Published online Aug 26, 2013. doi: 10.4331/wjbc.v4.i3.35
Revised: June 11, 2013
Accepted: June 18, 2013
Published online: August 26, 2013
Processing time: 107 Days and 18.3 Hours
The intrinsic physical properties of the noble metal nanoparticles, which are highly sensitive to the nature of their local molecular environment, make such systems ideal for the detection of molecular recognition events. The current review describes the state of the art concerning molecular recognition of Noble metal nanoparticles. In the first part the preparation of such nanoparticles is discussed along with methods of capping and stabilization. A brief discussion of the three common methods of functionalization: Electrostatic adsorption; Chemisorption; Affinity-based coordination is given. In the second section a discussion of the optical and electrical properties of nanoparticles is given to aid the reader in understanding the use of such properties in molecular recognition. In the main section the various types of capping agents for molecular recognition; nucleic acid coatings, protein coatings and molecules from the family of supramolecular chemistry are described along with their numerous applications. Emphasis for the nucleic acids is on complementary oligonucleotide and aptamer recognition. For the proteins the recognition properties of antibodies form the core of the section. With respect to the supramolecular systems the cyclodextrins, calix[n]arenes, dendrimers, crown ethers and the cucurbitales are treated in depth. Finally a short section deals with the possible toxicity of the nanoparticles, a concern in public health.
Core tip: The article is an in-depth review of the state of the art of molecular recognition processes involving hybrid nanoparticles and bio-molecular substrates. We describe the methods of preparation and physical characterization. The capping by proteins, DNA, peptides and supramolecular assemblies, including cyclodextrins, calix[n]arenes, cucurbitales, dendrimers and crown ethers is then discussed. There is a large analysis of the interactions of these systems with various substrates, such as complimentary oligo-nucleotides, antibodies, active pharmaceutical ingredients and polluants. Finally we discuss the problem of possible toxicity.
- Citation: Tauran Y, Brioude A, Coleman AW, Rhimi M, Kim B. Molecular recognition by gold, silver and copper nanoparticles. World J Biol Chem 2013; 4(3): 35-63
- URL: https://www.wjgnet.com/1949-8454/full/v4/i3/35.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v4.i3.35
Nanoparticle (NP) science and technology is considered to be a quintessimal aspect of 20th and 21st century science and research, however the use of Noble metal nanoparticles dates back to at least the Roman epoch[1]. At that time colloidal suspensions of gold were used to stain glass. Somewhat later, in ninth century Mesopotamia copper and silver nanoparticles were used to introduce a metallic luster into pottery glazes[2]. In parallel, in India noble metal nanoparticles were first applied in medicine. During the sixteenth and seventeenth centuries Cassius and Kunckel refined the process of glass staining although still without a fundamental understanding of the subject[3]. Similarly, Herschel developed a photographic process using colloidal gold[4]. It was in 1857 that Faraday first characterized the optical properties of nanoparticles[5]. Subsequent work has shown that the differing colours observed for various forms of noble metal nanoparticles are related to the particle size[6]. With the understanding of plasmon physics, a clear understanding of the behavior of nanoparticles has emerged[7].
In fact, nanoparticles are based on small well defined aggregates of the Noble metals in the zero valent state. The preparation of Noble metal nanoparticles is generally based on a wet chemical reduction of a suitable metal salt in the presence of a capping or stabilizing agent to prevent both aggregation and oxidation away from the reduced state[8]. The size and more importantly the shape of the nanoparticles can be controlled by the reducing agent, the capping agent and the reaction conditions used in the preparation[9]. While spherical forms are most commonly prepared, rod-like shapes, cubes, hexagonal and even hollow forms are known[10].
In this review we will concentrate on how the introduction of various capping agents allows the introduction of molecular recognition properties to the surface of the noble metal nanoparticles. The choice of the capping agent has opened up applications in biomedical science[11], antibacterial systems[12], drug carriers[13] and as sensing elements[14]. The last application, sensing via molecular recognition is greatly facilitated by the sensitivity of the wavelength and intensity of the Plasmon Resonance peak to the nature of the local environment around the nanoparticles[15] and also to the aggregation state of the colloidal system[16]. In a final section we will deal with some of the health concerns related to the use of such nanoparticles[17].
Numerous techniques have been developed to synthesize Noble metal nanoparticles, including both chemical methods (e.g., chemical reduction, photochemical reduction, co-precipitation, thermal decomposition, hydrolysis, etc.) and physical methods (e.g., vapor deposition, laser ablation, grinding, etc.) The ultimate goal is to obtain nanoparticles with a high level of homogeneity and provide fine control over size, shape and surface properties[18].
Table 1 presents the different strategies employed in the literature for preparing and functionalizing metallic nanoparticles.
| Metal | Molecule | Type of conjugation | Nanoparticle preparation | Ref |
| Gold | Oligonucleotide | Thiol labelled oligo | Citrate reduction | [35,41] |
| Gold | Aptamer | Thiol labelled oligo | Citrate reduction | [54,56] |
| Gold | Antisera | Electrostatic attraction | Reduction | [20] |
| Gold | Antibody : anti-epidermal growth factor receptor | Electrostatic attraction | Citrate reduction | [89] |
| Gold | Monoclonal antibody LCC (ALT04) | Electrostatic attraction | Citrate reduction | [204] |
| Gold | Antibody: antigoat, antimouse, anti-sheep | Covalent bond (protein/MUA SAM) | Seed-mediated growth | [91,22] |
| Gold | Avidin | Succinimyl labelled avidin | Frens-Turkevich method | [71] |
| Gold | Peptide | Colavelent bonds (peptide-NH2/COOH-PEG-NP) | seed-mediated growth and coated with tlyolated-PEG-COOH | [75] |
| Gold | Cyclodextrine | Thiol labelled cyclodextrin | NaBH4 reduction | [119,121] |
| Gold | Cyclodextrine | Hydrophobic | Laser-induced ablation | [205] |
| Gold | Calix[n]arene | Thiol labelled calix[n]arene | NaBH4 reduction | [136] |
| Gold | Calix[n]arene | Thiol labelled calix[n]arene | Citrate reduction | [135] |
| Gold | Calix[n]arene | Chemisorbtion (sulphonate/gold) | NaBH4 reduction | [138] |
| Gold | Dendron | Thiol labelled dendron | NaBH4 reduction | [160] |
| Gold | Dendron | Thiol labelled dendron | Thiol-Ligand Substitution | [161] |
| Gold | Dendrimer | Electrostatic attraction | Acetic Acid reduction | [158] |
| Gold | Crown Ether | Thiol labelled crown ether | NaBH4 reduction | [206] |
| Gold | Crown Ether | Thiol labelled crown ether | Citrate reduction | [178] |
| Silver | Oligonucleotide | Thiol labelled oligo | NaBH4 reduction | [39] |
| Silver | Oligonucleotide | Physical adsorption | Photo-Induced | [48] |
| Silver | Aptamer | Thiol labelled oligo | NaBH4 reduction | [159] |
| Silver | Antibody: IgG | Electrostatic attraction | Citrate reduction | [207] |
| Silver | Antibody: anti-ngn1 | Electrostatic attraction | Citrate reduction and coated with DL-mercaptosuccinic acid (MSA) | [101] |
| Silver | Norvancomycin | Covalent bond (peptide-NH2/COOH-MA-NP) | NaBH4 reduction stabilized with Mercaptoacetic Acid (MA) | [82] |
| Silver | Glutheraldehyde | Electrostatic attraction | NaBH4 reduction | [104] |
| Silver | Peptide | Electrostatic attraction | Ascorbate sodium reduction | [76] |
| Silver | Peptide | Electrostatic attraction | trisodium citrate and hydroxylamine hydrochloride reduction | [77] |
| Silver | Peptide | Electrostatic attraction | NaOH added to silver nitrate and peptid | |
| Silver | Cyclodextrin | Host-guest by Electrostatic interaction | NaBH4 reduction stabilized with Cetyl Trimethyl Ammonium (CTA) | [208] |
| Silver | Cyclodextrin | Thiol labelled cyclodextrin | NaBH4 reduction | [127] |
| Silver | Cyclodextrin | Thiol labelled cyclodextrin | Citrate reduction | [126] |
| Silver | Cyclodextrin | Electrostatic attraction | NaBH4 reduction | [124] |
| Silver | Calix[n]arene | Chemisorbtion (sulphonate/silver) | NaBH4 reduction | [145,147] |
| Silver | Calix[n]arene | Electrostatic attraction | Hydrogen gaz reduction | [143] |
| Silver | Calix[n]arene | Electrostatic attraction | Photo-chemical reduction | [144] |
| Silver | Dendrimer | Electrostatic attraction | UV reduction | [164,168] |
| Silver | Dendrimer | Electrostatic attraction | NaBH4 reduction | [166] |
| Silver | Crown Ether | Thiol labelled crown ether | NaBH4 reduction | [177] |
| Silver | Cucurbit[n]uril | Chemisorbtion | NaBH4 reduction | [179] |
| Silver | Cucurbit[n]uril | Chemisorbtion | NaOH induced | [180] |
| Copper | Oligonucleotide | Electrostatic attraction | Chemical reduction (ascorbic acid) | [53] |
| Copper | Antibody | Electrostatic attraction | Pyrometallurigically by heating copper metal (Sigma, China) | [109] |
| Copper | Peptide | Electrostatic attraction | NADH reduction by fungus F. oxysporum | [84] |
| Copper | Peptide/latex | Electrostatic attraction | Ascorbate sodium reduction | [83] |
| Copper | Cyclodextrin | Electrostatic attraction | ultrasound irradiation | [128] |
| Copper | Cyclodextrin | Electrostatic attraction | calcination | [129] |
| Copper | Calix[n]arene | Chemisorbtion (sulphonate/copper) | hydrazine reduction | [151] |
| Copper | Dendrimer | Electrostatic attraction | Ascorbic acid reduction | [171] |
| Copper | Dendrimer | Electrostatic attraction | Electrochemical reduction | [170] |
Molecular functionalization on inorganic supports has been made through a variety of techniques that includes physical adsorption, electrostatic binding, specific recognition, and covalent coupling. Recently, these immobilization techniques have been applied to bring together biomolecules and nanoparticles. Willener has reviewed[19] these methods of functionalization and has identified three types: (1) Electrostatic adsorption; (2) Chemisorption; and (3) Affinity-based methods.
The electrostatic adsorption method involves the adsorption of positively charged molecules on nanoparticles that are stabilized by anionic ligands such as carboxylic acid derivatives (citrate, ascorbate). Protein and in particular, antibodies have been used in this way, to functionalize nanoparticles since the work of Faulk and Taylor in 1971[20].
Chemisorption involves capping nanoparticles using the affinity of Noble metals for thiol-containing molecules or by covalent binding through bifunctional linkers. Nucleic acids can be prepared with pendant thiol groups using solid-phase synthesis, thus facilitating their attachment to the metal nanoparticle[21]. Otherwise, an anchor group can be used for covalent binding through bifunctional linkers. Such functionalization can be divided into a two-step process: in 1, the activation step, a chemical anchor layer is formed on the nanoparticle surface to provide active functional groups to which biological molecules (i.e., antibodies) can be covalently attached; and in 2, the functionalization step, biomolecules are covalently linked to the anchor layer to yield systems for molecular recognition[22]. The affinity based method is defined as the functionalization of nanoparticles with groups that provide affinity sites for the binding of biomolecules, and has been used for the specific attachment of proteins and oligonucleotides. For example, streptavidin-functionalized gold nanoparticles have been used for the affinity binding of biotinylated proteins (e.g., immunoglobulins and serum albumins) or biotinylated oligonucleotides[23]. Doria summarized the advantages and disadvantages of the three methods: the advantage of electrostatic adsorption is the ease of usage while chemisorption and affinity-based functionalisations are robust and allow an orientation of the capped molecules. The disadvantages of electrostatic adsorption include sensitivity to the external environment (pH, ionic strength) and the restricted choice of charged molecules for capping. Also, chemisorption and affinity-based functionalization usually require the modification of the capped molecules[24].
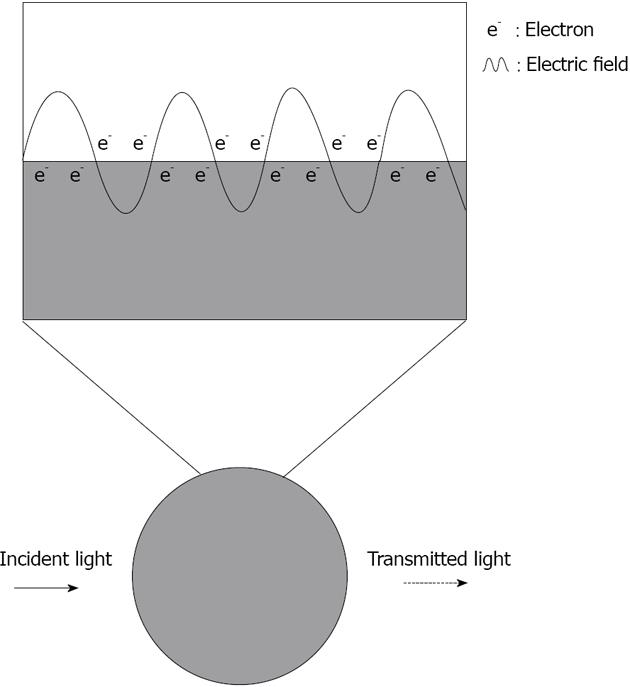
The optical attributes of metal nanoparticles, as reflected in their bright intense colors, are due to their unique interaction with light. In the presence of the oscillating electromagnetic field of light, the free electrons of the metal nanoparticle undergo a collective coherent oscillation (Figure 1). This motion is resonant at a particular light frequency and is termed the localized surface plasmon resonance (LSPR) oscillation[25]. The surface plasmon oscillation either decays by radiating its energy, resulting in light scattering, or decays non-radiatively as a result of conversion of absorbed light to heat. The electric field intensity and the scattering and absorption characteristics of the nanoparticles are all strongly enhanced at the LSPR frequency, which for gold, silver and copper lies in the visible region[26].
The plasmonic resonance of metallic nanoparticles will depend on several parameters: (1) the size of the nanoparticles; (2) the geometry of the nanoparticles (spherical, triangle, rods, etc.); (3) the physical properties of the medium in which the nanoparticles are dispersed (air, liquid, solid); and (4) the nature of the metallic nanoparticles.
In water, the absorption of spherical nanoparticles, with sizes ranging from 3 nm to 80 nm and composed of copper (Cu), silver (Ag) or gold (Au), lies in the visible range and gives rise to a narrow peak[27], respectively at 400, 520 and 570 nm. The metal plasmon absorption frequency for copper, silver and gold nanoparticles were 500-550 nm, 390-400 nm, 565-570 nm respectively.
The electrical properties of metal particles which have a size greater than 2 nm diameter, are similar to those of the corresponding bulk metals[28]. Electron transport is not confined to the discreet energy levels of several atoms but appears as a continuum energy level of a bulk metal. Hence, surface charging and electron transport processes in metal nanoparticles may be understood with relatively simple classical physical expressions, as for resistance/capacitor electronic circuit diagrams. In contrast to molecules and semiconductor nanoparticles whose electron transport properties require a quantum mechanical description, metal nanoparticles only require knowledge of their size and the dielectric properties of the surrounding medium to determine their properties[18]. The electronic properties of metallic nanoparticles have been used for many applications, such as electrical sensors using metal nanoparticle as a tag for recognizing a specific target molecule[29], and the development of new electronic chips[30].
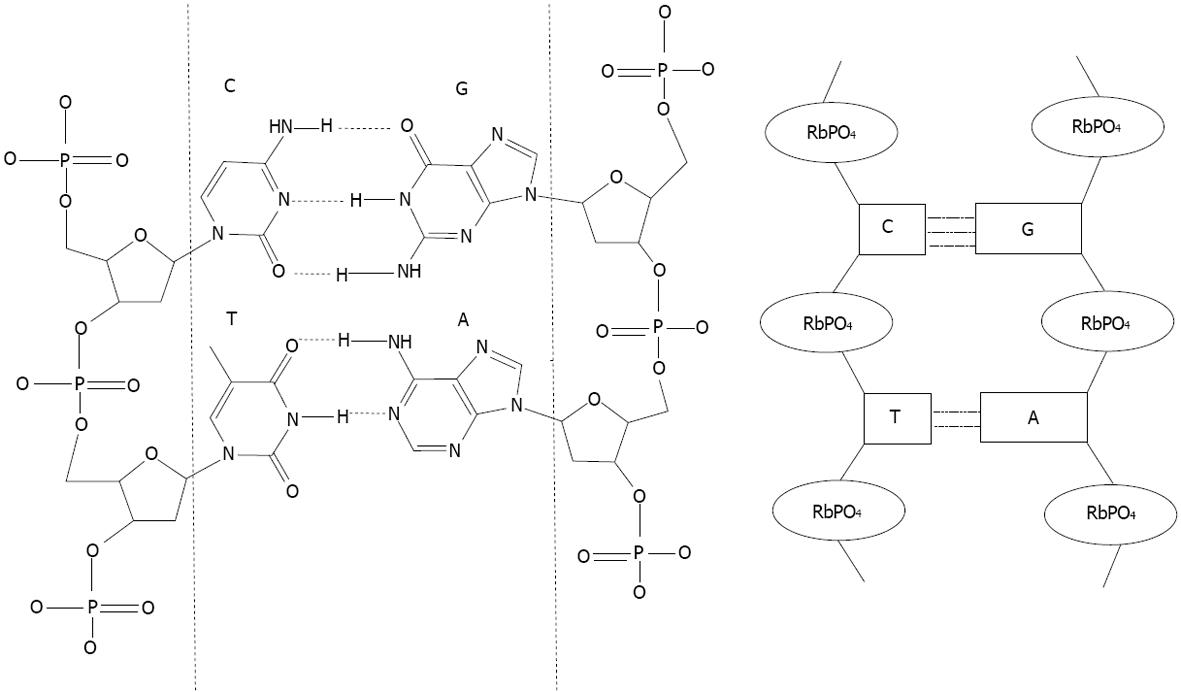
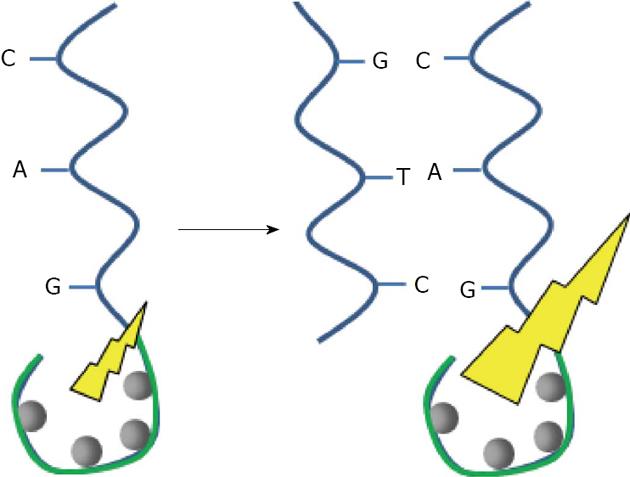
After proteins, the nucleic acids are the most studied biomolecules for capping Noble metal nanoparticles. Many reviews regarding gold nanoparticles, can be found in the literature[31-34]. Such hybrid systems have been extensively investigated since Mirkin, first used oligonucleotides as a capping agent to provide a basis for recognition[35]. Nucleic acid molecules consist of a sequence of nucleotides distinguished by which nucleobase they contain. In DNA, the four bases present are adenine (A), cytosine (C), guanine (G), and thymine (T), whereas RNA contains adenine (A), cytosine (C), guanine (G), and uracil (U). Nucleic acids have the property that two strands will only bind to each other to form a double helix if the two sequences are complementary, with A only binding to T in DNA A binds to U in RNA, and C only to G, linked by hydrogen bonds (Figure 2).
