose, the spermidine-HA complex was selected due to its superior balance between regenerative potency and biocompatibility. In fact, spermidine-HA was not inhibitory at any tested concentration (Figure 1C). The spermidine-supramolecular complex formed with a cationic to anionic ratio of 50:1 meq/meq, herein termed spermidine-HA 50, was evaluated in reconstituted human vaginal epithelium by examining the immunohistochemical expression of the mitotic marker Ki-67. In control samples, Ki-67, which is a nuclear protein associated with ribosomal RNA transcription and cell growth and is expressed in the G1, S, G2 and mitosis phases of the cell cycle[21,22], was observed only in a few apical cells (Figure 3A). However, many cells expressing Ki-67 homogeneously distributed from the apical to the basal stratum were found in samples exposed to spermidine-HA 50 (Figure 3B). The tissue had a normal cell morphology and extracellular matrix structure and adhered normally to support.
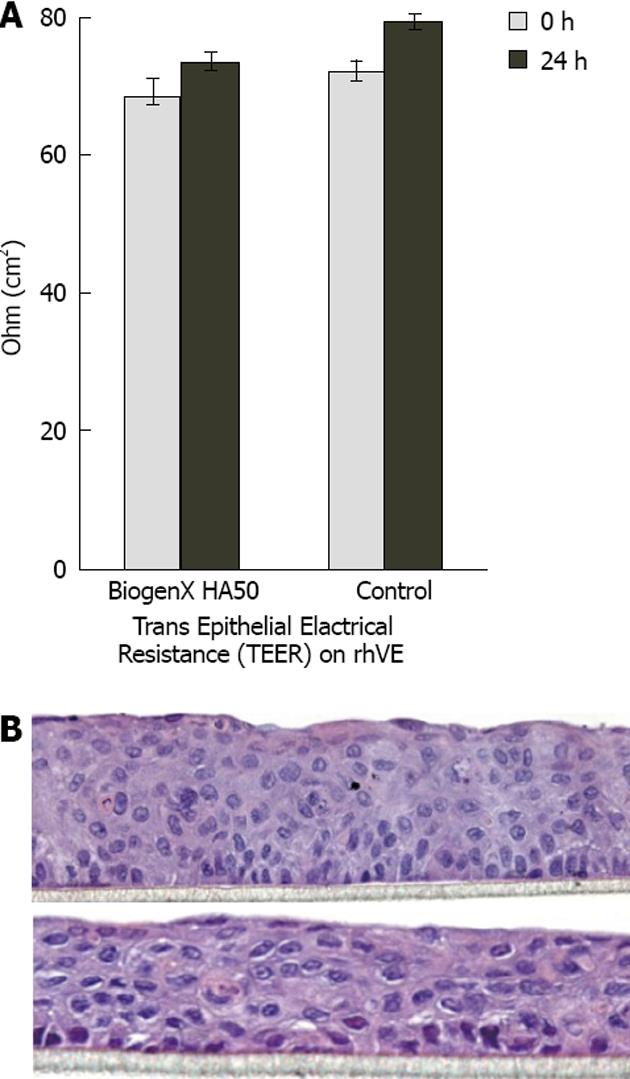
The transepithelial electrical resistance assay, which directly measures total resistivity due to the combined thickness and function of cell tight junctions, evaluates the degree of damage and functionality of tissues. We thus used transepithelial electrical resistance in reconstituted human vaginal epithelium to check for possible damaging effects of spermidine-HA 50. Figure 4A shows that after 24 h of treatment, the resistivity of control samples increased by about 10%, whereas that of the spermidine-supramolecular complex-treated samples increased by 7%. The values fell in the normal range of 60-80 Ω/cm2, and the difference, though not statistically significant, was regarded as a fluctuation without pathological significance. Baseline values accounted for less than 5% of the difference.
Histological examination did not show tissue alterations (Figure 4B). In samples exposed to spermidine-HA 50 for 24 h (upper panel), the tissue condition, cell morphology, adherence to support, appearance of the extracellular matrix, and continuity to the squamous external layer appeared essentially unchanged with respect to reconstituted human vaginal epithelium treated for 24 h with saline (lower panel).
In vivo, polyamine metabolism is tightly regulated by complex machinery involving biosynthesis, interconversion, catabolism, and cellular uptake to reach the appropriate levels of these regulatory factors[23]. Due to their cationic nature, polyamines, including spermidine, strongly bind to DNA, thereby changing its conformation. Moreover, spermidine, by modulating histone acetylase and deacetylase activity, can alter histone acetylation, which in turn will affect gene transcription in proliferating cells[24] that contain the amino acid residue hypusine, which is specifically synthesized from spermidine. Therefore, by modulating gene expression at multiple levels, polyamines (and spermidine in particular) trigger a variety of changes that can potentially lead to complex cellular responses[13,25]. Such a variety of different actions, together with the requirement for appropriate tissue concentrations, may explain how, in experimental studies, polyamines were able to promote both cell growth and cell death and showed complex involvement in various pathophysiological conditions[26]. This finding suggests that the proper concentrations are required to attain a high positive response for regenerative purposes.