Moreover, the ability of nucleic acids to self assemble extends their molecular recognition properties from complementary sequences to various molecular targets such as small molecules, proteins, and even cells, tissues and organisms[36]. Aptamers (Apt), oligo-nucleic acids engineered to bind a specific ligand, have shown considerable potential to be used as a capping agent for nanoparticles[21].
Oligonucleotides: As noted above, oligonucleotides bind, in a sequence-specific manner, to their respective complementary oligonucleotides, DNA, or RNA to form duplexes or, less often, hybrids of a higher order. This basic property serves as a foundation for the use of oligonucleotides as probes for detecting DNA or RNA. Many applications can be found in biology such as Polymerase Chain Reaction (PCR), DNA microarrays, Southern blots, fluorescent in situ hybridization (FISH)[37]. As an example, DNA microarrays use thousands of different oligonucleotides probes in order to measure the expression levels of large numbers of genes simultaneously or to genotype multiple regions of a genome. The fundamental idea behind most microarrays is to exploit complementary base pairing of the oligonucleotide probes to measure the amount of the different types of mRNA molecules in a cell, thus indirectly measuring the expression levels of the genes that are responsible for the synthesis of those particular mRNA molecules[38].
Gold: Oligonucleotide Gold Nanoparticle (OGN) conjugates are powerful tools for the detection of target DNA sequences by the complementary assemblage of double stranded DNA. Practically all the research and applications of these conjugates have used gold nanoparticles to the exclusion of other Noble metal nanoparticles[39]. Initially, Mirkin demonstrated the colorimetric detection of hybrid gold nanoparticles[35]. Subsequently Mirkin used non-complementary thiolated oligonucleotide probes attached by chemisorption on 13 nm gold nanoparticles[40], addition of DNA containing the complementary sequence for both oligonucleotide probes led to aggregation of the nanoparticles.
Subsequent biological applications led to the development of an oligonucleotide gold nanoparticle set for the detection of mutation of a polynucleotide sequence[41]. With the development of DNA arrays, oligonucleotide capped gold nanoparticles have been shown to be alternative markers to classical fluorophores, bringing very high sensitivity (50 fM of targeted DNA)[42].
Sun et al[43] demonstrated the ability to use such a hybrid system for multiple DNA sequence detection by the surface-enhanced raman scattering (SERS) technique. The assembly of nanoparticles provoked by molecule junctions, enhances strongly the Raman scattering[44]. This has been used for detecting and identifying each set of oligonucleotide capped gold nanoparticles which hybridize with the unknown DNA.
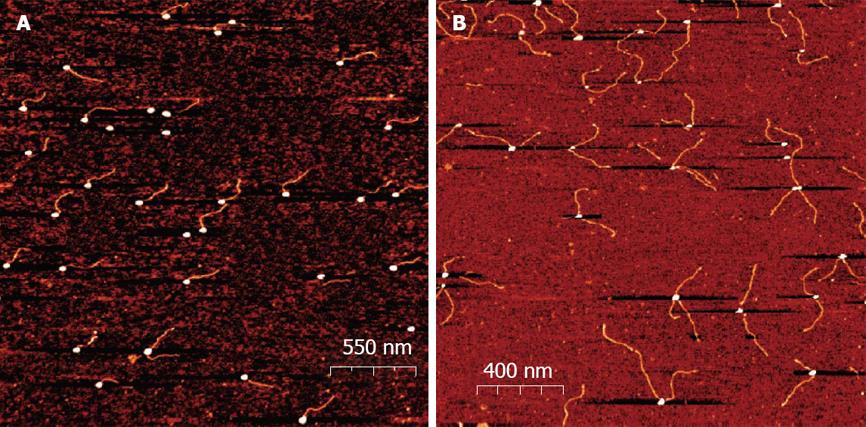
The emergence of DNA origami, first described by Rothemund[45], offered new applications of nucleic acid capped gold nanoparticles. In electronic and plasmonic applications, the self assembly properties of DNA can be associated with metallic nanoparticles to construct a variety of metallized and nanostructured shapes. Borokov has developed a method for controlling a specific number of short (25 base) ssDNA molecules[46]. This method allows variation in the size of nanoparticles, the distance between them (by changing the length of a DNA linker), and the number of connections that each particle establishes (Figure 3). Such work opens up horizons for nucleic acid capped gold nanoparticles to be used for developing new functional materials.
Silver: In 2008 Thompson reported the synthesis of oligonucleotide silver nanoparticle conjugates and demonstrated their use in a sandwich assay format[39] (Figure 4). These conjugates have practically identical properties to their gold analogues and due to their greater extinction coefficient, absorption analyses can occur at much lower concentrations.
Li et al[47] showed that oligonucleotide silver nanoparticle conjugates could be developed for multiplexed DNA electrochemical detection. Although the emergence of DNA chip technology has accelerated this process, it is still a challenge to perform ultrasensitive DNA assays at attomol concentrations. With the use of oligonucleotide silver nanoparticles and a suitable device, it is possible to detect concentrations as low as 5 aMol/L of viral DNA.
Recently Zon et al[48] reported the photo-induced nucleation and growth of silver nanoparticles in the presence of DNA oligomers. An organic dye (Cy5) was used as a photosensitizer to initiate nanoparticle growth. Irradiation of the precursor solutions with light at the Cy5 absorption maximum triggered the instantaneous formation of spherical particles with a metallic core of 15 nm in diameter.
The emergence of a new class of so-called Oligonucleotide Capped Silver Nano Clusters (OSNC), consisting of silver nanoparticles from 2 to 10 atoms of silver (2 nm maximum), has led to the observation of novel fluorescence properties, including tunable emission and high photostability. At these sizes, discrete atomic energy levels merge into highly polarizable, continuous, plasmon-supporting bands, thereby leading to very strong absorption and emission[49]. The preparation is very simple, requiring mixing of a suitable salt of silver with the desired oligonucleotide and addition of a reducing agent. Interestingly, the fluorescence properties depend on the sequence of the oligonucleotide. The main applications are sensors for genes, proteins, small molecules, or metal anions. For example, OSNC can be used instead of oligonucleotide probes tagged with a fluorophore to detect a targeted DNA sequence. Their hybridization with a targeted DNA sequence enhances the fluorescence (Figure 5). Chang has developed a system able to detect single point mutations in the gene involved in the hereditary disease tyrosinemia typeI[50]. Additionally, these structures promise potential for labeling biomolecules for imaging purposes, as they can be prepared to yield fluorescence in the red or NIR. Antibodies have been conjugated for imaging NIH 3T3 cells[51].
Copper: Because of their ease of back-oxidation, copper systems are still not widely used as nanoparticle cores. However, oligonucleotide capped copper nanoclusters have recently been developed and offer an excellent choice as functional biological probes. Rotaru showed that copper can selectively metallize a double stranded DNA on a oligonucleotide probe, allowing control of the size of the cluster[52]. Moreover, the fluorescence of DNA-hosted Cu nanoclusters is very sensitive to the base type located in the major groove. The advantages of this method over current fluorescence based assays employed for the detection of mismatches in DNA (as Real time quantitative PCR) are a simpler design of probes and the fact that no conjugation of fluorophore on the probes is required[53].
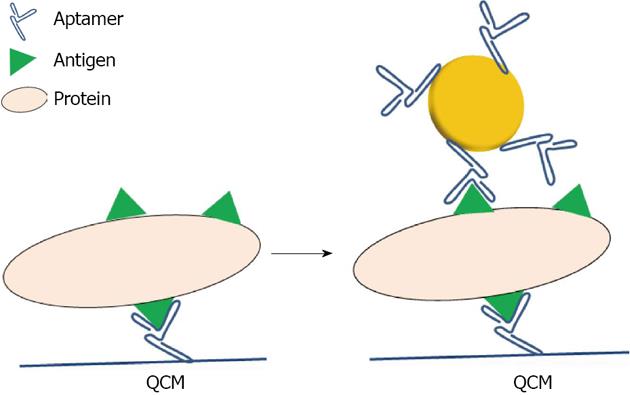
Apt: In comparison to antibodies, Apt possess certain advantages, including their relatively simple and inexpensive synthesis, tolerance to internal labeling, and long storage times without losing their biological activity. The use of Apt as capping agents for metal nanoparticles started in 2004, when Pavlov used such nanoparticles to detect thrombin with a Quartz Crystal Microbalance (QCM)[54]. The resonance of the QCM is disturbed by small mass changes due to film deposition on the surface of the acoustic resonator. Here, the advantage of using metallic particles is to give a weight effect so as to increase the sensitivity of the targeted molecule (Figure 6).
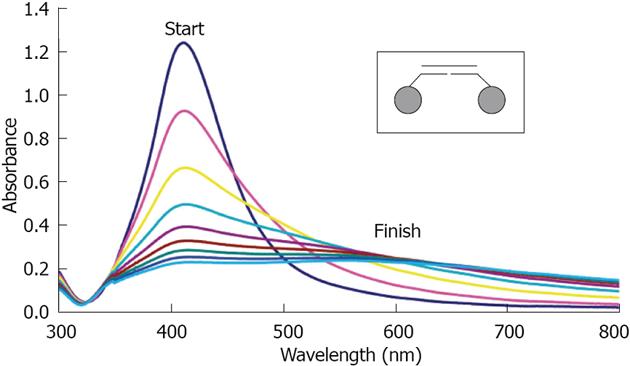
Chang has used Apt gold nanoparticles as biosensors for detecting Platelet-Derivated Growth Factor (PDGF)[55], which is overexpressed in some cancer cells. They proposed an aggregation-based assay using Apt gold nanoparticles to detect PDGF at the nM level. By the same principle, other Apt gold nanoparticles were developed toward adenosine triphosphate and glutathione[56].
The same group later used Apt gold nanoparticles as a contrast agent for detecting cancer cells over-expressing PDGF[57]. Nanoparticles enter the cell where PDGF induces aggregation, and produces a colour (Figure 7). When compared to immunostaining, this approach offers advantages of lower cost and minimum matrix interference. Similarly, Liu used Apt gold nanoparticles for recognizing cancer cells with a strip biosensor[58].
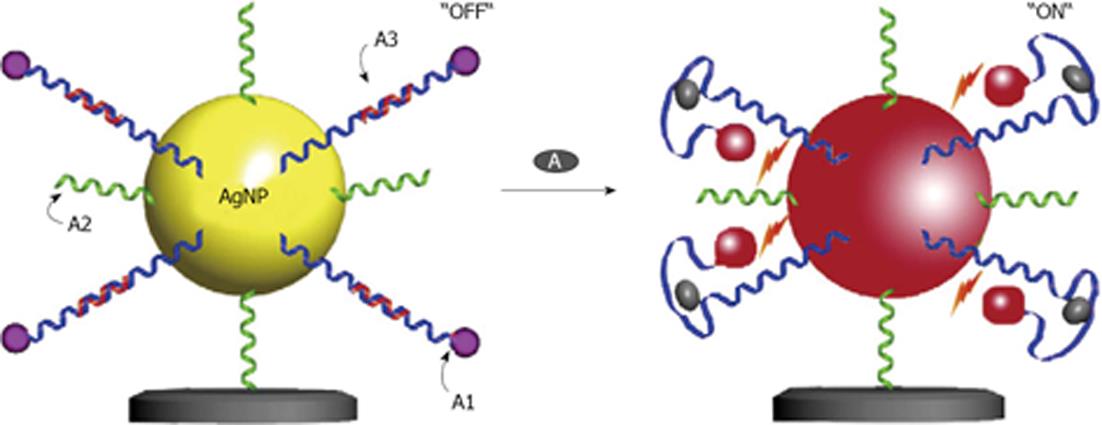
Very recently, one study described the use of silver as a nanoparticle material[59]. In this case silver was chosen because of its excellent optical properties for metal enhanced fluorescence[60]. Based on the results, an aptamer based fluorescent switch has been constructed. In the “OFF” state, without the target molecule, there is a greater spacing distance between the dye and the silver nanoparticle giving comparatively lower fluorescence intensity. However in the “ON” state, in the presence of target molecules, the fluorescence signal is increased due to a shortened distance between the dye and the nanoparticle (Figure 8). This Apt-sensor linearly detects adenosine concentrations from 200 nmol/L to 200 μmol/L with a detection limit of 48 nmol/L.
The structural versatility, biological importance and biomedical impact of proteins make them one of the most widely studied classes of molecules in bio-recognition. There has long been a major interest in the use of capping metallic nanoparticles with proteins[19,61]. Among proteins with molecular recognition abilities, antibodies, key elements of the immune response, are probably the most widely studied[62,63]. Enzymes have also been studied as capping agents for metallic nanoparticles. Willner et al[64] reviewed recent advances in the development of enzyme-metallic nanoparticle conjugates and their specific applications for biosensing and the generation of nanostructure.
Peptides: Peptides which are distinguished from proteins on the basis of size (typically containing fewer than 50 monomer units), and their folding, are well known to be involved in molecular recognition events. Peptide-capped metal nanoparticles combine several advantages. Peptide chemistry is versatile and provides the possibility to utilize functional groups found in the 20 naturally occurring amino acids plus the possibility to introduce non-natural amino acids[65]. Peptides and peptide conjugates are readily commercially available. The preparation of peptide-capped nanoparticles is rapid, simple, and amenable to high-throughput approaches. This allows, in a single step, the production of stable and functional nanoparticles. Peptide-capped gold nanoparticles and, more generally, peptide-capped nanomaterials have immediate applications as bioanalytical sensors and cell imaging, but, perhaps more importantly, they offer an almost unlimited range of possibilities for the design and preparation of the advanced functional nanomaterials of the future[66].
Gold: The use of peptide-capped gold nanoparticles as enzyme mimics, also called nanozymes has been previously reviewed[67,68]. The fundamental idea is to engineer a micro environment, within a self assembled monolayer, that resembles the catalytic site of natural enzyme. Pengo developed a functional artificial protein by grafting a thiol functionalized dodecapeptide onto the surface of gold nanoparticles. This system was able to catalyze the hydrolysis of carboxylate esters. It was found that certain substrates affected the structure of the catalytic site by altering its hydration, demonstrating that the nanozyme can regulate its own activity just as proteins do[69].
Brust used peptide-capped gold nanoparticles as artificial substrates for kinases to develop a colorimetric protocol for the evaluation of kinase activity and inhibition[70]. Sun reported the use of these hybrid particles in a microarray format[71]. The method is based on labeling peptide phosphorylation events on a microarray with gold nano-particles and using resonance light scattering (RLS) detection[72]. They demonstrated that it is possible to screen kinases with single or multiple inhibitors simultaneously on the same microarray[73].
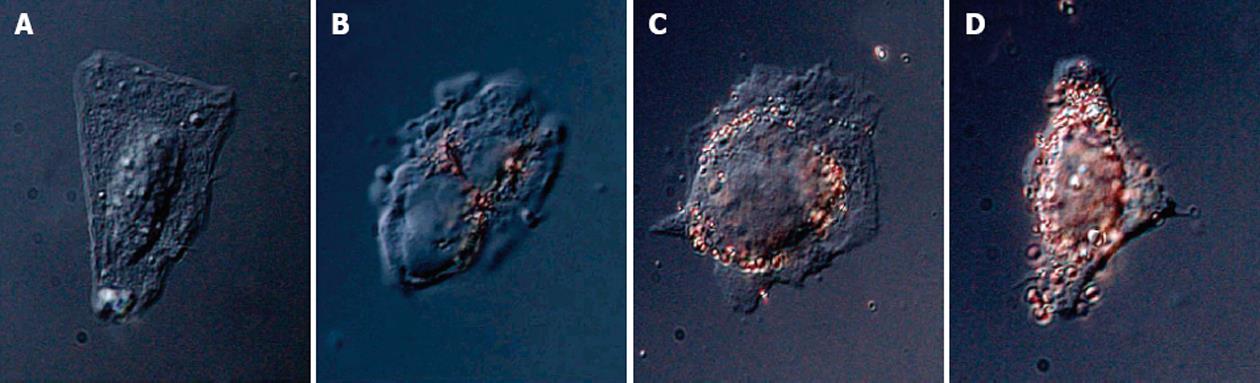
Targeting transmembrane transport is another field of application in which peptide-capped gold nanoparticles have been used. Franzen reported a method for assessing the efficiency of various combinations of targeting peptides using nanoparticle complexes for nuclear targeting. VEC-DIC combination microscopy permits observation of the localization of the peptide-capped gold nanoparticles according to the peptide sequence[74] (Figure 9). Recently El Sayed investigated the quantitative tumor uptake of a class of elongated gold nanoparticles (nanorods) that were covalently conjugated to tumor-targeting peptides[75]. The results suggest that for photothermal cancer therapy, the preferred route of gold nanorod administration is intratumoral injection. With direct tumor injection, the peptide-capped gold nanoparticles were mainly found in the tumor cells while peptide-capped gold nanoparticles injected with intravenous injection are mainly localized around blood vessels, in the tumor stromal matrix.
Silver: To date, few articles describe the use of peptide capped silver nanoparticle for colorimetric sensing. Most studies focus on the nature of the peptide/silver interaction and the effect of the peptide on the formation of the silver nanoparticles[76,77]. Recently, Cui has demonstrated that at basic pH the peptide secondary structure was modified, and could affect the size of the silver nanoparticles[78].
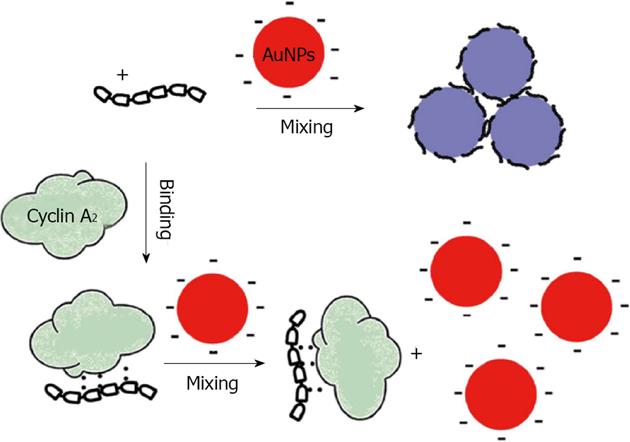
Qu reported a homogenous assay for colorimetric and quantitative detection of a cancer marker and the promising antitumor target, cyclin A2, using the aggregation of unmodified gold nanoparticles and/or silver nanoparticles[79]. They used the difference in coagulating ability of a cationic peptide probe (P1) and its binding form toward unmodified nanoparticles, in order to detect cyclin A2. In the absence of cyclin A2, P1 aggregates particles immediately, whereas cyclin A2 binding prevents the interaction of P1 with the surface, significantly reducing the aggregation. The extent of aggregation is dependent on the concentration of the target protein cyclin A2 and the difference in color can readily be distinguished by spectrometer (Figure 10).