In our skin fibroblast model, spermidine showed an expected growth-promoting effect, but polyanionic-type supramolecular complexes containing spermidine were overall much more effective in increasing cell viability, with an effect generally more pronounced at 24 h than at 48 h (Figure 2). Moreover, while the stimulating effect of spermidine alone on cell viability was evident only at low concentrations, time course and dose response studies showed that spermidine-supramolecular complexes promoted cell growth over a wide range of concentrations without inhibitory effects. The trends depicted bell-shaped curves with peaks exceeding an 80% increase over basal levels and showed different profiles (Figure 1). The strongest effect on cell viability was obtained by the association with a typical growth-enhancing biopolymer, such as HA, that resulted in a potent synergistic effect. The cumulated potency of spermidine-HA was 15-fold higher than that of HA alone, and it did not show the bimodal pattern of spermidine alone. Our interpretation of these data suggest that the effect of spermidine-supramolecular complexes might correlate with the kinetics of spermidine release in the culture medium; spermidine-supramolecular complexes may continuously release low amounts of spermidine that simulate the growth-stimulating effect of low doses of spermidine alone (Figure 1A) while avoiding the inhibitory effect of high doses.
Support for this hypothesis is provided by comparing the results obtained with spermidine complexed to polymeric HA to those obtained with a salt formed by spermidine and OHA, i.e., monomers or dimers of glucuronic acid/N-acetylglucosamine disaccharide. OHA showed a response comparable to that of spermidine, further indicating that spermidine needs to be complexed with either natural or synthetic polymers in spermidine-supramolecular complexes to effectively promote cell growth.
Further confirmation that the inclusion of spermidine in spermidine-supramolecular complexes may be a key factor in optimizing the growth-promoting properties of spermidine was provided by the results obtained with spermidine-aCD, i.e., a spermidine-supramolecular complex based on an uncharged type of inclusion complex. When serially diluted over a concentration range spanning from 1 to 0.1 nmol/L, these complexes also yielded excellent results that were comparable to those of the spermidine-supramolecular complexes formed with the polyanionic polymers.
The data obtained in cultured cells were supported by experiments performed in reconstituted human vaginal epithelium that was used as a validated model of human vaginal mucosa[27-30]. To explore the mechanism of action of spermidine-supramolecular complexes, reconstituted human vaginal epithelium was exposed to spermidine-HA, the complex of spermidine with HA that showed the best stimulation in fibroblasts. Upon treatment, the expression of the Ki-67 antigen, which is a nuclear protein associated with cell growth expressed in proliferating cells but absent in quiescent cells[21,22], was detected in many cells from the apical layer to the basal cells. Therefore, these results indicate that spermidine-supramolecular complexes provide a steady, mitogenic amount of spermidine (in the nmol/L range) released, thus eliciting a proliferative effect on epithelial cells and sub-seeding fibroblasts. These data indicated that spermidine-supramolecular complexes are effective in triggering regeneration by inducing cell proliferation, although other effects relying on the inhibition of cell death cannot be ruled out. Our results are in agreement with literature data showing that polyamines are essential factors in tissue remodeling[3,6,31-34].
Finally, because these results suggest that spermidine-supramolecular complexes may be used in restoring a healthy tissue condition in various age-related disorders characterized by a compromised trophic status of mucosae, e.g., in urogenital pathology, safety evidence is required. Transepithelial electrical resistance experiments using reconstituted human vaginal epithelium, which were performed to assess functional and morphological characteristics of the vaginal epithelium after exposure to spermidine-supramolecular complexes, showed only minor alterations after 24 h exposure, indicating that the spermidine-supramolecular complex tolerability is satisfactory.
In conclusion, our in vitro results indicate that spermidine-supramolecular complexes may elicit soft-tissue remodeling, and the complex potency seems to be linked to a very low, steady flux of spermidine. The broad range of stimulating activity that spanned 6 logs of concentration seems to indicate the presence of a non-receptorial mechanism leading to cell proliferation.
Spermidine is a master regulator of the cell cycle with a key role in tissue homeostasis, reparation and proliferation. In the cell, spermidine exerts its effects by interacting with polyanionic polymers and chlatrating moieties to form supramolecular complexes. However, its multifarious functions may lead to mitotic activity or to an opposite, pro-apoptotic pathway. In this respect, the local spermidine concentration seems to be the driving force, which could be modulated in pursuit of regenerative therapies.