Generally, peptide capped silver nanoparticles are used for their anti-microbial activity. Taglietti studied the mechanism of action of glutathione (GSH) capped nanoparticles. GSH peptide displays a thiol function, capable of being anchored to silver surfaces, and three pH-dependent, charged functional groups (carboxylates and amines), that promote water solubility and interactions with complex biostructures[80]. GSH capped nanoparticles have shown a bacteriocidal effect related to their penetration of bacterial cells. They show a stronger bacteriocidal effect on Escherichia coli (E. coli) (Gram negative strain) than on Staphylococcus aureus (gram positive strain). Moreover GSH capped Ag nanoparticles showed lower antibacterial activity when grafted onto functionalized glass surfaces, which prevent the nanoparticle from penetrating the bacterial cell[81]. Wei et al[82] investigated another mechanism of the antibacterial effect using the peptide norvancomycin, a treatment of choice for antibiotic resistant bacteria. The silver nanoparticles decrease the stability of the lipopolysaccharide (LPS) present in the outer membrane of gram negative strains. Using the molecular recognition ability of norvancomycin, the hybrid nanoparticles bind to the peptidoglycan inner membrane and improve the destruction of the bacteria by increasing access of norvancomycin.
Copper: Peptide capped copper nanoparticles have received less interest. Recently, Thakore produced peptide-capped copper nanoparticles of 12-16 nm by chemical reduction[83]. The synthesis was carried out using stem latex of the medicinal plant, Euphorbia nivulia. The nanoparticles were stabilized and subsequently capped by peptides and terpenoids present within the latex. The study demonstrated that peptide capped copper nanoparticles are toxic to A549 cells in a dose dependent manner. Cell viability assay (MTT) determined an LD50 concentration of 20 μg/mL. The dose dependent cytotoxicity (biocompatible below 1 μg/mL) suggests that these nanoparticles could be used in the future to induce apoptotic destruction of cancer cells. Hossieni reported a promising method for producing such hybrid compounds[84]. The group reported the bio-production of copper sulfide nanoparticles from CuSO4 solution by the reduction of NADH released from the fungus Fusarium oxysporum. transmission electron microscopy (TEM) images demonstrated that spherical particles of 2-5 nm, were enclosed in spherical peptide shells of about 20 nm in diameter.
Antibodies: Antibodies constitute one of the most important specific defense mechanisms in vertebrate animals. All of them are bifunctional molecules in a Y-form with two identical domains for antigen recognition (Fab fragment), and two identical domains with effector functions (Fc fragment). The antigen-binding region is highly specific to individual antibodies and large numbers of different antibodies are available[85]. Antibodies act as neutralizers of pathogens or toxins, as well as in the recruitment of immune elements (complement, phagocytosis, antibody dependent cytotoxicity by natural killer cells, etc.). In addition they may transport molecules including toxins, drugs, fluorophores, and be used in diagnostic procedures, or in therapy to destroy a specific target. The conjugation of antibodies to nanoparticles generates a versatile product that combines, the small size and their thermal, electrical, or optical characteristics of nanoparticles with the abilities of antibodies for specific and selective recognition[61,86].
Gold: So far, antibody capped gold particles are the most popular hybrid systems for the exploitation of the molecular recognition properties of the capping agent. In 1971, Faulk reported their use as a specific marker for Salmonella surface antigens using TEM because of their high electron-dense metal density[20]. Later Horisberger demonstrated their use for Scanning Electron Microscopy[87]. Subsequently, Sokolov showed that when 12 nm gold nanoparticles were conjugated to anti-epidermal growth factor receptor (anti-EGFR) antibodies, they specifically bound to EGFR proteins overexpressed on the surfaces of cervical cancer cells[88]. Illumination of nanoparticle-labeled cells with laser light lit up the gold nanoparticles, and thus, the associated cancer cells. Later, El-Sayed used simple dark field optical microscopy to detect gold nanoparticle-labeled cancer cells. Anti-EGFR antibody conjugated gold nanoparticles were incubated with a nonmalignant epithelial cell line (HaCaT) and two malignant oral epithelial cell lines. The hybrid nanoparticles bound to the surface of the cancer type cells with 600% greater affinity than to the noncancerous cells[88].
Within the last decade, there has been substantial interest in antibody capped gold based contrast agents for in vivo X-ray imaging[89,90]. They are a promising candidate for next generation X-ray contrast materials, and their use in combined radiotherapy is under evaluation[91].
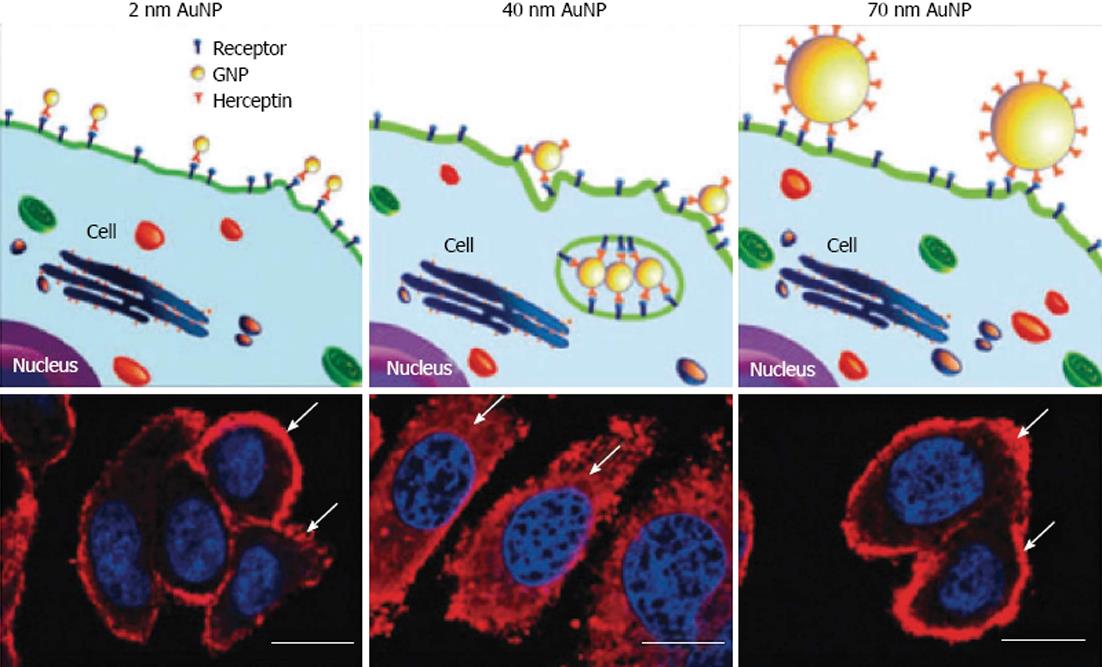
One of the most promising aspects of gold nanoparticle use in medicine, is targeted drug delivery. The most popular objects for targeted delivery are antitumor drugs[33]. Combination with antibody capping has been demonstrated to increase the intracellular uptake of gold nanoparticle carriers as compared to non-functionalized conjugates[92]. Chan investigated the cellular uptake of antibody-functionalized gold nanoparticles of different sizes, 2-100 nm[93]. It was found that nanoparticles with diameter of 40-50 nm enter cells more efficiently than either smaller and larger sized gold nanoparticles. At this size the antibody-capped gold nanoparticles maximize interactions with cell surface receptors and thus enter via receptor-mediated endocytosis (Figure 11)[94].
Another promising therapeutic application of antibody capped gold nanoparticles concerns photothermal damage to cells. Current research is focused on the treatment of cancer and infectious diseases[95]. Gold nanoparticles have an absorption maximum in the visible or near infra-red region and become very hot when irradiated at the resonant frequency. If the gold nanoparticles are located inside or at the surface of target cells, these cells will overheat and die. Such work has been reviewed in depth[96,97].
Finally, gold nanoparticle-antibody conjugates have been used for as biosensors for their complementary antigens. A wide variety of such antibody-antigen interactions been reviewed[19,24,33]. As an illustrative example, the aggregation of gold nanoparticles by antigen-antibody interactions in solution was applied to develop immunoassay procedures with optical detection of the association process[98,99]. A laser based double-beam absorption detection system for aggregation immunoassays has been developed. The assay is based on the aggregation of antigen capped gold nanoparticles, in the presence of the corresponding antibodies. The aggregation of the gold nanoparticles resulted in a change in the absorption bands with a detection limit for an antibody of 3 × 10-8 M.
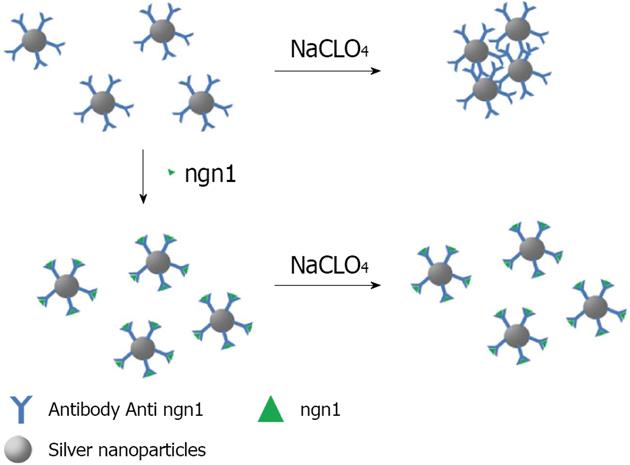
Silver: A range of highly sensitive biosensing methods using antibodies have been developed by exploring different physicochemical properties of the Noble metal nanoparticles, such as LSPR, metal fluorescence enhancement/quenching, SERS, electrochemical activity, etc[24]. To date, a number of colorimetric sensors using silver nanoparticles as probes have been developed[100]. In an effort to overcome the lower stability of silver nanoparticles Yuan reported a colorimetric sensing scaffold for neurogenin 1 (ngn1), a peptide expressed in neuronal precursor cells with the function of controlling the differentiation of neurons[101]. The detection procedure is based on an anti-aggregation mechanism, by which ngn1 inhibits the aggregation of the probe in the presence of NaClO4. The anti-ngn1 antibody conjugated silver nanoparticles (AgNP-Ab) is negatively charged, and binding of the negatively charged ngn1 to the probe enhances interparticle electrostatic repulsion. Accompanying the increase of ngn1 concentration, the color of the solution varies from red to yellow, presenting an approach for the detection of ngn1. This assay exhibits a linear response range over nearly two orders of magnitude, from 50 to 800 ng/mL, and a detection limit of 30 ng/mL (Figure 12).
In the area of micro- and nanotechnology-derived biosensors the use of antibody capped silver nanoparticles has received attention[102]. Silver nanoparticles are promising as the detection agent for electrochemical sensors. Porter highlighted the fact that although the number of assays reported with gold nanoparticles is much higher than those with silver, it is the silver nanoparticles that exhibit the better electrochemical properties[103]. Chen has developed the most sensitive electrochemical immunosensing method with a dynamic concentration range of 1-1000 ng/mL and a detection limit of 0.4 ng/mL[104].
The anti-microbial properties of silver are well documented[105], and the antimicrobial activity of silver nanoparticles dominates research in this area[106]. Recently, Singh reported the use of these hybrid compounds as anti-viral agents. The addition of silver nanoparticles to antibodies significantly increased the neutralizing potency in prevention of cell-associated human immunodeficiency virus (HIV)-1 transmission/infection (from 10% of inhibition for antibodies alone to 60%-71% for antibody capped silver nanoparticles)[107].
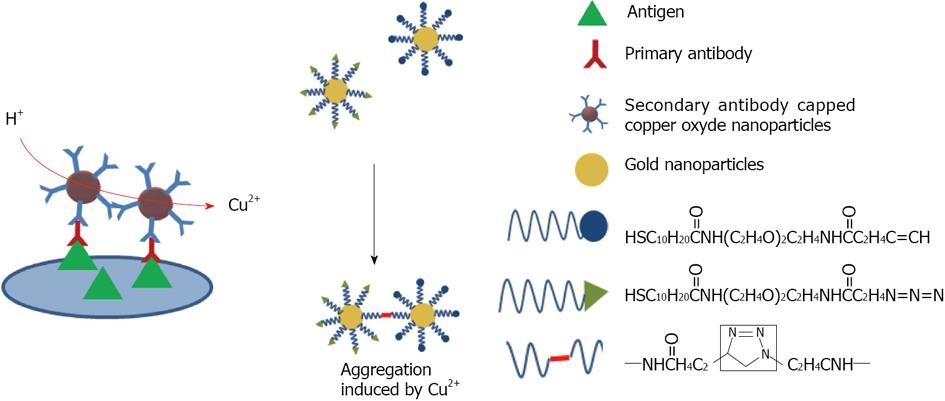
Copper: Since copper is easily oxidized, gold and silver nanostructures are more attractive for optical applications. In an effort to overcome this issue, research has been undertaken using different strategies. Zhang et al[108] proposed to cover the surface of copper nanoparticles with gold in order to combine the voltammetric activity of copper and the stability and biocompatibility of gold. The functionalization with antibody permits the detection of E. coli with a detection limit of 30 CFU/mL. More recently, Wang developed a method using antibody capped copper nanoparticles[109]. When Cu2+ is released into solution by HCl treatment, it can be assayed (Figure 13). The limit of detection for the GP41 glycoprotein of HIV was about 150 ng/mL.
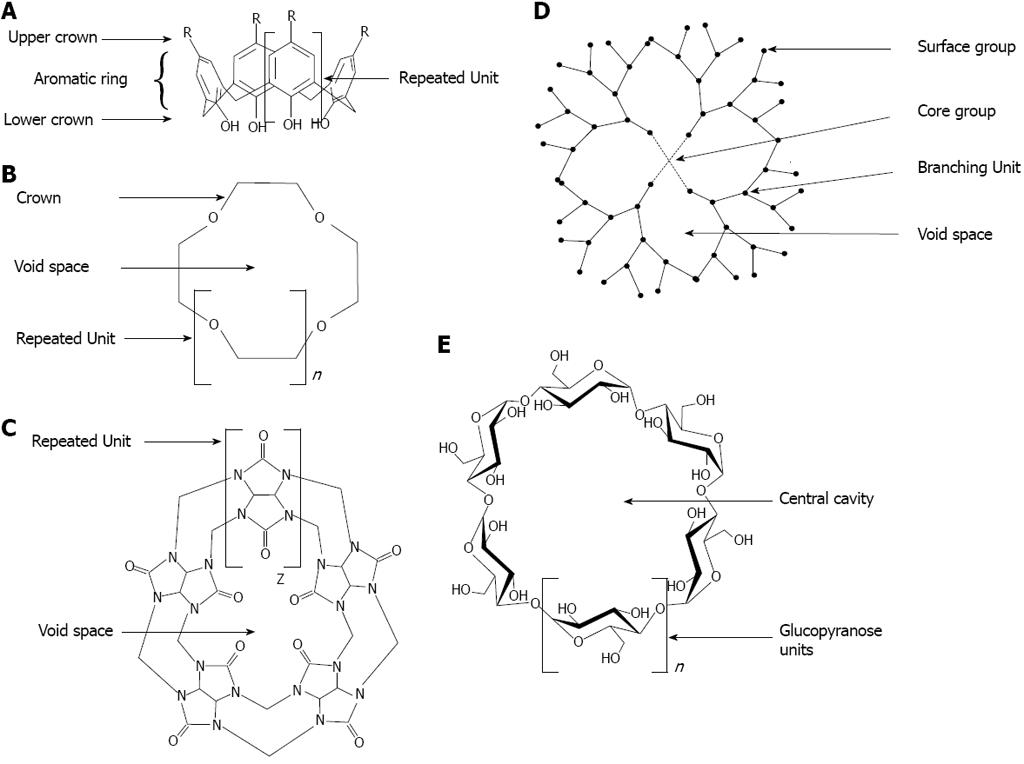
Supramolecular chemistry concerns the domain of chemistry beyond that of the covalent bond and focuses on chemical systems made up of a discrete number of non-covalently assembled molecular subunits or components. The forces responsible for the spatial organization may vary from weak (intermolecular forces, van der Waals), through medium (aromatic-aromatic stacking or dipole-dipole) to strong (electrostatic, coordination bonds or hydrogen bonding)[110]. The assembly of the molecules will depend on molecular recognition events. Because of their recognition capability, molecules such cyclodextrins (CDs), calix[n]arenes, dendrimers, crown ethers or cucurbiturils have attracted interests as capping agents on metallic nanoparticles[111] (Figure 14).
Cyclodextrins: The CDs are a family of soluble molecules generally consisting of 6, 7 or 8 D-glucopyranosyl residues (denoted as α-CD, β-CD and γ-CD, respectively) linked in a cyclic structure by α-1,4 glycosidic bonds. They can form inclusion complexes incorporating various molecular guests within their hollow, truncated cone shaped cavity structures, enabling them to be used as drug carriers. The host-guest interactions involved have been attributed to a combination of weak interactions such as van der Waals forces, and hydrophobic interactions and stronger interactions including hydrogen bonds[112,113]. Their combination with metal nanoparticles was the first reported among the supramolecular family[114].
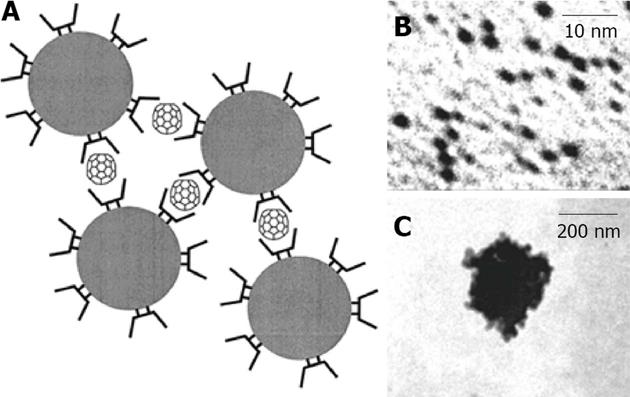
Gold: CD capped gold nanoparticles were initially investigated by Kaifer, in 1998, with the development of a new method based on the aqueous solubilization of aliphatic thiols by α-CD (Figure 15), which effectively binds to the aliphatic chains and carries the hydrophobic thiol molecules to the surface of the gold particles, where they undergo chemisorption[115]. This method can be used to prepare gold colloidal particles (diameter > 10 nm) modified with long chain alkanethiols. If the alkanethiol contains a bulky terminal group, such as ferrocene, the α-CD host is trapped after surface attachment, yielding CD-based rotaxanes supported on the gold nanoparticles. With the use of thiolated CDs, Liu et al[116] functionalized gold nanospheres (2-7 nanometers). The resulting monolayer-protected nanoparticles behave as multisite hosts in aqueous media, engaging in host-guest interactions with conventional guest molecules for CDs. Similarly, the group of Liu used the recognition ability of γ-CD for C60 fullerene, in order to create γ-CD -capped gold nanoparticles as a C60 extracting agent. Even though C60 is extremely insoluble, an aqueous suspension containing γ-CD-capped gold nanoparticles (3.2 nm diameter) can partially solubilize C60[117]. This solubilization involves the formation of complexes between one molecule of C60 and two γ-CD hosts attached to different nanoparticles (Figure 16). Therefore, the fullerenes act as a sort of “molecular glue”, leading to the formation of soluble nanoparticle aggregates with sizes around 290 nm.
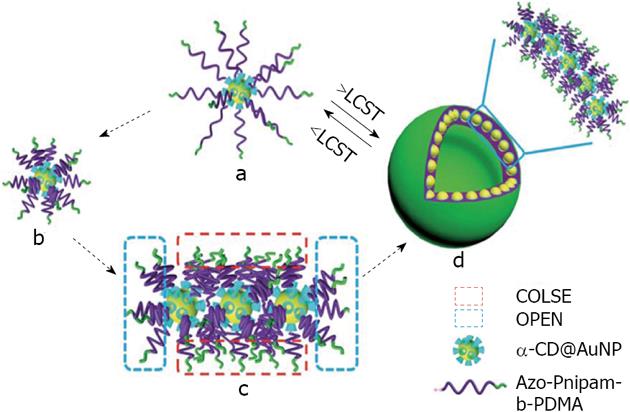
A number of studies describe the use of CDs as capping agents for gold nanoparticles in order to construct nanoparticular superstructures in a reversible way[118,119]. In this area, Chen and Jiang have developed a reversible self-assembly of α-CD capped gold nanoparticles to vesicles, mediated by a guest (azobenzene) conjugated to the double hydrophilic block copolymers Poly-Isopropylacrylamide (PNIPAM) and poly dimethyl acrylamide[120]. This assembly mechanism occurs in pure water under the stimulus of temperature. A possible mechanism is via the thermal responsive coil-to-globule transition of the PNIPAM block (Figure 17).
Recently, CD-capped gold nanoparticles have been used as nanozymes[121]. They were utilized as a backbone to install metal catalytic centers by supramolecular assembly of the copper complex of triethylnetetramine-adamantane and 6-thio-β-CD 15.2 receptors immobilized on the gold surface by thiol groups. The catalytic behaviour of β-CD-15.2-modified gold nanoparticles with adjacent multi-metal catalytic centers were investigated as an esterase mimic. Strong hydrolase activity for cleavage of an active ester 4,4’-dinitrodiphenyl carbonate (DNDPC) was observed. The rate acceleration is approximately 2600-fold with CD-capped gold nanoparticles compared to the reaction rate for the non-catalyzed hydrolysis of DNDPC in the same buffer solution.
Silver: CD capped silver nanoparticles were developed more recently than CD capped gold nanoparticles, being first demonstrated by Fan[122]. The method is simple, addition of NaBH4 to an aqueous solution of silver nitrate and α-CD. The authors explain the stabilization of the silver nanoparticles by hydrophobic interactions of α-CD primary faces. Then, hydrogen-bonding interactions between the exposed secondary -OH groups facilitates the threading of neighboring CDs, leading to the self-assembly of the silver nanoparticles into 1-D “pearl necklace” arrays.
Other studies have focused on the use of such hybrid systems to develop new antibacterial agents[123,124]. Wang developed silver nanoparticle-embedded one-dimensional β-CD-Poly-Vinyl-Pyrrolidone composite nanofibers using a one-step electrospinning technique. This composite exhibited good antibacterial properties against E. coli and Staphylococcus aureus[125].
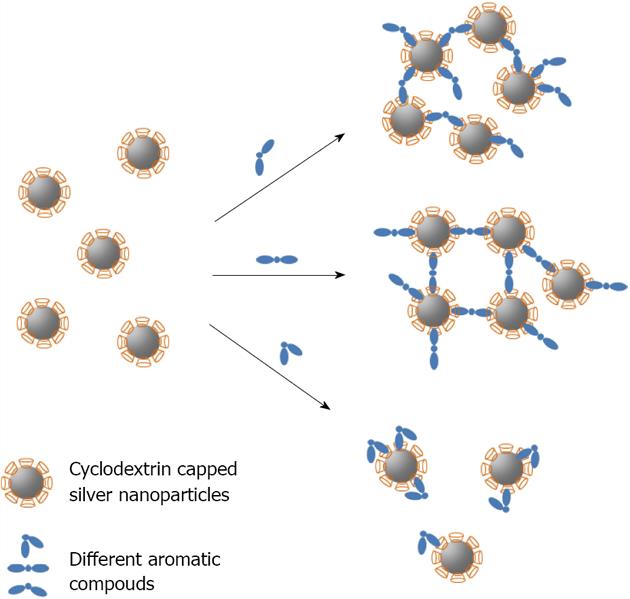
CD capped silver nanoparticle have also been used as biosensors by using SERS[126] or visual inspection[127]. In the latter case, Chen described the development of a robust, colorimetric detection method, sensitive to different isomers of aromatic compounds. 6-thio-β-CD-15.2 was capped by chemisorbtion of the thiol function on 10 nm sized silver nanoparticles. The assay relies on the distance-dependent optical properties of silver nanoparticles and the different inclusion binding strength of the aromatic guests to CD. In the presence of different isomers of aromatic compounds, silver nanoparticles could be rapidly induced to aggregate, thereby resulting in an apricot to red colour change. With a spectrophotometer, this method is quantitative for monitoring the behaviour of the CD-modified silver nanoparticles as a function of the aromatic (Figure 18). The cited detection limit for different isomers of aromatic compounds is 5 × 10-5 mol/L.
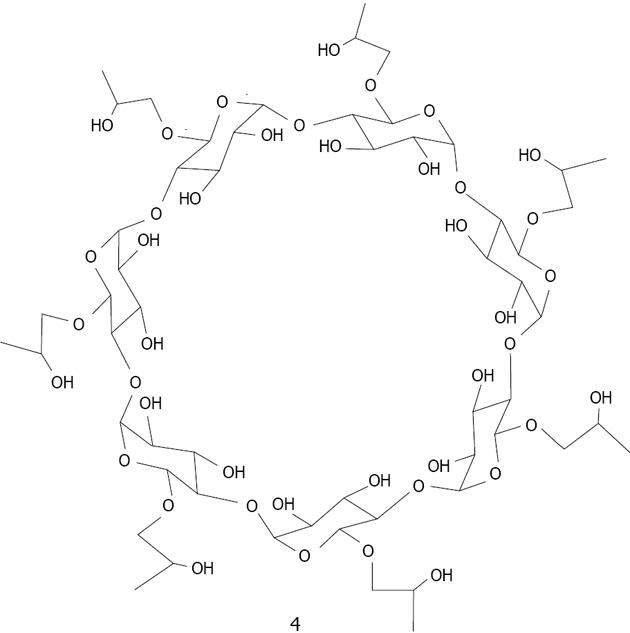
Copper: CD based copper nanoparticles have been developed according to various methods. Zhu used 2-Hydroxypropyl-β-CD 4 (Figure 19) as a template to fabricate hollow spherical copper sulfide nanoparticle assemblies using sonication. The average size of the prepared copper sulfide nanoparticles was estimated to be 10 nm[128]. Geckeler reported the preparation of uniform copper oxide (tenorite) nanoparticles via a green pathway by thermal decomposition, using a novel supramolecular complex, in which β-CD is selected to encapsulate the precursor copper(II) acetate[129].
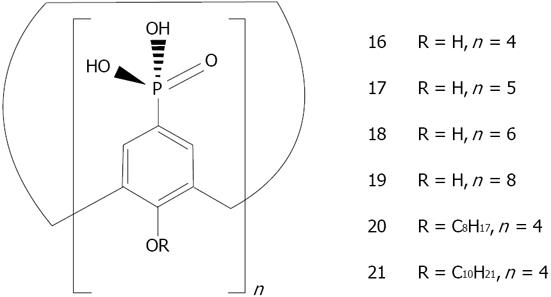
Calix[n]arenes: The calix[n]arenes are one the most widely studied classes of organic supramolecular hosts[111]. This popularity arises from their ease of synthesis and the fact that they contain two very different chemistries, one at the phenolic functions and a second at the para-position on the aromatic ring. This is coupled with the possibility to region-selectively modify them with varying degrees of controlled substitution at either face (Figure 20). Finally, their molecular recognition abilities added to their lack of toxicities[130] have given calix[n]arenes many applications in biology from protein sensors, stabilizers, enzyme inhibitors to active pharmaceutical ingredient (API) solubilizers[131]. A secondary property that has proved highly advantageous is the propensity of the calix[n]arenes to crystallize, allowing solid-state studies of a wide range of their complexes with bio-active molecules[132]. Combined with the electronic and optical properties of metal nanoparticles, calixarene capped nanoparticles have already started to show new applications, as recently described[133,134].
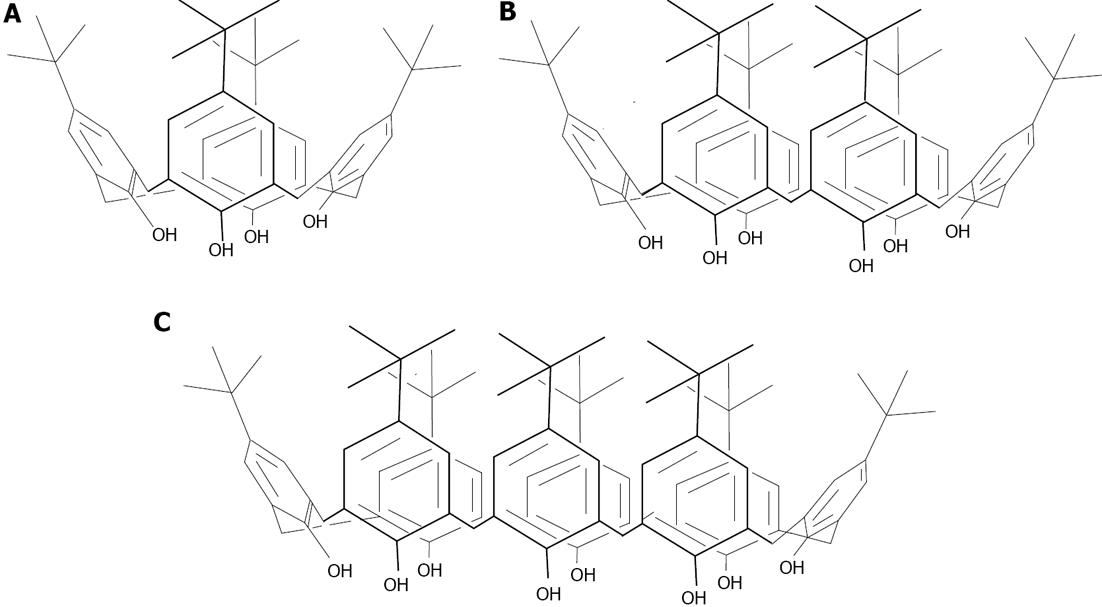
Gold: So far, calixarene based gold nanoparticles have been mainly applied as colorimetric sensors. Pochini has carried out a large body of work in the use of thiolated derivatives of calix[4]arene for capping gold nanoparticles (Figure 21). The recognition of immobilized cationic pyridinium moieties with 5_GNP[135], or quaternary ammonium salts with 5_GNP or 6_GNP[136] by the unmodified upper rim of the calix[4]arene present on the gold nanoparticles was demonstrated.
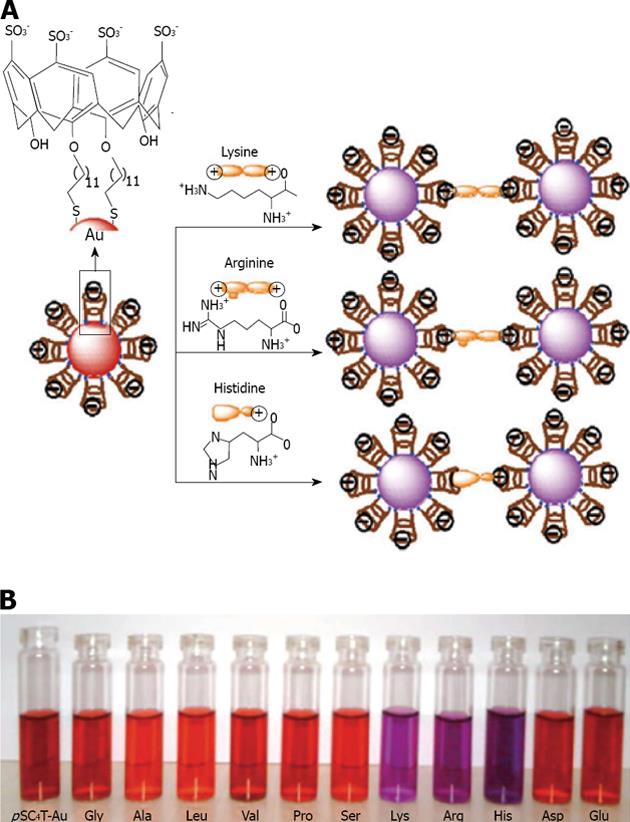
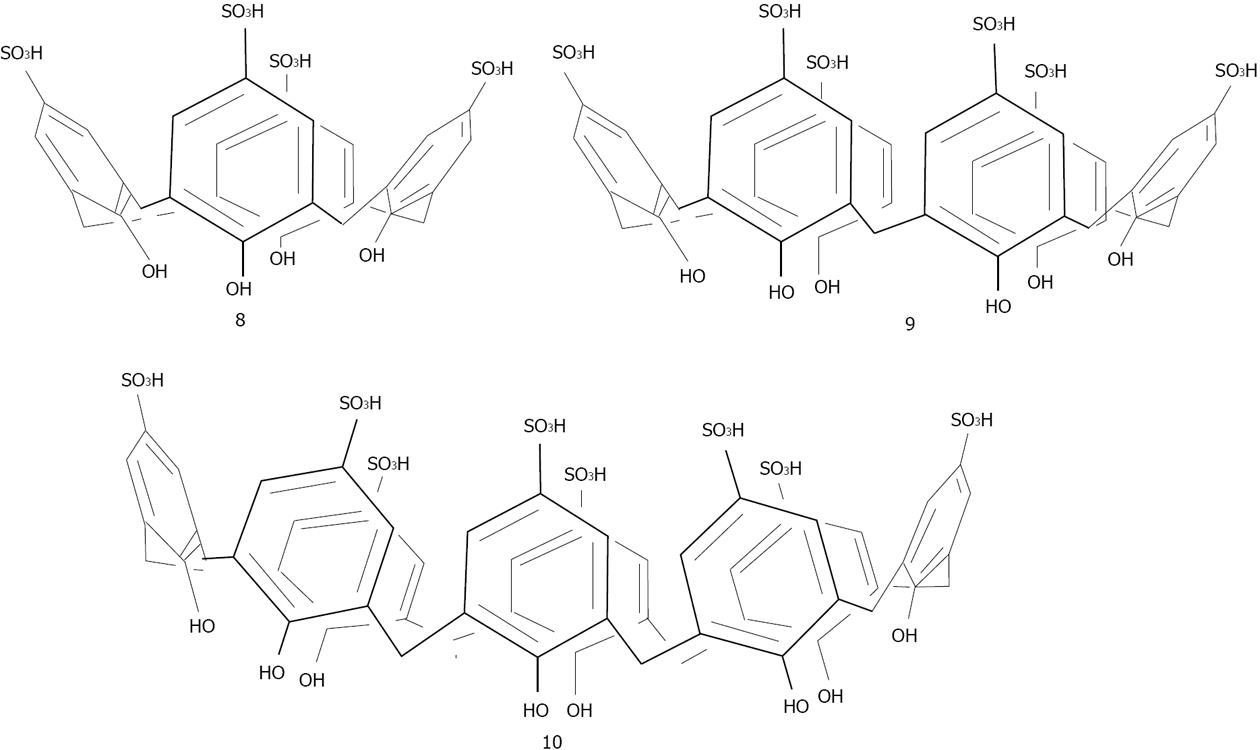
Menon has introduced a simple route for the preparation of water soluble para-sulphonatocalix[4]arene thiol 7 capped gold nanoparticles[137] (Figure 22). 7 possesses an electron-rich cyclic cavity that can attract specifically cationic amino acids (lysine, arginine and histidine). These interactions between one amino acid molecule and two calix-modified gold nanoparticles tend to aggregate the assemblies more than the other amino acids (Figure 23). Similarly Han et al[138] used para-sulphonato-calix[6]arene 9, gold nanoparticles in order to detect pollutant aromatic amines isomers (Figure 24).
Other studies have focused on the recognition of cations. Yan et al[139] have developed 6 nm sized gold nanoparticles capped with methylthio-para-tert-butyl-calixarene derivatives 11, 12 and 13 (Figure 25). They used the cationic recognition of structurally-tailored para-tert-butylcalixarenes to control access of the cationic guest to the cone cavity. This novel strategy has been shown to yield a specific red shift of the surface plasmon resonance band of gold nanoparticles, according to the cationic metal added. Solution of 0.4 μmol/L, calix GNP showed apparent rates of 1.4 × 10-2 s-1 for Cu2+ and 2.5 × 10-1 s-1 for Cs2+.
Recently, Menon modified para-sulphonatocalix[4]arene with dithiocarbamate 14, for capping gold nanoparticles[140]. Sulfide ion recognition triggers particle aggregation through N-H-S hydrogen bonds and provides an easy way to measure color change (Figure 26). The lower detection limit was 10 nmol/L. This result validates the use of calix[n]arene capped gold nanoparticles in applications requiring high sensitivity and specificity.
Silver: Sanchez-Cortez used 25,27-diethyl-dithiocarbamic 26,28-dihydroxy para-tert-butylcalix[4]arene 15 in the functionalization of silver nanoparticles for pyrene detection by SERS[141]. SERS spectra provided information about the calix[4]arene orientation on the metal surface and the interaction mechanism (Figure 27).
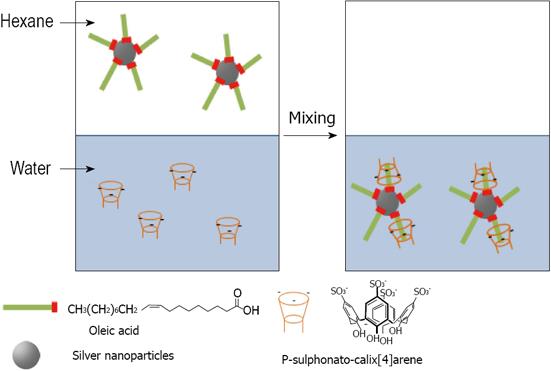
Later Diao proposed a new method to change the surface properties of oleic acid stabilized silver nanoparticles and was successful in transferring silver nanoparticles from an organic phase into an aqueous phase[142]. By vigorous shaking of a biphasic mixture of the silver organosol protected with oleic acid and an aqueous solution of para-sulphonato-calix[4]arene 8, it is believed that an inclusion complex is formed between oleic acid molecules and 8, and the protective layer of the silver nanoparticles shifts from hydrophobic to hydrophilic in nature, which drives the transfer of silver nanoparticles from the organic phase into the aqueous phase. The 8-oleic acid inclusion complexation stabilized the nanoparticles for several weeks in the aqueous phase under ambient atmospheric conditions (Figure 28). Raston proposed new methods for environmentally friendly capping of silver nanoparticles with phosphonated derivatised calixarenes. Para-phosphonatedcalix[n]arenes 16, 17, 18 and 19 were used as stabilizers for evaluating the effect of hydrogen gas as an environmentally benign reductant of silver nanoparticles[143] (Figure 29). Other phosphonated derivatized calixarenes 20 and 21 have been tested for possible their effect on the growth of silver nanoparticles by photochemical synthesis[144] (Figure 29).