Senescence conditions in women, such as vaginal atrophy and stress urinary incontinence, are a largely unmet medical need occurring in post-menopausal and elderly women or after pregnancy and labor. Both require an effective, more appropriate, and less hazardous therapy than estrogenic treatment. Biogenic polyamines play a key role in tissue homeostasis, but due to their bimodal effects on cell viability and tissue growth, it is of the utmost importance to find appropriate conditions for attaining a positive and strong response for regenerative purposes.
Innovation entails demonstrating that the inclusion of spermidine in supramolecular complexes optimizes its trophic activity. Safety and efficacy evidence supports the assessment of the regenerative properties of spermidine-supramolecular complexes in damaged or senescent tissues.
These results represent a promising basis for in vivo investigations into whether spermidine-supramolecular complexes are able to restore a healthy condition in disorders characterized by the overall senescence or damage of soft tissues or in age-related diseases in general. Women’s urogenital diseases, such as vaginal atrophy and stress urinary incontinence, may represent potential applications.
Spermidine belongs to the family of biogenic amines, i.e., polyamines, which include its metabolic precursor putrescine and the higher homolog spermine. Polyamines are organic polycations that interact with polyanionic polymers by multiple acid-base interactions. The minimal steric hindrance and the low molecular weight of polyamines also allow for the formation of inclusion complexes with cyclodextrins. Authors studied both types of supramolecular complexes at broad concentration ranges in vitro. The thiazolyl blue assay measured the effect of the tested substances on cell viability as a function of mitochondrial function. Hence, the expressions “growth promoting activity”, “proliferative effects”, “cell proliferation”, “stimulatory activity”, “stimulatory effect”, and “regenerative properties”, among others, are used synonymously, i.e., to encompass different possibilities of increased cell viability, including proliferation through mitosis, reduced apoptosis and prolonged life-span by autophagy.
This is an interesting study in which the authors analyzed the effect of various spermidine-supramolecular complexes on fibroblast and vaginal epithelium proliferation. The results are sound and suggest that spermidine-supramolecular complexes are potential therapeutic substances that could be used for treating tissue senescence associated with urogenital pathological conditions.
P- Reviewer Ariga K S- Editor Gou SX L- Editor A E- Editor Yan JL
| 1. | Pegg AE. Mammalian polyamine metabolism and function. IUBMB Life. 2009;61:880-894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 630] [Cited by in RCA: 590] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 2. | Perez-Leal O, Merali S. Regulation of polyamine metabolism by translational control. Amino Acids. 2012;42:611-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Oredsson SM. Polyamine dependence of normal cell-cycle progression. Biochem Soc Trans. 2003;31:366-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 114] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Mitchell JLA. Regulation of polyamine metabolism. Luxemburg: Office for Official Publications of the European Communities 2003; 89-100. |
| 5. | Minois N, Carmona-Gutierrez D, Madeo F. Polyamines in aging and disease. Aging (Albany NY). 2011;3:716-732. [PubMed] |
| 6. | Nishimura K, Shiina R, Kashiwagi K, Igarashi K. Decrease in polyamines with aging and their ingestion from food and drink. J Biochem. 2006;139:81-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 205] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 7. | Soda K, Dobashi Y, Kano Y, Tsujinaka S, Konishi F. Polyamine-rich food decreases age-associated pathology and mortality in aged mice. Exp Gerontol. 2009;44:727-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 8. | Eisenberg T, Knauer H, Schauer A, Büttner S, Ruckenstuhl C, Carmona-Gutierrez D, Ring J, Schroeder S, Magnes C, Antonacci L. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11:1305-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 1221] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 9. | Deignan JL, Livesay JC, Shantz LM, Pegg AE, O’Brien WE, Iyer RK, Cederbaum SD, Grody WW. Polyamine homeostasis in arginase knockout mice. Am J Physiol Cell Physiol. 2007;293:C1296-C1301. [PubMed] |
| 10. | Binh PNTSK, Maruyama C. Relationship between food polyamines and gross domestic product in association with longevity in Asian countries. Health. 2010;2:1390-1396. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Kaeberlein M. Spermidine surprise for a long life. Nat Cell Biol. 2009;11:1277-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Johnson DA, Fields C, Fallon A, Fitzgerald ME, Viar MJ, Johnson LR. Polyamine-dependent migration of retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2002;43:1228-1233. [PubMed] |