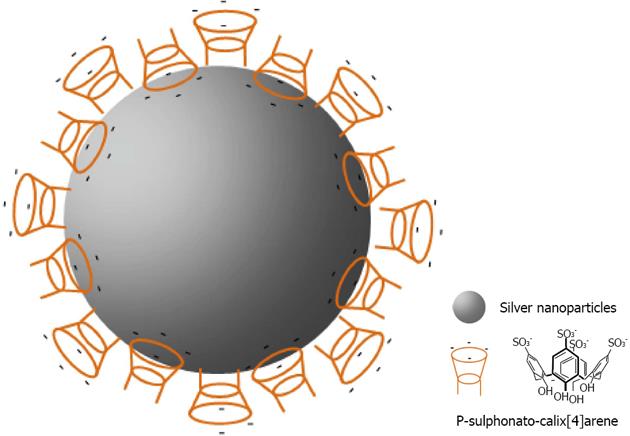
It is the work of Li which has made popular calix[n]arene capped silver nanoparticles with his easy route for producing them[145]. The group demonstrated the application of 8 capped silver nanoparticles for the recognition of cationic amino acids (Histidine, Lysine, Arginine) and pesticides including methyl parathion[146].
Later, Coleman gave a more reasonable explanation for the structure of the assembly of 8 on the surface of the silver nanoparticles[147]. It lies in the formation of the classic bilayer solid-state structure, where alternate coordinated para-sulphonato-calix[4]arene molecules give available cavities at the surface of the nanoparticle (Figure 30). Such assembly allows differentiation between the interactions, nucleic acids and nucleotides with 8 capped silver nanoparticles. Subsequently, the group investigated the assembly of the calixarene with one nucleotide (cytosine) in different states: in solution, in the solid-state and on the surface of silver nanoparticle[148]. The assembly was quite different according to the states involved, and needed the use of multiple physical methods to probe the complex assembly process. Recently, the group of Coleman has shown that 8 capped silver nanoparticles could interact with active pharmaceutical ingredients[149]. More interesting was the use of calixarene capped silver nanoparticles for the determination of the Critical Micellar Concentration (CMC) of some cationic surfactants[150]. This method generates a new means of studying CMC in media containing proteins and in particular membrane proteins.
Copper: Interestingly, only one study from 2007 reports on the use of copper as material support for calix[n]arenes[151]. Uniform cuprous oxide nanospheres with diameter of 10 nm were prepared by the reduction of CuSO4 in para-sulphonato-calix[8]arene 10 aqueous solution using hydrazine as a reducing agent. The host molecule, 10, was used as a bridge linker to make nanoparticles connect to each other and form large aggregations, which possess two types of properties: the photonic, catalytic and semiconductor properties of copper oxide and the supramolecular recognition function of 10-modified nanoparticles. As yet, there are no reports of any applications of this type of particle.
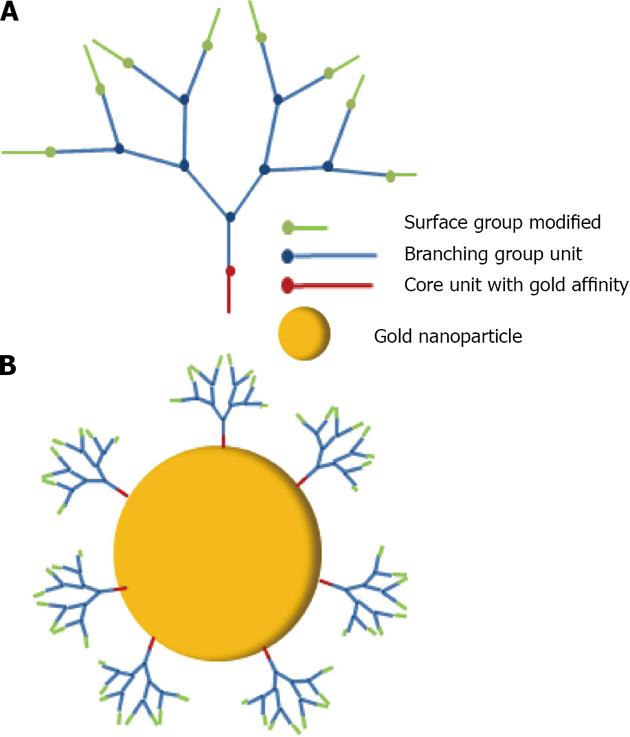
Dendrimers: Dendrimers are a class of hyper branched oligomeric materials. Dendrimers are large and complex molecules having defined chemical structures. They possess three distinguishing architectural components, namely (a) an initiator core, (b) an interior layer (generations), composed of repeating units, radially attached to the initiator core and (c) exterior (terminal) functionality attached to the outermost interior generation (Figure 14). Dendrimers have been applied in biomedical applications, for drug-delivery systems and also for cancer therapy[152]. At this time, the major prospective applications of nanoparticle-dendrimer composites are in catalysis, biomedical research and electronic devices. The catalytic applications are mainly determined by properties of various mono- and bimetallic nanoparticles, while the dendrimer role is in templating or stabilization of nanoparticles, which, in turn, controls the nanoparticle size and morphology. There are a few examples where dendrimer generation (size) puts a limitation on the accessibility of the active centers for reacting molecules, due to a different size cavity in the dendrimer, thus creating size selectivity for a catalytic reaction. A similar effect was demonstrated for mesoporous catalysts with reactants of different sizes. The biomedical applications become possible due to the biocompatibility of many nanoparticle-dendrimer composites and their optical, magnetic and sensor properties[153].
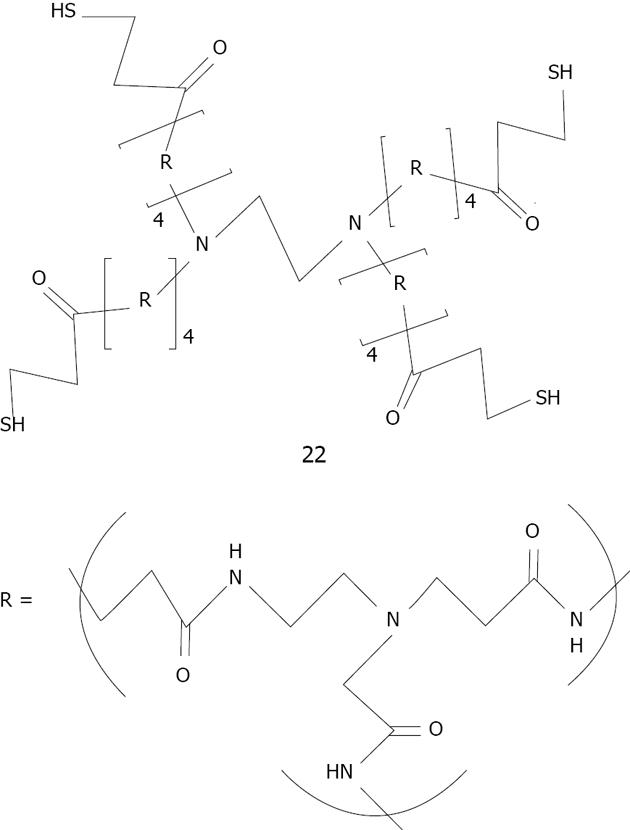
In the last decade dendrimeric gold nanoparticle systems have been widely studied. Crooks was the first to first encapsulated small gold nanoparticles (1-2 nm of diameter) by using a thiolated fourth-generation poly (amido-amine) (PAMAM) dendrimer 22 mixed with tetrachloroauric acid and reduced with an excess of NaBH4[154] (Figure 31). Crooks also used dendrimeric gold nanoparticles in catalysis including intradendrimer hydrogenation and carbon-carbon coupling reactions in water, organic solvents, biphasic fluorous/organic solvents and supercritical CO2[155].
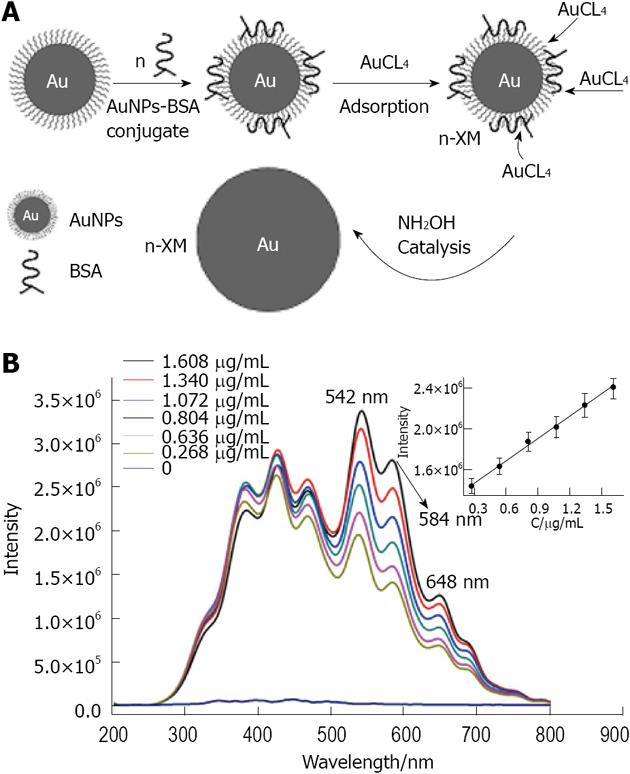
Lu et al[156] reported the use of RLS as a method for detecting trace quantities of protein. Here, RLS measures the change of light intensity scattered from the gold nanoparticles. The signal is known to be amplified upon aggregation. Generation of polypropylene imine hexadecane amine dendrimers 23 (PPIHA) was employed to synthesize uniform gold nanoparticles modified with amine groups on their surface (Figure 32). The amine groups strengthen the covalent coupling between gold nanoparticles and bovine serum albumin (BSA). As illustrated in Figure 33, the size of the gold nanoparticle-BSA conjugates was increased in the HAuCl4-NH4OH_HCl reaction system, enhancing the RLS intensity of the bioconjugates. The RLS intensity is related to the concentration of gold nanoparticle-BSA and has a lower detection limit of 0.090 μg/mL. By employing BSA as a model protein, this work introduced a novel method for the quantitative detection of trace proteins[157]. Recently, Baker has developed a simple approach to fabricating multifunctional dendrimer-stabilized gold nanoparticles for cancer cell targeting and imaging[158]. In this work, amine-terminated generation 5 (G5) poly(amidoamine) (PAMAM) dendrimers pre-functionalized with folic acid and fluorescein isothiocyanate are complexed with Au (III) ions, followed by acetylation of the amine groups on the dendrimer surfaces. This one-step process leads to the spontaneous formation of 6 nm Au nanoparticles stabilized by multifunctional dendrimers bearing both targeting and imaging functionalities.
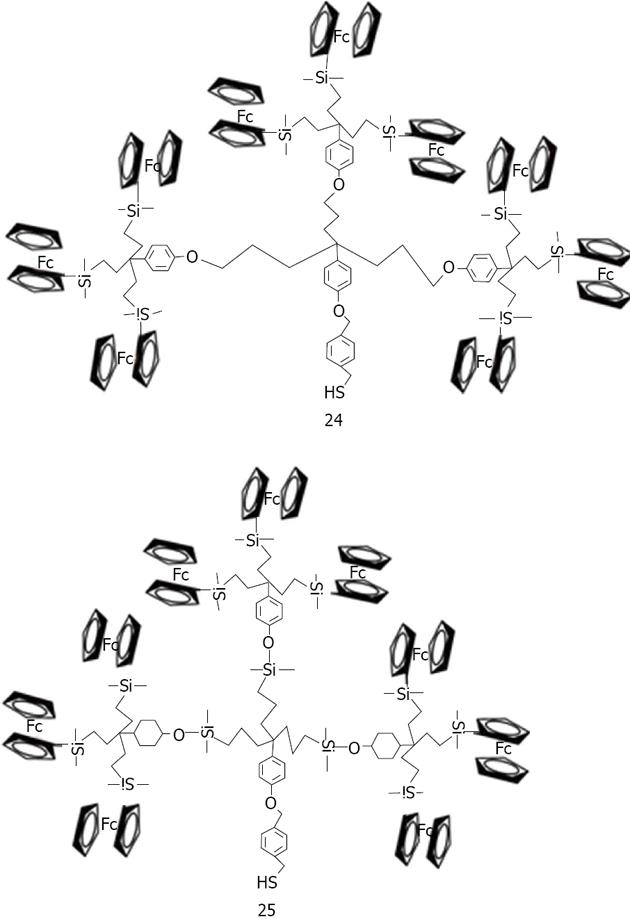
Wang used dendrons, segments of dendrimers that possesses a focal point onto which the branching units of a dendritic architecture are attached to associated gold nanoparticles[159] (Figure 34). It was demonstrated that dendrons modified with a metal-coordinating functionality can be utilized as stabilizing media for the controlled growth of nanocrystals. The average size of the resulting nanoparticles is a direct function of the generation number of the capping dendron, with higher generation dendrons producing larger particles. Astruc has made a large contribution in the research and development of dendronized gold nanoparticles[160]. Dendrons have been synthesized and used to assemble dendronized gold nanoparticles either by the ligand-substitution method from dodecanethiolate-gold nanoparticles (AB3 units) or Brust-type direct synthesis from a 1:1 mixture of dodecanethiol and dendronized thiol (AB9 units). Two nanoparticles have been made containing a nona-silylferrocenyl dendron 24 and 25 (Figure 35)[161], bear ing respectively 180 and 360 ferrocenyl units at the periphery. These colloids selectively recognize the anions H2PO4- and adenosine-5’-triphosphate (ATP2-). Recognition has been monitored by cyclic voltammetry.
Silver: Recently Kakar reviewed dendrimer templated construction of silver nanoparticles. Up to now, synthesis assisted by dendrimers has led almost exclusively to formation of spherical-shaped silver nanoparticles. It is believed that dendrimers are likely to be more valuable for modulating the size of silver nanoparticles than their shape[162].
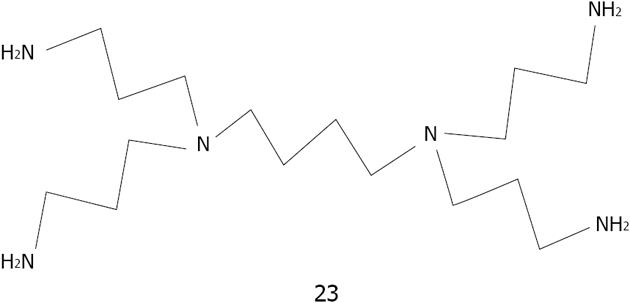
Balogh et al[163] reported that PAMAM Dendrimer 23 attached silver nanoparticles display considerable activity against Staphylococcus aureus, Pseudomonas aeruginosa and E. coli bacteria without the loss of solubility and activity in the presence of sulfate or chloride ions. Balogh has shown that dendrimer silver nanoparticles may find potential application as cell biomarkers[164]. They have synthesized hydroxyl-, and carboxyl-terminated ethylenediamine core generation 5 poly(amidoamine) dendrimers which were utilized to prepare aqueous silver-dendrimer nanoparticles. The hybrid particles are water-soluble, biocompatible, fluorescent, and stable below pH 7.5 The cellular uptake of nanoparticles was examined by transmission electron microscopy and confocal microscopy. Overall, cytotoxicity analysis indicates that the uptake of the hybrid particles is correlated with the surface charge of dendrimer and that the silver has no effect.
Mali reported the synthesis and characterization of a novel electrochemical label for sensitive electrochemical stripping metallo-immunoassays based on silver dendrimer-encapsulated nanoparticles[165]. Several fixed ratios of Ag+/dendrimer were prepared with the aim of obtaining stable nanocomposites with maximal silver loading in the interior of a polymeric shell. By combination of differential pulse voltammetry and anodic stripping analysis on a carbon electrode, individual silver dendrimer-encapsulated nanoparticles (limit of detection is 0.9 pMol) were detected down to 1.35 × 1010 after the dissolution of silver nanoparticles in dilute nitric acid.
Dendrimer silver nanoparticles have been shown to be good catalysts for reactions, such as the reduction of nitrophenol[166], chloronitrobenzene[167] or the 2,7-dicholoroflurescein dye[168].
Copper: Various methods can be found in the literature to produce dendrimer copper nanoparticles, ranging from classical metal reduction using dendrimer as stabilizer[169] to more original electrochemical preparation[170].
Using molecular assembly properties, Moore showed that dendrimer associated copper nanoparticles could be used as a catalyst of Cu+-catalyzed azide-alkyne cycloaddition[171]. Reactivity was tested on a model reaction between azido propanol and propargyl alcohol in aqueous solution. The authors observed up to 120 fold faster conversion using PAMAM dendrimers as macromolecular Cu+ ligands compared to traditional small molecular ligand systems, and demonstrated that the macromolecular catalyst can be removed by ultrafiltration.
Huang has successfully synthesized mono-, di-, and tri-functionalized G5 PAMAM dendrimer conjugates with a copper-free click conjugation method[172]. An azido modified targeting moiety, a therapeutic drug and an imaging reagent were mixed with a G5 PAMAM dendrimer nanoplatform, simultaneously or sequentially, to give mono-, di- and tri-functional conjugates.
Crown ethers: Since Pedersen first reported the synthesis and cation complexation properties the crown ethers in 1967, these neutral synthetic heterocyclic compounds have attracted extensive and continuous attention through their unusual and powerful non-covalent cation binding properties. Classical crown ethers are macrocyclic polyethers that contain 3-20 oxygen atoms, each separated from the next by two or more carbon atoms (Figure 14)[173]. The most effective complexation agents, however, are macrocyclic oligomers of ethyleneoxy units, either substituted or unsubstituted, that contain 5-10 oxygen atoms. They are exceptionally versatile in selectively, binding a range of metal ions and a variety of organic neutral and ionic species. Crown ethers are currently being studied and used in a variety of applications beyond their traditional place in chemistry (used in the laboratory as phase transfer catalysts). In the biological context, they are being investigated as a promising anti-cancer compound[174].
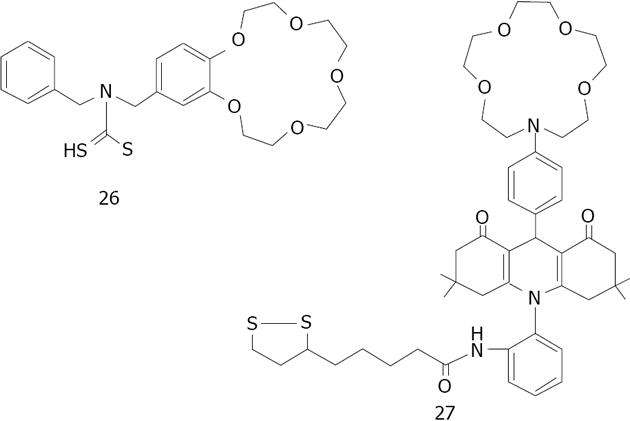
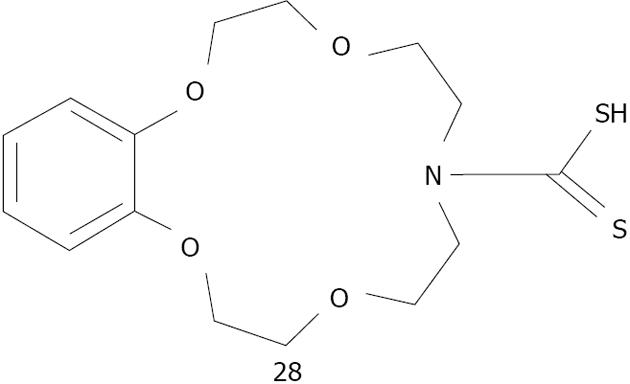
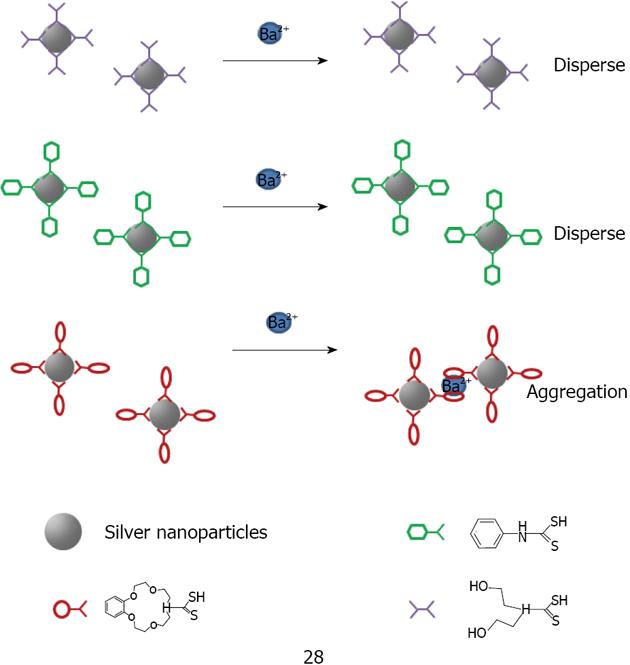
The combination of crown ethers with metallic nanoparticles has been mainly employed as a cation sensor. So far, crown ether capped gold nanoparticles have been used for cation detection using dithiocarbamate modified N-benzyl-4-aminobenzo-15-crown-5-ether 26 for K+[175,176] or aza-15-crown-5-ether acridinedione 27 for Ca2+ and Mg2+[176] (Figure 36). Li et al[177] used silver because of its higher optical extinction ratio (stronger than gold). He capped dithiocarbamate modified aza-15-crown-5-ether 28 on silver nanoparticles with an average size of 8 nm of diameter (Figure 37). Li was able to detect Ba2+ with a detection limit of 1 × 10-8 mol/L. A possible explanation of the aggregation induced with Ba2+ is given by the formation of the sandwich structure with crown ether (Figure 38).
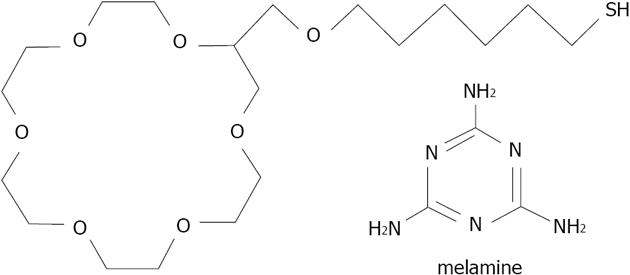
Kwang has developed a colorimetric method of melamine detection, based on 18-crown-6 ether 29 (thiol derivatized) functionalized gold nanoparticles with an average diameter of 20 nm[178] (Figure 39). Melamine is a plant metabolite of cyromazine pesticides and a common chemical. It is also a highly toxic agent used fraudulently in the food industry. Crown ether capped gold nanoparticles enables the detection of melamine in milk after a pre-treatment consisting of centrifugation and purification. The crown ether GNP aggregation induced by the melanine is then monitored by UV-visible spectra with a LOD as low as 6 ppb, a wide linear range from 10 to 500 ppb, and acceptable reproducibility and specificity. This method could be extended to other toxins which show sufficient specificity in relation to the crown ethers and assembly ability (creating bridge between nanoparticles for aggregation).
Cucurbiturils: Cucurbiturils (CBs), are macrocycles derived from glycoluril units, which form stable host-guest complexes with various guest cationic molecules (Figure 14). Cucurbiturils have gained attention due to their unique structure and multiple recognition properties, as well as their potential applications for constructing sensors, drug delivery and biomimetic systems. Geckeler and collaborators initially reported a simple, green, one-pot synthesis of well-dispersed CB capped silver nanoparticles by the reaction of an aqueous silver nitrate solution with CB[7] in the presence of NaOH at room temperature[179]. Furthermore, they have investigated the in vitro cytotoxic properties of the prepared silver nanoparticles against two different human cancer cell types, namely human breast adenocarcinoma (MCF-7) and human lung bronchoalveolar (NCIH358) cells. It was demonstrated that the prepared CB[7]-protected silver nanoparticles, with an average size of about 5 nm, could be suitable candidates for cancer therapy applications. Mason prepared a series of CB capped silver nanoparticles and aggregates by reduction of silver nitrate with sodium borohydride in the presence of different CB[180]. They addressed the impact of CB[n] macrocycles (n = 5-8) on the formation and stabilization of aqueous silver nanoparticles and Ag nanoaggregates obtained from silver nitrate and sodium borohydride, in the absence and presence of a set of positively charged guests shielding one or both portals of the cavitand. While CB[5] and CB[6] caused rapid aggregation and precipitation of Ag aggregates (diameters >13 nm), CB[7] and CB[8] allowed the formation and stabilization of monocrystalline, narrowly dispersed silver nanoparticles (diameters 5.3 and 3.7 nm, respectively). This could be explained by the rigidity of CB[5] and CB[6], and their possible lack of suitable arrangement at the silver surface, giving a poor stabilization of these silver assemblies, while the more flexible CB[7] and CB[8] may undergo some minor distortions and better adapt to the requirements of the metallic surface.
Since hybrid nanoparticles have been investigated as therapeutic agents, a number of studies have investigated their toxicity[181]. More importantly, because of their numerous applications (described above) already high level of production of hybrid nanoparticles is growing and this inevitably leads to their appearance in air, water, soil and organisms[182]. As a consequence the risks to the environment and to human health are significant and should be treated as a concern.
Ray reviewed the different effects on health of different metal nanoparticle preparations[183]. Nanotoxicity studies revealed that the physicochemical characteristics of engineered nanomaterials play an important role in their interactions with living cells[184]. Physicochemical properties that affect the biological activity of hybrid nanoparticles include particle size, shape, surface chemistry, surface area, surface charge and their metallic composition[185].
While bulk gold is considered as “safe”, nanoscale particles of gold need to be examined for biocompatibility and environmental impact if they are to be manufactured on a large scale for in vivo usage[186]. Several researchers have reviewed the toxicity of gold nanoparticles in cells (cytoxicity) and in vivo[187,188]. Murphy reviewed these studies and highlighted some key parameters in the toxicity process[189]. First, the cell type is of critical importance. Patra studied the cell selective response to gold nanoparticles[190] and found that nanoparticles induced death in the A549 human carcinoma lung cell line while two other cell lines tested, BHK21 (baby hamster kidney) and HepG2 (human hepatocellular liver carcinoma), remained unaffected. The second key parameter is the surface charge. Cationic nanoparticles are much more cytotoxic than anionic particles. This may be related to their electrostatic interaction with the negatively charged cell membrane[191]. Size and shape are further critical parameters regarding potential toxicity. Chithrani et al[192] observed that the cellular uptake of gold nanoparticles was greatly size dependent. Spheres of 50 nm were taken up more quickly by cells than either smaller or larger spheres in the 10-100 nm range and spheres were taken up more efficiently than nanorods that had dimensions in the 10-100 nm range[193]. Dikman has reviewed recently in vivo studies concerning gold nanoparticles and came to three conclusions[193]. Firstly, the dose and possible inflammatory processes are of paramount importance for the clearance (process avoiding accumulation in the organs of the reticuloendothelial system, such as spleen or liver) of 10-100 nm gold nanoparticles; Secondly, the effect of nanoparticle penetration via the hematoencephalic barrier depends critically on their size; 5-20 nm being the upper limit; Thirdly, gold nanoparticles of 1-2 nm in diameter could be more toxic due to the possibility of irreversible binding to biopolymers in cells. Also, numerous experiments on cell cultures have revealed no observable toxicity in colloidal particles with a size of 3-100 nm.
Silver was originally used as an effective antimicrobial agent and as a disinfectant, as it was relatively free of adverse effects[194]. However even if silver is believed to be relatively nontoxic to mammalian cells, many in vitro studies have been performed to determine if this is still the case for silver nanoparticles. Several reviews summarize their effect on cells[195-197] in vitro studies have shown that silver nanoparticles have potential to induce toxicity in cells derived from a variety of organs. Chueh proposed a mechanism of cytotoxicity for fibroblast cells[198]. Silver nanoparticles induce cellular death (called apoptosis) by the generation of reactive oxygen species (ROS) and the activation of a specific biochemical pathway, JNK, via the mitochondria.
Despite the fact that silver nanoparticles have been increasingly applied in the biomedical and pharmacological fields, relatively little research has been done into their possible side-effects in clinical medicine[197]. Silver ingestion and topical application can induce the benign condition known as “argyria”, a grey-blue discoloration of the skin and liver caused by deposition of silver particles in the basal laminae of such tissues. Although argyria is not a life-threatening condition it is, however, cosmetically undesirable[199]. Wong et al[200] reviewed and themselves investigated the effect on health of silver nanoparticles. They injected silver nanoparticles intravenously into experimental mice and did not observe any overt systemic effects, despite the silver nanoparticle solution used being at the relatively high concentration of 100 mmol/L. With to this administration route and dose application, silver nanoparticles can be potentially toxic. Intravenous administration may have more implications for adverse effects than dermal application[195].
Compared to gold and silver, the cytotoxicity of copper has been less studied[83]. However, Kim has recently investigated the toxicity of different metal nanoparticles[201]. They used the laser ablation method to generate Ag, Au, Co and Cu NPs in biocompatible aqueous solution. This method consists of intense laser irradiation of a material in solution and leads to an ejection of its constituents and the formation of nanoparticles[202]. This method allows generation of uncapped nanoparticles, allowing investigation of the toxicity of the metal itself. The cytotoxicity assay results demonstrate that nanoparticles possess moderate cytotoxicity to human cells in a cell-dependent manner. The half maximal inhibitory concentration (IC50) has been respectively determined for Hela and PC3 cell lines: 78.9 and 88.6 μg/mL of silver NP; 66.4 and 82.9 μg/mL of gold NP and 85.5 and 91.7 μg/mL of copper NP. Interestingly, the copper nanoparticles showed the lowest cytotoxicity. Valodkar produced 10 nm water soluble copper nanoparticles using starch as stabilizing agent and ascorbic acid as reducing agent[203]. These nanoparticles showed relatively low cytotoxicity (half maximal lethal dose of 100 μg/mL) and a strong bactericidal effect. The Minimum Bactericidal Concentration, the lowest concentration of antibiotic required to kill a particular bacterium, was determined as 3.2 μg/mL for S. Aureus and 1.6 μg/mL for E. coli. Because of their bactericidal effect at non-cytotoxic dose, these copper nanoparticles seem promising as possible therapeutic agents. However, clinical studies on silver and gold nanoparticles are required before considering copper nanoparticles as safe.
In this review we have presented the preparation and physical properties of Noble metal capped nanoparticles. All aspects of molecular recognition of the various types of capping agent derived nanoparticles have been discussed in depth. Finally notice has been taken of the concerns of many people with respect to the possible toxic effects of capped Noble metal nanoparticles.
P- Reviewer Tanaka T S- Editor Wen LL L- Editor A E- Editor Yan JL
| 1. | Hornyak GL, Patrissi CJ, Oberhauser EB, Martin CR, Valmalette JC, Lemaire L, Dutta J, Hofmann H. Effective medium theory characterization of Au/Ag nanoalloy-porous alumina composites. NanoStructured Materials. 1997;9:571-574. [RCA] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Chabanne D, Bobin O, Schvoerer M, Ney C, Sciau PH. Metallic lustre of glazed ceramics: evolution of decorations in search for discriminating elements: Proceedings of 34th International Symposium on Archaeometry; 2004 May 3-7; Zaragoza, Spain. Zaragowza: Sociedad Cooperativa, Libreria General 1997; 327-332. |
| 3. | Needham J. Science and civilisation in China. 4th ed. Cambridge: Cambridge University Press 1997; 268-269. |
| 4. | Kerker M. Founding fathers of light scattering and surface-enhanced Raman scattering. Appl Opt. 1991;30:4699-4705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. |
Faraday M; The Bakerian Lecture: Experimental Relations of Gold (and Other Metals) to Light. |
| 6. |
Strutt W, Rayleigh L; On the scattering of light by small particles. |
| 7. | Mie G. Contribution to the optics of turbid media particulary of colloidal metal solutions. Ann Phys (Leipzig). 1908;25:377-445. |
| 8. | Turkeviche J, Stevenson PC, Hillier J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss Faraday Soc. 1951;11:55-75. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5454] [Cited by in RCA: 5482] [Article Influence: 74.1] [Reference Citation Analysis (0)] |
| 9. | Grzelczak M, Pérez-Juste J, Mulvaney P, Liz-Marzán LM. Shape control in gold nanoparticle synthesis. Chem Soc Rev. 2008;37:1783-1791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1577] [Cited by in RCA: 1122] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 10. | Tréguer-Delapierre M, Majimel J, Mornet S, Duguet E, Ravaine S. Synthesis of non-spherical gold nanoparticles. Gold Bulletin. 2008;41:195-207. [DOI] [Full Text] |
| 11. | Agasti SS, Rana S, Park MH, Kim CK, You CC, Rotello VM. Nanoparticles for detection and diagnosis. Adv Drug Deliv Rev. 2010;62:316-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 201] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 12. | Hajipour MJ, Fromm KM, Ashkarran AA, Jimenez de Aberasturi D, de Larramendi IR, Rojo T, Serpooshan V, Parak WJ, Mahmoudi M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012;30:499-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1641] [Cited by in RCA: 1303] [Article Influence: 100.2] [Reference Citation Analysis (0)] |
| 13. | Arruebo M. Drug delivery from structured porous inorganic materials. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;4:16-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 14. | Upadhyayula VK. Functionalized gold nanoparticle supported sensory mechanisms applied in detection of chemical and biological threat agents: a review. Anal Chim Acta. 2012;715:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 15. | Jain PK, Huang X, El-Sayed IH, El-Sayed MA. Review of some interesting Surface Plasmon Resonance-enhanced properties of noble metal nanoparticles and their applications to biosystems. Plasmonics. 2007;2:107-118. [DOI] [Full Text] |
| 16. | Wang LH, Li J, Song SP, Li D, Fan C. Biomolecular Sensing via Coupling DNA-based Recognition with Gold Nanoparticles. J Phys D Appl Phys. 2009;42:203001. [DOI] [Full Text] |
| 17. | Li XM, WangL , Fan YB, Feng QL, Cui FZ. Biocompatibility and Toxicity of Nanoparticles and Nanotubes. J Nanomater. 2012;2012:1-19. [RCA] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 18. | Feldheim DL, Foss CA. Metal nanoparticles synthesis, characterization, and applications. New York: Marcel Dekker Inc 2001; 360. |
| 19. | Katz E, Willner I. Integrated nanoparticle-biomolecule hybrid systems: synthesis, properties, and applications. Angew Chem Int Ed Engl. 2004;43:6042-6108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2129] [Cited by in RCA: 1643] [Article Influence: 82.2] [Reference Citation Analysis (0)] |
| 20. | Faulk WP, Taylor GM. An immunocolloid method for the electron microscope. Immunochemistry. 1971;8:1081-1083. [PubMed] |
| 21. | Wilson R. The use of gold nanoparticles in diagnostics and detection. Chem Soc Rev. 2008;37:2028-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 434] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 22. | Yu C, Irudayaraj J. Multiplex biosensor using gold nanorods. Anal Chem. 2007;79:572-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 306] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 23. | Niemeyer CM. Nanoparticles, Proteins, and Nucleic Acids: Biotechnology Meets Materials Science. Angew Chem Int Ed. 2001;40:4128-4158. [DOI] [Full Text] |
| 24. | Doria G, Conde J, Veigas B, Giestas L, Almeida C, Assunção M, Rosa J, Baptista PV. Noble metal nanoparticles for biosensing applications. Sensors (Basel). 2012;12:1657-1687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 480] [Cited by in RCA: 344] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 25. | Jain PK, Huang X, El-Sayed IH, El-Sayed MA. Noble metals on the nanoscale: optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc Chem Res. 2008;41:1578-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3225] [Cited by in RCA: 2331] [Article Influence: 137.1] [Reference Citation Analysis (0)] |
| 26. | Chen Y, Ming H. Review of surface plasmon resonance and localized surface plasmon resonance sensor. Photonic Sensors. 2012;2:37-49. [DOI] [Full Text] |
| 27. | Colomban P. The Use of Metal Nanoparticles to Produce Yellow, Red and Iridescent Colour, from Bronze Age to Present Times in Lustre Pottery and Glass: Solid State Chemistry, Spectroscopy and Nanostructure. Nano Res. 2009;8:109-132. [DOI] [Full Text] |
| 28. | Schmid G. Nanoparticles from Theory to Application. Electrical Properties of Metal Nanoparticles. 2nd ed. Germany: Wiley-VCH 2004; 401-454. |
| 29. | Malaquin L, Vieu C, Genevieve M, Tauran Y, Carcenac F, Pourciel ML, Leberre V, Trevisiol V. Nanoelectrode-based devices for electrical biodetection in liquid solution. Microelectron Eng. 2004;73-74:887-892. |
| 30. | Chen YS, Hong MY, Huang GS. A protein transistor made of an antibody molecule and two gold nanoparticles. Nat Nanotechnol. 2012;7:197-203. [PubMed] [DOI] [Full Text] |
| 31. | Sato K, Hosokawa K, Maeda M. Colorimetric biosensors based on DNA-nanoparticle conjugates. Anal Sci. 2007;23:17-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Baptista P, Pereira E, Eaton P, Doria G, Miranda A, Gomes I, Quaresma P, Franco R. Gold nanoparticles for the development of clinical diagnosis methods. Anal Bioanal Chem. 2008;391:943-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 304] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 33. | Dykman L, Khlebtsov N. Gold nanoparticles in biomedical applications: recent advances and perspectives. Chem Soc Rev. 2012;41:2256-2282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1384] [Cited by in RCA: 1188] [Article Influence: 84.9] [Reference Citation Analysis (0)] |
| 34. | Tiwari PM, Vig K, Dennis VA, Singh SR. Functionalized gold nanoparticles and their biomedical applications. Nanomaterials. 2011;1:31-63. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 505] [Cited by in RCA: 470] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 35. | Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ. A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature. 1996;382:607-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5553] [Cited by in RCA: 4120] [Article Influence: 142.1] [Reference Citation Analysis (0)] |
| 36. | Stoltenburg R, Reinemann C, Strehlitz B. SELEX--a (r)evolutionary method to generate high-affinity nucleic acid ligands. Biomol Eng. 2007;24:381-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 981] [Cited by in RCA: 972] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 37. | Nelson DL, Lehninger AL, Cox MM. Lehninger principles of biochemistry. 3rd ed. New York: Worth Publishers, 2008: 1100 44 Pustovit VN, Shahbazyan TV. SERS from molecules adsorbed on small Ag nanoparticles: a microscopic model. Chem Phys Lett. 2006;420:469-473. |
| 38. | Heller MJ. DNA microarray technology: devices, systems, and applications. Annu Rev Biomed Eng. 2002;4:129-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 831] [Cited by in RCA: 618] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 39. | Thompson DG, Enright A, Faulds K, Smith WE, Graham D. Ultrasensitive DNA detection using oligonucleotide-silver nanoparticle conjugates. Anal Chem. 2008;80:2805-2810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 167] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 40. | Grabar KC, Griffith Freeman R, Hommer MB, Natan MJ. Preparation and characterization of Au colloid monolayers. Anal Chem. 1995;67:735-743. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2342] [Cited by in RCA: 1760] [Article Influence: 76.5] [Reference Citation Analysis (0)] |
| 41. | Storhoff JJ, Elghanian R, Mucic RD, Mirkin CA, Letsinger RL. One-Pot colorimetric differentiation of polynucleotides with single base imperfections using gold nanoparticle probes. J Am Chem Soc. 1998;120:1959-1964. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1907] [Cited by in RCA: 1536] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 42. | Taton TA, Mirkin CA, Letsinger RL. Scanometric DNA array detection with nanoparticle probes. Science. 2000;289:1757-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2090] [Cited by in RCA: 1589] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 43. | Sun L, Yu C, Irudayaraj J. Surface-enhanced Raman scattering based nonfluorescent probe for multiplex DNA detection. Anal Chem. 2007;79:3981-3988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 44. | Pustovit VN, Shahbazyan TV. SERS from molecules adsorbed on small Ag nanoparticles: a microscopic model. Chem Phys Lett. 2006;420:469-473. |