| 13. | Takeuchi O, Matsushita K, Akira S. [Control of inflammatory responses by a novel RNase, Zc3h12a]. Tanpakushitsu Kakusan Koso. 2009;54:1837-1841. [PubMed] |
| 14. | Vuohelainen S, Pirinen E, Cerrada-Gimenez M, Keinänen TA, Uimari A, Pietilä M, Khomutov AR, Jänne J, Alhonen L. Spermidine is indispensable in differentiation of 3T3-L1 fibroblasts to adipocytes. J Cell Mol Med. 2010;14:1683-1692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Kucharzewska P, Welch JE, Svensson KJ, Belting M. The polyamines regulate endothelial cell survival during hypoxic stress through PI3K/AKT and MCL-1. Biochem Biophys Res Commun. 2009;380:413-418. [PubMed] |
| 16. | Flamigni F, Stanic’ I, Facchini A, Cetrullo S, Tantini B, Borzì RM, Guarnieri C, Caldarera CM. Polyamine biosynthesis as a target to inhibit apoptosis of non-tumoral cells. Amino Acids. 2007;33:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Igarashi K, Kashiwagi K. Characteristics of cellular polyamine transport in prokaryotes and eukaryotes. Plant Physiol Biochem. 2010;48:506-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 18. | Seiler N, Raul F. Polyamines and apoptosis. J Cell Mol Med. 2005;9:623-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 229] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 19. | Mechulam A, Chernov KG, Mucher E, Hamon L, Curmi PA, Pastré D. Polyamine sharing between tubulin dimers favours microtubule nucleation and elongation via facilitated diffusion. PLoS Comput Biol. 2009;5:e1000255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Igarashi K, Kashiwagi K. Modulation of cellular function by polyamines. Int J Biochem Cell Biol. 2010;42:39-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 634] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 21. | Schonk DM, Kuijpers HJ, van Drunen E, van Dalen CH, Geurts van Kessel AH, Verheijen R, Ramaekers FC. Assignment of the gene(s) involved in the expression of the proliferation-related Ki-67 antigen to human chromosome 10. Hum Genet. 1989;83:297-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Bullwinkel J, Baron-Lühr B, Lüdemann A, Wohlenberg C, Gerdes J, Scholzen T. Ki-67 protein is associated with ribosomal RNA transcription in quiescent and proliferating cells. J Cell Physiol. 2006;206:624-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 286] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 23. | Alm K, Oredsson S. Cells and polyamines do it cyclically. Essays Biochem. 2009;46:63-76. [PubMed] |
| 24. | Landau G, Bercovich Z, Park MH, Kahana C. The role of polyamines in supporting growth of mammalian cells is mediated through their requirement for translation initiation and elongation. J Biol Chem. 2010;285:12474-12481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 25. | Stabellini G, Moscheni C, Gagliano N, Dellavia C, Calastrini C, Ferioli ME, Gioia M. Depletion of polyamines and increase of transforming growth factor-beta1, c-myc, collagen-type I, matrix metalloproteinase-1, and metalloproteinase-2 mRNA in primary human gingival fibroblasts. J Periodontol. 2005;76:443-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Moinard C, Cynober L, de Bandt JP. Polyamines: metabolism and implications in human diseases. Clin Nutr. 2005;24:184-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 317] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 27. | Moinard C; ECVAM. ECVAM Scientific Advisory Committee (ESAC) statement. Statement on the scientific validity of in-vitro tests for skin irritation testing. In: ESAC 29th Meeting; 2008; November 4-5. |
| 28. | Kandárová H, Liebsch M, Schmidt E, Genschow E, Traue D, Spielmann H, Meyer K, Steinhoff C, Tornier C, De Wever B. Altern Lab Anim. 2006;34:393-406. [PubMed] |
| 29. | Costin GE, Raabe HA, Priston R, Evans E, Curren RD. Vaginal irritation models: the current status of available alternative and in vitro tests. Altern Lab Anim. 2011;39:317-377. [PubMed] |
| 30. | Meloni M, Dalla Valle P, Cappadoro M, de Wever B. The importance of Multiple endpoint analysis (MEA) using reconstituted human tissue models for irritation and biocompatibility assays. Italy: Formia 2002; 4-7. |
| 31. | Madeo F, Eisenberg T, Büttner S, Ruckenstuhl C, Kroemer G. Spermidine: a novel autophagy inducer and longevity elixir. Autophagy. 2010;6:160-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 32. | Bachrach U. The early history of polyamine research. Plant Physiol Biochem. 2010;48:490–495. [PubMed] |
| 33. | Hussain SS, Ali M, Ahmad M, Siddique KH. Polyamines: natural and engineered abiotic and biotic stress tolerance in plants. Biotechnol Adv. 2011;29:300-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 248] [Article Influence: 17.7] [Reference Citation Analysis (0)] |