| 45. | Rothemund PW. Folding DNA to create nanoscale shapes and patterns. Nature. 2006;440:297-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5119] [Cited by in RCA: 4781] [Article Influence: 251.6] [Reference Citation Analysis (0)] |
| 46. | Borovok N, Gillon E, Kotlyar A. Synthesis and Assembly of Conjugates Bearing Specific Numbers of DNA Strands per Gold Nanoparticle. Bioconjug Chem. 2012;Apr 30; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 47. | Li H, Sun Z, Zhong W, Hao N, Xu D, Chen HY. Ultrasensitive electrochemical detection for DNA arrays based on silver nanoparticle aggregates. Anal Chem. 2010;82:5477-5483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 48. | Zon VB, Burley GA, Rant U. Photo-induced growth of DNA-capped silver nanoparticles. Nanotechnology. 2012;23:115607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 49. | Latorre A, Somoza Á. DNA-mediated silver nanoclusters: synthesis, properties and applications. Chembiochem. 2012;13:951-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 50. | Lan GY, Chen WY, Chang HT. Characterization and application to the detection of single-stranded DNA binding protein of fluorescent DNA-templated copper/silver nanoclusters. Analyst. 2011;136:3623-3628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 51. | Richards CI, Hsiang JC, Senapati D, Patel S, Yu J, Vosch T, Dickson RM. Optically modulated fluorophores for selective fluorescence signal recovery. J Am Chem Soc. 2009;131:4619-4621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 52. | Rotaru A, Dutta S, Jentzsch E, Gothelf K, Mokhir A. Selective dsDNA-templated formation of copper nanoparticles in solution. Angew Chem Int Ed Engl. 2010;49:5665-5667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 260] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 53. | Jia X, Li J, Han L, Ren J, Yang X, Wang E. DNA-hosted copper nanoclusters for fluorescent identification of single nucleotide polymorphisms. ACS Nano. 2012;6:3311-3317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 183] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 54. | Pavlov V, Xiao Y, Shlyahovsky B, Willner I. Aptamer-functionalized Au nanoparticles for the amplified optical detection of thrombin. J Am Chem Soc. 2004;126:11768-11769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 511] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 55. | Huang CC, Huang YF, Cao Z, Tan W, Chang HT. Aptamer-modified gold nanoparticles for colorimetric determination of platelet-derived growth factors and their receptors. Anal Chem. 2005;77:5735-5741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 378] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 56. | Huang YF, Chang HT. Analysis of adenosine triphosphate and glutathione through gold nanoparticles assisted laser desorption/ionization mass spectrometry. Anal Chem. 2007;79:4852-4859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 202] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 57. |
Huang YF, Lin YW, Lin ZH, Chang HT; Aptamer-modified gold nanoparticles for targeting breast cancer cells through light scattering. |
| 58. | Liu G, Mao X, Phillips JA, Xu H, Tan W, Zeng L. Aptamer-nanoparticle strip biosensor for sensitive detection of cancer cells. Anal Chem. 2009;81:10013-10018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 244] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 59. | Wang Y, Li Z, Li H, Vuki M, Xu D, Chen HY. A novel aptasensor based on silver nanoparticle enhanced fluorescence. Biosens Bioelectron. 2012;32:76-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 60. | Aslan K, Holley P, Geddes CD. Metal-enhanced fluorescence from silver nanoparticle-deposited polycarbonate substrates. J Mater Chem. 2006;16:2846-2852. [RCA] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 61. | Dreaden EC, Alkilany AM, Huang X, Murphy CJ, El-Sayed MA. The golden age: gold nanoparticles for biomedicine. Chem Soc Rev. 2012;41:2740-2779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2693] [Cited by in RCA: 2097] [Article Influence: 161.3] [Reference Citation Analysis (0)] |
| 62. | Kim SB, Hattori M, Ozawa T. Intelligent design of nano-scale molecular imaging agents. Int J Mol Sci. 2012;13:16986-17005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 63. | Van Dorst B, Mehta J, Bekaert K, Rouah-Martin E, De Coen W, Dubruel P, Blust R, Robbens J. Recent advances in recognition elements of food and environmental biosensors: a review. Biosens Bioelectron. 2010;26:1178-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 64. | Willner I, Basnar B, Willner B. Nanoparticle-enzyme hybrid systems for nanobiotechnology. FEBS J. 2007;274:302-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 121] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 66. | Kogan MJ, Olmedo I, Hosta L, Guerrero AR, Cruz LJ, Albericio F. Peptides and metallic nanoparticles for biomedical applications. Nanomedicine (Lond). 2007;2:287-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 67. | Pasquato L, Pengo P, Scrimin P. Nanozymes: Functional nanoparticle-based catalysts. Supramol Chem. 2005;17:163-171. [RCA] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 68. | Lévy R. Peptide-capped gold nanoparticles: towards artificial proteins. Chembiochem. 2006;7:1141-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 69. | Pengo P, Baltzer L, Pasquato L, Scrimin P. Substrate modulation of the activity of an artificial nanoesterase made of peptide-functionalized gold nanoparticles. Angew Chem Int Ed Engl. 2007;46:400-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 70. | Wang Z, Lévy R, Fernig DG, Brust M. Kinase-catalyzed modification of gold nanoparticles: a new approach to colorimetric kinase activity screening. J Am Chem Soc. 2006;128:2214-2215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 209] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 71. | Sun L, Liu D, Wang Z. Microarray-based kinase inhibition assay by gold nanoparticle probes. Anal Chem. 2007;79:773-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 72. | Yguerabide J, Yguerabide EE. Resonance light scattering particles as ultrasensitive labels for detection of analytes in a wide range of applications. J Cell Biochem Suppl. 2001;Suppl 37:71-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 89] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 73. | Wang Z, Lévy R, Fernig DG, Brust M. The peptide route to multifunctional gold nanoparticles. Bioconjug Chem. 2005;16:497-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 74. | Tkachenko AG, Xie H, Coleman D, Glomm W, Ryan J, Anderson MF, Franzen S, Feldheim DL. Multifunctional gold nanoparticle-peptide complexes for nuclear targeting. J Am Chem Soc. 2003;125:4700-4701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 509] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 75. | Huang X, Peng X, Wang Y, Wang Y, Shin DM, El-Sayed MA, Nie S. A reexamination of active and passive tumor targeting by using rod-shaped gold nanocrystals and covalently conjugated peptide ligands. ACS Nano. 2010;4:5887-5896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 319] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 76. | Graf P, Mantion A, Foelske A, Shkilnyy A, Masić A, Thünemann AF, Taubert A. Peptide-coated silver nanoparticles: synthesis, surface chemistry, and pH-triggered, reversible assembly into particle assemblies. Chemistry. 2009;15:5831-5844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 77. | Garrido C, Aliaga AE, Gomez-Jeria JS, Clavijo RE, Campos-Vallette MM, Sanchez-Cortes S. Adsorption of oligopeptides on silver nanoparticles: surface-enhanced Raman scattering and theoretical studies. J Raman Spectrosc. 2010;41:1149-1155. [DOI] [Full Text] |
| 78. | Cui Y, Wang Y, Liu R, Sun Z, Wei Y, Zhao Y, Gao X. Serial silver clusters biomineralized by one peptide. ACS Nano. 2011;5:8684-8689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 79. | Wang X, Wua L, Ren J, Miyoshi D, Sugimoto N, Qu X. Label-free colorimetric and quantitative detection of cancer marker protein using noncrosslinking aggregation of Au/Ag nanoparticles induced by target-specific peptide probe. Biosens Bioelectron. 2011;26:4804-4809. [RCA] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 80. | Amato E, Diaz-Fernandez YA, Taglietti A, Pallavicini P, Pasotti L, Cucca L, Milanese C, Grisoli P, Dacarro C, Fernandez-Hechavarria JM. Synthesis, characterization and antibacterial activity against Gram positive and Gram negative bacteria of biomimetically coated silver nanoparticles. Langmuir. 2011;27:9165-9173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 81. | Taglietti A, Diaz Fernandez YA, Amato E, Cucca L, Dacarro G, Grisoli P, Necchi V, Pallavicini P, Pasotti L, Patrini M. Antibacterial activity of glutathione-coated silver nanoparticles against Gram positive and Gram negative bacteria. Langmuir. 2012;28:8140-8148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 199] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 82. | Wei QS, Ji J, Fu JH, Shen JC. Norvancomycin-capped silver nanoparticles: Synthesis and antibacterial activities against E. coli. Sci China B. 2007;50:418-424. [RCA] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 83. | Valodkar V, Jadeja RN, Thounaojam MC, Devkar RV, Thakore S. Biocompatible synthesis of peptide capped copper nanoparticles and their biological effect on tumor cells. Mater Chem Phys. 2011;128:83-89. [DOI] [Full Text] |
| 84. | Hosseini MR, Schaffie M, Pazouki M, Darezereshki E, Ranjbar M. Biologically synthesized copper sulfide nanoparticles: Production and characterization. Materials Science in Semiconductor Processing. 2012;15:222-225. [DOI] [Full Text] |
| 85. | Lesk AM, Chothia C. Evolution of proteins formed by β-sheets: II. The core of the immunoglobulin domains. J Mol Biol. 1982;160:325-342. [RCA] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 212] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 86. | Arruebo M, Valladares M, Gonzalez-Fernandez A. Antibody-conjugated nanoparticles for biomedical applications. J Nanomater. 2009;2009:1-24. [DOI] [Full Text] |
| 87. | Horisberger M. Colloidal gold as a cytochemical marker in electron microscopy. Gold Bull. 1981;14:90-94. [DOI] [Full Text] |
| 88. | Sokolov K, Follen M, Aaron J, Pavlova I, Malpica A, Lotan R, Richards-Kortum R. Real-time vital optical imaging of precancer using anti-epidermal growth factor receptor antibodies conjugated to gold nanoparticles. Cancer Res. 2003;63:1999-2004. [PubMed] |
| 89. | El-Sayed IH, Huang X, El-Sayed MA. Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: applications in oral cancer. Nano Lett. 2005;5:829-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1524] [Cited by in RCA: 1043] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 90. | Popovtzer R, Agrawal A, Kotov NA, Popovtzer A, Balter J, Carey TE, Kopelman R. Targeted gold nanoparticles enable molecular CT imaging of cancer. Nano Lett. 2008;8:4593-4596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 671] [Cited by in RCA: 529] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 91. | Ankri R, Peretz V, Motiei M, Popovtzer R, Fixler D. A new method for cancer detection based on diffusion reflection measurements of targeted gold nanorods. Int J Nanomedicine. 2012;7:449-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 92. | Huang HC, Barua S, Sharma G, Dey SK, Rege K. Inorganic nanoparticles for cancer imaging and therapy. J Control Release. 2011;155:344-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 364] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 93. | Park C, Youn H, Kim H, Noh T, Kook YH, Oh ET, Park HJ, Kim C. Cyclodextrin-covered gold nanoparticles for targeted delivery of an anti-cancer drug. J Mater Chem. 2009;19:2310-2315. [RCA] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 151] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 94. | Jiang W, Kim BY, Rutka JT, Chan WC. Nanoparticle-mediated cellular response is size-dependent. Nat Nanotechnol. 2008;3:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2173] [Cited by in RCA: 1979] [Article Influence: 116.4] [Reference Citation Analysis (0)] |
| 95. | Kennedy LC, Bickford LR, Lewinski NA, Coughlin AJ, Hu Y, Day ES, West JL, Drezek RA. A new era for cancer treatment: gold-nanoparticle-mediated thermal therapies. Small. 2011;7:169-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 543] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 96. | Akhter S, Ahmad MZ, Ahmad FJ, Storm G, Kok RJ. Gold nanoparticles in theranostic oncology: current state-of-the-art. Expert Opin Drug Deliv. 2012;9:1225-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 97. | Alkilany AM, Thompson LB, Boulos SP, Sisco PN, Murphy CJ. Gold nanorods: their potential for photothermal therapeutics and drug delivery, tempered by the complexity of their biological interactions. Adv Drug Deliv Rev. 2012;64:190-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 517] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 98. | Thanh NT, Rees JH, Rosenzweig Z. Laser-based double beam absorption detection for aggregation immunoassays using gold nanoparticles. Anal Bioanal Chem. 2002;374:1174-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 99. | Thanh NT, Rosenzweig Z. Development of an aggregation-based immunoassay for anti-protein A using gold nanoparticles. Anal Chem. 2002;74:1624-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 303] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 100. | Murphy CJ, Gole AM, Hunyadi SE, Stone JW, Sisco PN, Alkilany A, Kinard BE, Hankins P. Chemical sensing and imaging with metallic nanorods. Chem Commun (Camb). 2008;544-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 334] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 101. | Yuan Y, Zhang J, Zhang H, Yang X. Silver nanoparticle based label-free colorimetric immunosensor for rapid detection of neurogenin 1. Analyst. 2012;137:496-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 102. | Wang J. Electrochemical biosensing based on noble metal nanoparticles. Microchim Acta. 2012;177:245-270. [RCA] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 103. | Szymanski M, Turner APF, Porter R. Electrochemical dissolution of silver nanoparticles and its application in metalloimmunoassay. Electroanalysis. 2010;22:191-198. [RCA] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 104. | Hao N, Li H, Long Y, Zhang L, Zhao X, Xu D, Chen HY. An electrochemical immunosensing method based on silver nanoparticles. J Electroanal Chem. 2011;656:50-54. [RCA] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 105. | Duncan TV. Applications of nanotechnology in food packaging and food safety: barrier materials, antimicrobials and sensors. J Colloid Interface Sci. 2011;363:1-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1538] [Cited by in RCA: 784] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 106. | Gilmartin N, O’Kennedy R. Nanobiotechnologies for the detection and reduction of pathogens. Enzyme Microb Technol. 2012;50:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 107. | Lara HH, Ixtepan-Turrent L, Garza Treviño EN, Singh DK. Use of silver nanoparticles increased inhibition of cell-associated HIV-1 infection by neutralizing antibodies developed against HIV-1 envelope proteins. J Nanobiotechnology. 2011;9:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 108. | Zhang X, Geng P, Liu H, Teng Y, Liu Y, Wang Q, Zhang W, Jin L, Jiang L. Development of an electrochemical immunoassay for rapid detection of E. coli using anodic stripping voltammetry based on Cu@Au nanoparticles as antibody labels. Biosens Bioelectron. 2009;24:2155-2159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 109. | Qu W, Liu Y, Liu D, Wang Z, Jiang X. Copper-mediated amplification allows readout of immunoassays by the naked eye. Angew Chem Int Ed Engl. 2011;50:3442-3445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 173] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 110. | Lehn JM. Supramolecular chemistry. Science. 1993;260:1762-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 497] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 111. | Steed JW, Atwood JL. Supramolecular Chemistry. 2nd ed. UK: John Wiley and Sons 2009; . [DOI] [Full Text] |
| 112. | Saenger W. Cyclodextrin inclusion compounds in research and industry. Angew Chem Int Ed. 1980;19:344-362. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1605] [Cited by in RCA: 1386] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 113. | Atwood JL, Davies JED, MacNicol DD, Votgle F. Comprehensive Supramolecular Chemistry: Cyclodextrine. Vol. 3. 1 st ed. Oxford: Pergamon 1996; . |
| 114. | Liu J, Alvarez J, Kaifer AE. Metal nanoparticles with a knack for molecular recognition. Adv Mater. 2000;12:1381-1383. [DOI] [Full Text] |
| 115. | Liu J, Renliang X, Kaifer AE. In Situ modification of the surface of gold colloidal particles. Preparation of cyclodextrin-based rotaxanes supported on gold nanospheres. Langmuir. 1998;14:7337-7339. [DOI] [Full Text] |
| 116. | Liu J, Ong W, Roman E, Lynn MJ, Kaifer AE. Cyclodextrin-modified gold nanospheres. Langmuir. 2000;16:3000-3002. [DOI] [Full Text] |
| 117. | Liu J, Alvarez J, Ong W, Kaifer AE. Network aggregates formed by C60 and gold nanoparticles capped with gamma-Cyclodextrin hosts. Nano Lett. 2001;1:57-60. [DOI] [Full Text] |
| 118. | Liu Z, Jiang M. Reversible aggregation of gold nanoparticles driven by inclusion complexation. J Mater Chem. 2007;17:4249-4254. [RCA] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 119. | Chen Z, Li J, Zhang X, Wu Z, Zhang H, Sun H, Yang B. Construction of nanoparticle superstructures on the basis of host-guest interaction to achieve performance integration and modulation. Phys Chem Chem Phys. 2012;14:6119-6125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 120. | Wei K, Li J, Liu J, Chen G, Jiang M. Reversible vesicles of supramolecular hybrid nanoparticles. Soft Matter. 2012;8:3300-3303. [DOI] [Full Text] |
| 121. | Li X, Qi Z, Liang K, Bai X, Xu J, Liu J, Shen J. An artificial supramolecular nanozyme based on b-Cyclodextrin-modified gold nanoparticles. Catal Letters. 2008;124:413-417. [RCA] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 122. | Ng CHB, Yang J, Fan WY. Synthesis and self-assembly of One-dimensional sub-10 nm Ag nanoparticles with cyclodextrin. J Phys Chem C. 2008;112:4141-4145. [DOI] [Full Text] |
| 123. | Hebeish A, El-Shafei A, Sharaf S, Zaghloul S. Novel precursors for green synthesis and application of silver nanoparticles in the realm of cotton finishing. Carbohydr Polym. 2011;84:605-613. [RCA] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 124. | Jaiswal S, Duffy B, Jaiswal AK, Stobie N, McHale P. Enhancement of the antibacterial properties of silver nanoparticles using beta-cyclodextrin as a capping agent. Int J Antimicrob Agents. 2010;36:280-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 125. | Wang S, Bai J, Li C, Zhang Y, Zhang J. Ag nanoparticle-embedded one-dimensional β-CD/PVP composite nanofibers prepared via electrospinning for use in antibacterial material. Colloid Polym Sci. 2012;290:667-672. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 126. | Xie Y, Wang X, Han X, Xue X, Ji W, Qi Z, Liu J, Zhao B, Ozaki Y. Sensing of polycyclic aromatic hydrocarbons with cyclodextrin inclusion complexes on silver nanoparticles by surface-enhanced Raman scattering. Analyst. 2010;135:1389-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 127. | Chen X, Parker SG, Zou G, Su W, Zhang Q. β-cyclodextrin-functionalized silver nanoparticles for the naked eye detection of aromatic isomers. ACS Nano. 2010;4:6387-6394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 128. | Xu JZ, Xu S, Geng J, Li GX, Zhu JJ. The fabrication of hollow spherical copper sulfide nanoparticle assemblies with 2-hydroxypropyl-beta-cyclodextrin as a template under sonication. Ultrason Sonochem. 2006;13:451-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 129. | Premkumar T, Geckeler KE. A green approach to fabricate CuO nanoparticles. J Phys Chem Solids. 2006;67:1451-1456. [DOI] [Full Text] |
| 130. | Coleman AW, Jebors S, Cecillon S, Perret P, Garin D, Marti-Battle D, Moulin M. Toxicity and biodistribution of para-sulfonato-calix[4]arene in mice. New J Chem. 2008;32:780-782. [RCA] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 131. | Perret F, Coleman AW. Biochemistry of anionic calix[n]arenes. Chem Commun (Camb). 2011;47:7303-7319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 205] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 132. | Danylyuk O, Suwinska K. Solid-state interactions of calixarenes with biorelevant molecules. Chem Commun (Camb). 2009;5799-5813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 133. | Acharya A, Samanta K, Pulla Rao C. Conjugates of calixarenes emerging as molecular entities of nanoscience. Coord Chem Rev. 2012;256:2096-2125. [RCA] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 134. | Kim HJ, Lee MH, Mutihac L, Vicens J, Kim JS. Host-guest sensing by calixarenes on the surfaces. Chem Soc Rev. 2012;41:1173-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 135. | Tshikhudo TR, Demuru D, Wang Z, Brust M, Secchi A, Arduini A, Pochini A. Molecular recognition by calix[4]arene-modified gold nanoparticles in aqueous solution. Angew Chem Int Ed Engl. 2005;44:2913-2916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 136. | Arduini A, Demuru D, Pochini A, Secchi A. Recognition of quaternary ammonium cations by calix[4]arene derivatives supported on gold nanoparticles. Chem Commun (Camb). 2005;645-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 137. | Patel G, Menon S. Recognition of lysine, arginine and histidine by novel p-sulfonatocalix[4]arene thiol functionalized gold nanoparticles in aqueous solution. Chem Commun (Camb). 2009;3563-3565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 138. | Han C, Zeng L, Li H, Xie G. Colorimetric detection of pollutant aromatic amines isomers with p-sulfonatocalix[6]arene-modified gold nanoparticles. Sens Actuators B Chem. 2009;137:704-709. [DOI] [Full Text] |
| 139. | Yan H, Luo J, Xie HM, Xie DX, Su Q, Yin J, Wanjala BN, Diao H, An DL, Zhong CJ. Cationic recognition by tert-butylcalix[4]arene-functionalized nanoprobes. Phys Chem Chem Phys. 2011;13:5824-5830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 140. | Pandya A, Joshi KV, Modi NR, Menon SK. Rapid colorimetric detection of sulfide using calix[4]arene modified gold nanoparticles as a probe. Sens Actuators B Chem. 2012;168:54-61. [DOI] [Full Text] |
| 141. | Guerrini L, Garcia-Ramos JV, Domingo C, Sanchez-Cortes S. Functionalization of Ag nanoparticles with dithiocarbamate calix[4]arene as an effective supramolecular host for the surface-enhanced Raman scattering detection of polycyclic aromatic hydrocarbons. Langmuir. 2006;22:10924-10926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 142. | Chen M, Ding W, Kong Y, Diao G. Conversion of the surface property of oleic acid stabilized silver nanoparticles from hydrophobic to hydrophilic based on host-guest binding interaction. Langmuir. 2008;24:3471-3478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 143. | Hartlieb KJ, Saunders M, Raston CL. Templating silver nanoparticle growth using phosphonated calixarenes. Chem Commun (Camb). 2009;3074-3076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 144. | Hartlieb KJ, Martin AD, Saunders M, Raston CL. Photochemical generation of small silver nanoparticles involving multi-functional phosphonated calixarenes. New J Chem. 2010;34:1834-1837. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 145. | Xiong D, Chen M, Li H. Synthesis of para-sulfonatocalix[4]arene-modified silver nanoparticles as colorimetric histidine probes. Chem Commun (Camb). 2008;880-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 151] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 146. | Bian Y, Li C, Li H. para-Sulfonatocalix[6]arene-modified silver nanoparticles electrodeposited on glassy carbon electrode: preparation and electrochemical sensing of methyl parathion. Talanta. 2010;81:1028-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 147. | Tauran Y, Grosso M, Brioude A, Kassab R, Coleman AW. Colourimetric and spectroscopic discrimination between nucleotides and nucleosides using para-sulfonato-calix[4]arene capped silver nanoparticles. Chem Commun (Camb). 2011;47:10013-10015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 148. | Tauran Y, Rhimi M, Ueno R, Grosso M, Brioude A, Janneau E, Suwinska K, Kassab R, Shahgaldian P, Cumbo A. Cytosine: para-sulphonato-calix[4]arene assemblies: in solution, in the solid-state and on the surface of hybrid silver nanoparticles. J Incl Phenom Macrocycl Chem. 2012;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 149. | Perret F, Tauran Y, Suwinska K, Kim BJ, Chassain-Nely C, Boulet M, Coleman AW. Molecular recognition and transport of active pharmaceutical ingredients on anionic calix[4]arene capped silver nanoparticles. J Chem. 2013;2013:1-9. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 150. | Tauran Y, Brioude A, Shahgaldian P, Cumbo A, Kim B, Perret F, Coleman AW, Montasser I. Calix-arene silver nanoparticles interactions with surfactants are charge, size and critical micellar concentration dependent. Chem Commun (Camb). 2012;48:9483-9485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 151. | Chen M, Diao G, Zhou X. Formation of nanospheres of cuprous oxide with a bridge linker of p-sulfonated calix[8]arene host. Nanotechnology. 2007;18:1-10. [DOI] [Full Text] |
| 152. | Nanjwade BK, Bechra HM, Derkar GK, Manvi FV, Nanjwade VK. Dendrimers: emerging polymers for drug-delivery systems. Eur J Pharm Sci. 2009;38:185-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 307] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 153. | Bronstein LM, Shifrina ZB. Dendrimers as encapsulating, stabilizing, or directing agents for inorganic nanoparticles. Chem Rev. 2011;111:5301-5344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 212] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 154. | Chechik V, Crooks RM. Monolayers of thiol-terminated dendrimers on the surface of planar and colloidal gold. Langmuir. 1999;15:6364-6369. [DOI] [Full Text] |
| 155. | Crooks RM, Zhao M, Sun L, Chechik V, Yeung LK. Dendrimer-encapsulated metal nanoparticles: synthesis, characterization, and applications to catalysis. Acc Chem Res. 2001;34:181-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1834] [Cited by in RCA: 1371] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 156. | Lu W, Shang J. A resonance light-scattering (RLS) serving for various quantitative events since 1995: a comment proposed towards how to apprehend well the meaning of RLS and its corresponding guiding role. Spectrochim Acta A Mol Biomol Spectrosc. 2009;74:285-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 157. | Liu Z, Luo L, Dong Y, Weng G, Li J. Resonance scattering amplification assay of biomolecules based on the biomineralization of gold nanoparticles bioconjugates. J Colloid Interface Sci. 2011;363:182-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 158. | Shi X, Wang SH, Van Antwerp ME, Chen X, Baker JR. Targeting and detecting cancer cells using spontaneously formed multifunctional dendrimer-stabilized gold nanoparticles. Analyst. 2009;134:1373-1379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 159. | Wang R, Yang J, Zheng Z, Carducci MD, Jiao J, Seraphin S. Dendron-Controlled Nucleation and Growth of Gold Nanoparticles We thank the University of Arizona and the Research Corporation for financial support. Acknowledgement is also made to the donors of the Petroleum Research Fund, administered by the American Chemical Society, for partial support of this research. The X-ray diffractometer was purchased with support from the U.S. National Science Foundation (CHEM-9610374). We thank Dr. K. Nebesny for help with the XPS experiments. Angew Chem Int Ed Engl. 2001;40:549-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 160. | Astruc D, Daniel MC, Ruiz J. Dendrimers and gold nanoparticles as exo-receptors sensing biologically important anions. Chem Commun (Camb). 2004;2637-2649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 150] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 161. | Daniel MC, Ruiz J, Nlate S, Blais JC, Astruc D. Nanoscopic assemblies between supramolecular redox active metallodendrons and gold nanoparticles: synthesis, characterization, and selective recognition of H2PO4-, HSO4-, and adenosine-5’-triphosphate (ATP2-) anions. J Am Chem Soc. 2003;125:2617-2628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 174] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 162. | Castonguay A, Kakkar AK. Dendrimer templated construction of silver nanoparticles. Adv Colloid Interface Sci. 2010;160:76-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 163. | Balogh L, Swanson DR, Tomalia DA, Hagnauer GL, McManus AT. Dendrimer-silver complexes and nanocomposites as antimicrobial agents. Nano Letters. 2001;1:18-21. [DOI] [Full Text] |
| 164. | Lesniak W, Bielinska AU, Sun K, Janczak KW, Shi X, Baker JR, Balogh LP. Silver/dendrimer nanocomposites as biomarkers: fabrication, characterization, in vitro toxicity, and intracellular detection. Nano Lett. 2005;5:2123-2130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 163] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 165. | Stofik M, Strýhal Z, Malý J. Dendrimer-encapsulated silver nanoparticles as a novel electrochemical label for sensitive immunosensors. Biosens Bioelectron. 2009;24:1918-1923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 166. | Esumi K, Isono R, Yoshimura T. Preparation of PAMAM- and PPI-metal (silver, platinum, and palladium) nanocomposites and their catalytic activities for reduction of 4-nitrophenol. Langmuir. 2004;20:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 394] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 167. | Manna A, Imae T, Aoi K, Okada M, Yogo T. Synthesis of dendrimer-passivated noble metal nanoparticles in a polar medium: comparison of size between silver and gold particles. Chem Mater. 2001;13:1674-1681. [DOI] [Full Text] |
| 168. | Li L, Cao X, Yu F, Yao Z, Xie Y. G1 dendrimers-mediated evolution of silver nanostructures from nanoparticles to solid spheres. J Colloid Interface Sci. 2003;261:366-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 169. | Wei X, Zhu B, Xu Y. Preparation and stability of copper particles formed using the template of hyperbranched poly(amine-ester). Colloid Polym Sci. 2005;284:102-107. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 170. | Berchmans S, Vergheese TM, Kavitha AL, Veerakumar M, Yegnaraman V. Electrochemical preparation of copper-dendrimer nanocomposites: picomolar detection of Cu2+ ions. Anal Bioanal Chem. 2008;390:939-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 171. | Moore E, McInnes SJ, Vogt A, Voelcker NH. Rapid aqueous ‘click chemistry’ using Cu(I)-loaded dendrimers as macromolecular catalysts. Tetrahedron Lett. 2011;52:2327-2329. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 172. | Huang B, Kukowska-Latallo JF, Tang S, Zong H, Johnson KB, Desai A, Gordon CL, Leroueil PR, Baker JR. The facile synthesis of multifunctional PAMAM dendrimer conjugates through copper-free click chemistry. Bioorg Med Chem Lett. 2012;22:3152-3156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 173. | Kralj M, Tusek-Bozić L, Frkanec L. Biomedical potentials of crown ethers: prospective antitumor agents. ChemMedChem. 2008;3:1478-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 174. | Patel G, Kumar A, Pal U, Menon S. Potassium ion recognition by facile dithiocarbamate assembly of benzo-15-crown-5-gold nanoparticles. Chem Commun (Camb). 2009;1849-1851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 175. | Ho ML, Hsieh JM, Lai CW, Peng HC, Kang CC, Wu IC, Lai CH, Chen YC, Chou PT. 15-Crown-5 Functionalized Au nanoparticles synthesized via single molecule exchange on silica nanoparticles: Its application to probe 15-crown-5/K+/15-crown-5 "sandwiches" as linking mechanisms. J Phys Chem C. 2009;113:1686-1693. [DOI] [Full Text] |
| 176. | Velu R, Ramakrishnan VT, Ramamurthy P. Colorimetric and fluorometric chemosensors for selective signaling toward Ca2 and Mg2 by aza-crown ether acridinedione-functionalized gold nanoparticles. Tetrahedron Lett. 2010;51:4331-4335. [RCA] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 177. | Li H, Zhang L, Yao Y, Han C, Jin S. Synthesis of aza-crown ether-modified silver nanoparticles as colorimetric sensors for Ba2. Supramol Chem. 2010;22:544-547. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 178. | Kuang H, Chen W, Yan W, Xu L, Zhu Y, Liu L, Chu H, Peng C, Wang L, Kotov NA. Crown ether assembly of gold nanoparticles: melamine sensor. Biosens Bioelectron. 2011;26:2032-2037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 179. | Premkumar T, Lee Y, Geckeler KE. Macrocycles as a tool: a facile and one-pot synthesis of silver nanoparticles using cucurbituril designed for cancer therapeutics. Chemistry. 2010;16:11563-11566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 180. | Lu X, Masson E. Formation and Stabilization of Silver Nanoparticles with Cucurbit[n]urils (n = 5-8) and Cucurbituril-Based Pseudorotaxanes in Aqueous Medium. Langmuir. 2011;Feb 15; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 181. | Klien K, Godnić-Cvar J. Genotoxicity of metal nanoparticles: focus on in vivo studies. Arh Hig Rada Toksikol. 2012;63:133-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 182. | Elsaesser A, Howard CV. Toxicology of nanoparticles. Adv Drug Deliv Rev. 2012;64:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 489] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 183. | Ray PC, Yu H, Fu PP. Toxicity and environmental risks of nanomaterials: challenges and future needs. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27:1-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 660] [Cited by in RCA: 333] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 184. | Auffan M, Rose J, Wiesner MR, Bottero JY. Chemical stability of metallic nanoparticles: a parameter controlling their potential cellular toxicity in vitro. Environ Pollut. 2009;157:1127-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 312] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 185. | Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823-839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5732] [Cited by in RCA: 4148] [Article Influence: 207.4] [Reference Citation Analysis (0)] |
| 186. | Fadeel B, Garcia-Bennett AE. Better safe than sorry: Understanding the toxicological properties of inorganic nanoparticles manufactured for biomedical applications. Adv Drug Deliv Rev. 2010;62:362-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 425] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 187. | Lewinski N, Colvin V, Drezek R. Cytotoxicity of nanoparticles. Small. 2008;4:26-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2181] [Cited by in RCA: 1768] [Article Influence: 104.0] [Reference Citation Analysis (0)] |
| 188. | Papasani MR, Wang G, Hill RA. Gold nanoparticles: the importance of physiological principles to devise strategies for targeted drug delivery. Nanomedicine. 2012;8:804-814. [PubMed] |
| 189. | Murphy CJ, Gole AM, Stone JW, Sisco PN, Alkilany AM, Goldsmith EC, Baxter SC. Gold nanoparticles in biology: beyond toxicity to cellular imaging. Acc Chem Res. 2008;41:1721-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1453] [Cited by in RCA: 1165] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 190. | Patra HK, Banerjee S, Chaudhuri U, Lahiri P, Dasgupta AK. Cell selective response to gold nanoparticles. Nanomedicine. 2012;8:897-900. [RCA] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 191. | Goodman CM, McCusker CD, Yilmaz T, Rotello VM. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug Chem. 2004;15:897-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1174] [Cited by in RCA: 1019] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 192. | Chithrani BD, Ghazani AA, Chan WC. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6:662-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3715] [Cited by in RCA: 3237] [Article Influence: 170.4] [Reference Citation Analysis (0)] |
| 193. | Dykman LA, Khlebtsov NG. Gold nanoparticles in biology and medicine: recent advances and prospects. Acta Naturae. 2011;3:34-55. [PubMed] |
| 194. | Uygur F, Oncül O, Evinç R, Diktas H, Acar A, Ulkür E. Effects of three different topical antibacterial dressings on Acinetobacter baumannii-contaminated full-thickness burns in rats. Burns. 2009;35:270-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 195. | Yildirimer L, Thanh NT, Loizidou M, Seifalian AM. Toxicology and clinical potential of nanoparticles. Nano Today. 2011;6:585-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 394] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 196. | Chernousova S, Epple M. Silver as antibacterial agent: ion, nanoparticle, and metal. Angew Chem Int Ed Engl. 2013;52:1636-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1516] [Cited by in RCA: 1343] [Article Influence: 103.3] [Reference Citation Analysis (0)] |
| 197. | Ahamed M, Alsalhi MS, Siddiqui MK. Silver nanoparticle applications and human health. Clin Chim Acta. 2010;411:1841-1848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 887] [Cited by in RCA: 732] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 198. | Hsin YH, Chen CF, Huang S, Shih TS, Lai PS, Chueh PJ. The apoptotic effect of nanosilver is mediated by a ROS- and JNK-dependent mechanism involving the mitochondrial pathway in NIH3T3 cells. Toxicol Lett. 2008;179:130-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 605] [Cited by in RCA: 561] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 199. | Fung MC, Bowen DL. Silver products for medical indications: risk-benefit assessment. J Toxicol Clin Toxicol. 1996;34:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 131] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 200. | Wong KKY, Liu X. Silver nanoparticles-the real ‘’silver bullet’’ in clinical medicine. Med Chem Commun. 2010;1:125-131. [RCA] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 236] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 201. | Kim YS, Kim KK, Shin S, Park SM, Hah SS. Comparative toxicity studies of ultra-pure Ag, Au, Co, and Cu nanoparticles generated by Laser Ablation in biocompatible aqueous solution. Bul Korean Chem Soc. 2012;33:3265-3268. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 202. | Ullmann M, Friedlander SK, Schmidt-Ott A. Nanoparticle formation by laser ablation. J Nanopart Res. 2002;4:499-509. [DOI] [Full Text] |
| 203. | Valodkar M, Rathore PS, Jadeja RN, Thounaojam M, Devkar RV, Thakore S. Cytotoxicity evaluation and antimicrobial studies of starch capped water soluble copper nanoparticles. J Hazard Mater. 2012;201-202:244-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 204. | Ma Z, Wu J, Zhou T, Chen Z, Dong Y, Tang J, Sui SF. Detection of human lung carcinoma cell using quartz crystal microbalance amplified by enlarging Au nanoparticles in vitro. New J Chem. 2002;26:1795-1798. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 205. | Sylvestre JP, Kabashin AV, Sacher E, Meunier M, Luong JH. Stabilization and size control of gold nanoparticles during laser ablation in aqueous cyclodextrins. J Am Chem Soc. 2004;126:7176-7177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 164] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 206. | Biji P, Patnaik A. Novel crown ether-capped cationic gold nanoclusters in an aqueous medium and their single-electron charging features. Langmuir. 2007;23:12048-12054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 207. | Yeh CH, Chen WT, Lin HP, Chang TC, Lin YC. Development of an immunoassay based on impedance measurements utilizing an antibody-nanosilver probe, silver enhancement, and electro-microchip. Sens Actuators B Chem. 2009;139:387-393. [DOI] [Full Text] |
| 208. | Yang Y, Liu S, Kimura K. Cyclodextrin as a capturing agent for redundant surfactants on Ag nanoparticle surface in phase transfer process. Colloids Surf A Physicochem Eng Asp. 2006;290:143-149. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |