Published online May 26, 2013. doi: 10.4331/wjbc.v4.i2.18
Revised: March 19, 2013
Accepted: April 28, 2013
Published online: May 26, 2013
Processing time: 116 Days and 9.1 Hours
AIM: To explore the possibility that nucleotide oligomerization domain 1 (NOD1) pathway involved in refractoriness of interferon-β signaling in mouse respiratory epithelial cells induced by the anticancer xanthone compound, 5,6-dimethylxanthenone-4-acetic acid (DMXAA).
METHODS: C10 mouse bronchial epithelial cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 2 mmol/L glutamine, 100 units/mL penicillin, 100 g/mL streptomycin. Pathogen-free female BALB/c mice were used to explore the mechanisms of refractoriness of interferon-signaling. Mouse thioglycollate-elicited peritoneal macrophages, bone marrow derived macrophages and bone marrow derived dendritic cells were collected and cultured. The amount of interferon (IFN)-inducible protein-10 (IP10/CXCL10), macrophage chemotactic protein (MCP1/CCL2) and interleukin (IL)-6 secreted by cells activated by DMXAA was quantified using enzyme-linked immunosorbent assay kits according to the instructions of the manufacturers. Total RNA was isolated from cells or nasal epithelium with RNeasy Plus Mini Kit, and cDNA was synthesized. Gene expression was checked using Applied Biosystems StepOne Real-Time Polymerase Chain Reaction System. Transfection of small interfering RNA (siRNA) control, NOD1 duplexed RNA oligonucleotides, and high-mobility group box 1/2/3 (HMGB1/2/3) siRNA was performed using siRNA transfection reagent.
RESULTS: DMXAA activates IFN-β pathway with high level of IFN-β dependent antiviral genes including 2’, 5’-oligoadenylate synthetase 1 and myxovirus resistance 1 in mouse thioglycollate-elicited peritoneal macrophages, bone marrow derived macrophages and bone marrow derived dendritic cells. Activation of IFN-β by DMXAA involved in NOD1, but not HMGB1/2/3 signal pathway demonstrated by siRNA. NOD1 pathway plays an important role in refractoriness of IFN-β signaling induced by DMXAA in mouse C10 respiratory epithelial cells and BALB/c mice nasal epithelia. These data indicate that DMXAA is not well adapted to the intrinsic properties of IFN-β signaling. Approaches to restore sensitivity of IFN-β signaling by find other xanthone compounds may function similarly, could enhance the efficacy of protection from influenza pneumonia and potentially in other respiratory viral infections.
CONCLUSION: NOD1 pathway may play an important role in refractoriness of IFN-β signaling in mouse respiratory epithelial cells induced by DMXAA.
Core tip: We recently demonstrated that a small, cell-permeable compound, 5,6-dimethylxanthenone-4-acetic acid (DMXAA), was able to induce production of interferon (IFN)-γ and IFN-β-dependent proteins and protect epithelial cells in vitro from virally-induced cell death and to protect mice from a lethal dose of H1N1 influenza A virus. DMXAA that activates multiple antiviral pathways including IFN-β pathway is an attractive strategy in antiviral therapies. Nucleotide oligomerization domain 1 pathway may play an important role in refractoriness of IFN-β signaling in mouse respiratory epithelial cells induced by DMXAA.
- Citation: Yu Z, Predina JD, Cheng G. Refractoriness of interferon-beta signaling through NOD1 pathway in mouse respiratory epithelial cells using the anticancer xanthone compound. World J Biol Chem 2013; 4(2): 18-29
- URL: https://www.wjgnet.com/1949-8454/full/v4/i2/18.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v4.i2.18
The influenza pandemic of 1918 occurred suddenly and killed over 50 million people worldwide to be followed by subsequent two major pandemic in 1957 and 1968[1,2]. The 2009 H1N1 influenza A pandemic initially raised similar concerns, but luckily, the pathogenicity of this virus was relatively less severe.
Two approaches currently available for use against influenza are antiviral drugs and vaccines. The use of antiviral drugs is the first line of defense against a new influenza pandemic. Four antiviral drugs, including zanamivir, oseltamivir, amantadine and rimantadine are currently approved by Food and Drug Administration in United States to treat acute, uncomplicated influenza. Although extremely valuable, their widespread use will likely be limited by concerns over side effects, drug resistance, etc.[2,3]. The best defense against influenza is vaccinated with appropriate vaccines. However, it is the most difficult defense to achieve. The 2009 pandemic of H1N1 influenza made it painfully clear how difficult it will be to generate vaccine quickly enough and in sufficient quantities for use during the initial stages of a pandemic. The need for new anti-influenza treatment or prophylactic strategies that can be employed rapidly is obvious.
To explore a different antiviral strategy by using a drug that activates antiviral innate immunity in a very rapid and non-specific fashion allowing for protection from any newly arising strain of influenza or other viral respiratory pathogen such as SARS will be important. The primary targets of respiratory viruses are respiratory tract mucosal epithelial cells, which form the majority of the cells lining the epithelial tract and lungs, and usually resistant to viral infection due to a complex defense system involving physical barriers, innate immune responses of the epithelial cells and resident leukocytes, and finally the development of acquired immune responses. One of the most important protections against viral infections is the release of a variety of immunostimulatory cytokines and chemokines by epithelial cells, macrophages and neutrophils, the most important being interferon (IFN)-α and IFN-γ[4]. These type I IFNs trigger the upregulation of a cascade of antiviral genes such as 2’,5’-oligoadenylate synthetase 1 (OAS1) and myxovirus resistance 1 (Mx1) that protect cells from viral replication[5-7]. It has been reported that 5,6-di-methylxanthenone-4-acetic acid (DMXAA) can induce protection against vesicular stomatitis virus and H1N1 influenza A respiratory viral infections through innate immune activation, supported by inducing type I IFN signaling[8-13].
Despite much research that DMXAA can activate multiple innate immune pathways, the effects of DMXAA to activate IFN-β mediated antiviral signaling have never been studied in mouse thioglycollate-elicited peritoneal macrophages, bone marrow derived macrophages (BMDM) and bone marrow derived dendritic cells (BMDDC). Given its ability that type I IFN signaling became desensitization to DMXAA re-exposure in mouse macrophages cell line, we evaluated the hypothesis that DMXAA may also induce type I IFN signaling refractoriness in mouse respiratory epithelial cells. This was tested in the C10 mouse bronchial epithelial cells in vitro and nasal mucosa epithelial cells in vivo. Using siRNA targeting nucleotide oligomerization domain 1 (NOD1) or high-mobility group box 1/2/3 (HMGB1/2/3), we demonstrated that activation of IFN-β by DMXAA involved in NOD1 but not HMGB1/2/3 signal. The findings provide clear evidence that development of this approach, could offer an alternative therapeutic strategy in hosts refractory to IFN-β signaling or paralyzed by viral infection, may be especially valuable. This study also gives a hint that whether refractoriness also occurs during DMXAA treatment in lung cancer of clinical trials.
C10 cells are a nontumorigenic murine alveolar type II-like epithelial cell line that show the presence of lamellar bodies and the biosynthesis of surfactant[13]. Cells were grown in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA, United States) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, 100 μg/mL streptomycin. Mouse thioglycollate-elicited peritoneal macrophages were collected and cultured as previously described[14]. The cells were maintained at 37 °C in an atmosphere containing 5%CO2 and were regularly tested and maintained negative for Mycoplasma spp. Ultrapure lipopolysaccharide (LPS) was purchased from Invivogen (Carlsbad, CA, United States). The CpG oligodeoxynucleotide TCCATGACGTTCCTG ATGCT, known as CpG 1668, was synthesized on a phosphorothioate backbone (Integrated DNA technologies, Coralville, IA, United States).
Pathogen-free female BALB/c mice (6-8 wk old) were purchased from Charles River Laboratories (Wilmington, MA, United States). Animals were housed in the animal facility at the Wistar Institute (Philadelphia, PA, United States). The animal use committees of the Wistar Institute and University of Pennsylvania approve all protocols in compliance with the care and the use of animals[8,9,13].
After mice euthanized by CO2 asphyxiation, pelvic and femoral bones of mice were removed. The marrow was flushed from the bones with 10 mL of culture medium and then centrifuged at 1000 rpm for 10 min at 4 °C. Cells were resuspended in 10 mL L929 hybridoma (secreted macrophage colony-stimulating factor, M-CSF) conditioned medium after washed twice with Dulbecco’s modified Eagle’s medium plus 10%FBS and gently aspirated and expelled using needles until the cell aggregates broken up. Cells were incubated at 37 °C with 5%CO2 in L929 conditioned medium for 1-2 wk. The BMDM were stained with CD11b and determined to be more than 92% pure macrophages by flow cytometry.
BMDDC were cultured as previously described[9]. Briefly, in 24-well plates, 1 × 106 total bone marrow cells per well were seeded in 1 mL Iscove’s modified Dulbecco’s medium (IMDM, Invitrogen, Carlsbad, CA, United States) with 10%FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mmol/L L-glutamine, 1.5 μmol/L 2-ME, 3 ng/mL granulocyte M-CSF and 3 ng/mL interleukin (IL)-4. After 7 d of culture, the cells were enriched using magnetic beads to a purity of at least greater than 93% CD11c+ cells. Briefly, 1 μL biotinylated anti-CD11c antibodies (BD PharMingen) per 15 × 106 cells was added to cells suspended in at least 500 μL buffer comprised of PBS with 2 mmol/L EDTA and 0.5% bovine serum albumin. After 30 min at 4 °C, cells were washed with buffer and labeled with MACS Streptavidin MicroBeads (Miltenyi Biotec) per manufacturer’s instructions. Cells were then positively selected using MACS LS columns (Miltenyi Biotec) and washed.
The amount of IFN-inducible protein-10 (IP10/CXCL10), macrophage chemotactic protein (MCP1/CCL2) and IL-6 secreted by cells activated by DMXAA was quantified using enzyme-linked immunosorbent assay (ELISA) kits to detect murine IP10 (R and D Systems, Inc, Minneapolis, MN, United States), MCP1 and IL-6 (BD Biosciences Pharmingen, San Diego, CA, United States) according to the instructions of the manufacturers[12,13].
Total RNA was isolated from cells or nasal epithelium with RNeasy Plus Mini Kit (Qiagen, Valencia, CA, United States), and cDNA was synthesized. Gene expression was checked using Applied Biosystems StepOne Real-time polymerase chain reaction (PCR) System following the manufacturer’s protocol[12,13]. Primer sequences can be obtained from the authors on request.
Transfection of siRNA control and NOD1 duplexed RNA oligonucleotides was performed using siRNA transfection reagent (Santa Cruz Biotechnology, Santa Cruz, CA, United States) according to the manufacturer’s instructions[12]. After varying amounts of time, C10 cells were stimulated with DMXAA 100 μg/mL for 6 h, supernatants were collected for IP10 ELISA, and cells were harvested for RNA isolation. Transfection of siRNA control and HMGB1/2/3 siRNA (customized pan-HMGB siRNA, Santa Cruz Biotechnology, Santa Cruz, CA, United States) that targeting the sequence for 5’-GTA TGA GAA GGA TAT TGC T-3’ was performed using siRNA transfection reagent (Santa Cruz Biotechnology, Santa Cruz, CA, United States)[12]. After amount of time, C10 cells were stimulated with DMXAA 100 μg/mL or Poly I-C 2 μg/mL for 6 h, then harvested for RNA isolation.
Unless otherwise noted, data comparing differences between two groups were assessed using unpaired Student’s t test. Comparisons with more than two groups were done using one way ANOVA with appropriate post hoc testing. Differences were considered significant when P < 0.05. Data are presented as mean ± SE. Results are representative of two to three independent experiments.
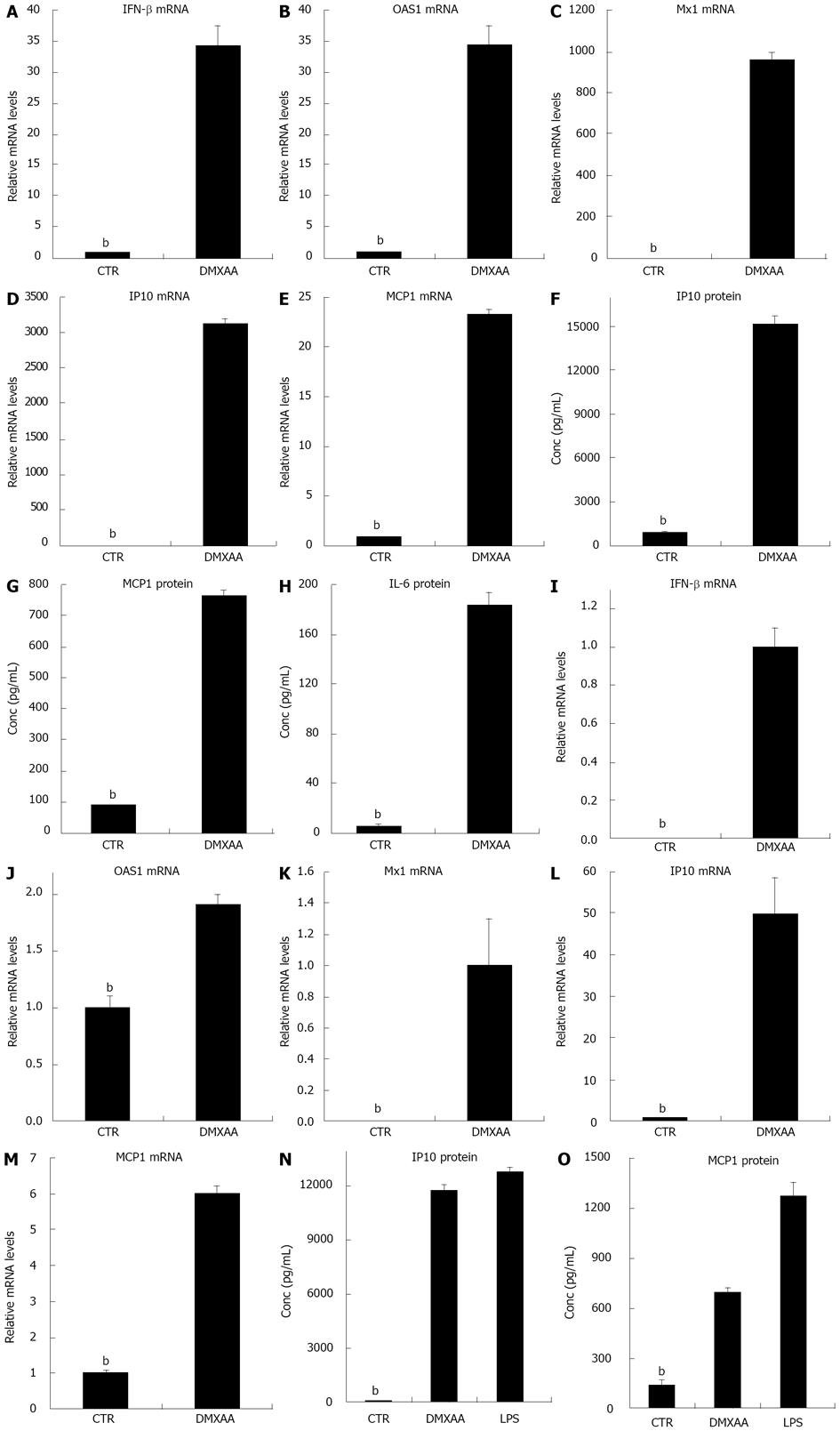
Macrophages have a central role in innate immunity. To investigate the ability of DMXAA activation of IFN-β and IFN-γ responsive genes in different sources of macrophages, thioglycollate-stimulated peritoneal macrophages isolated by lavage from the peritonea of mice injected with thioglycollate for 3 d, cells were exposed to DMXAA. The mRNA expression profiles were evaluated using real time RT-PCR. IFN-β and IFN-γ mediated antiviral genes including OAS1 and Mx1 were significantly upregulated by DMXAA[13,15]. Marked and significant increases in IP10 and MCP-1 mRNA levels were also noted (Figure 1A-E). The concentrations of IP10, MCP1 and IL-6 proteins in the supernatants evaluated by ELISA, consistent with the mRNA data, were strongly elevated (Figure 1F-H). The mRNA levels of IFN-β OAS1 and Mx1, mRNA and protein levels of IP10 and MCP1, and protein level of IL-6 were significantly upregulated by DMXAA in thioglycollate-stimulated mouse peritoneal macrophages. To provide additional evidence for activation of IFN-β and IFN-γ mediated antiviral genes in macrophages by DMXAA, BMDM were exposed to DMXAA or TLR4 ligand LPS. IFN-β and IFN-γ mediated antiviral genes OAS1 and Mx1 were significantly increased by DMXAA (Figure 1I-M). IP10 and MCP-1 inflammatory cytokine/chemokine mRNA levels were also significant upregulated by DMXAA. Protein levels of IP10 and MCP1 in the supernatants evaluated by ELISA were significantly upregulated by DMXAA or LPS in BMDM (Figure 1N and O). The data presented here indicated that DMXAA activated IFN-β and IFN-γ mediated antiviral genes OAS1, Mx1, inflammatory cytokine/chemokine IP10 and MCP-1 in BMDM. LPS activated inflammatory cytokine/chemokine IP10 and MCP-1 in BMDM.
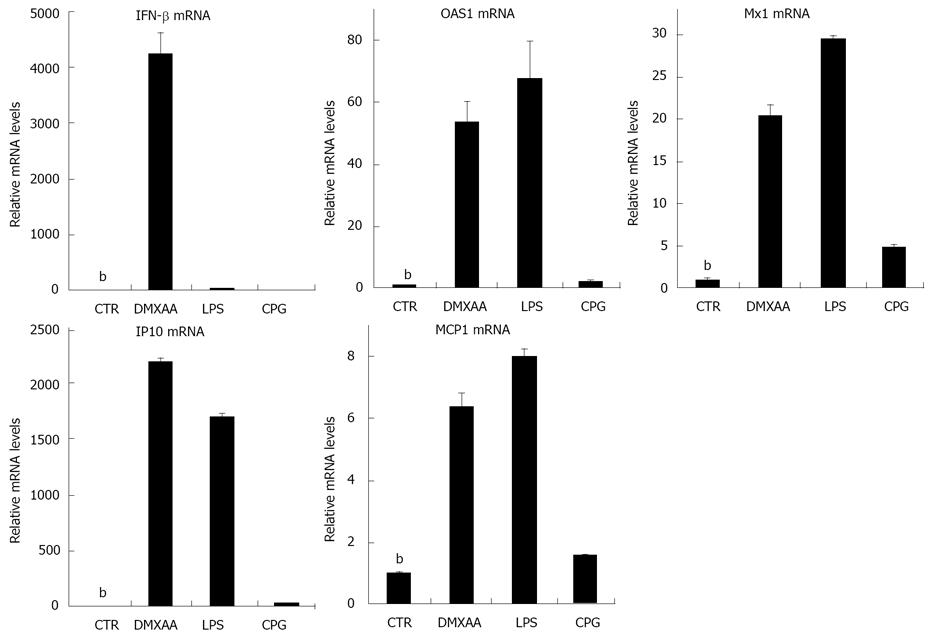
Dendritic cells are antigen-presenting cells that process antigen materials and present them on the surface to other cells of the immune system function as messengers between the innate and adaptive immunity. Our previous data have shown that DMXAA can directly activate mouse BMDDC inducing proinflammatory cytokine production and costimulatory molecule expression in vitro in a MyD88-independent fashion. Next, we investigate whether DMXAA can effectively activate IFN-β and IFN-γ responsive genes in mouse BMDDC, cells were exposed to DMXAA, LPS and TLR9 agonist CpG DNA, respectively[9]. DMXAA, LPS or CpG DNA caused rapid, and in some cases, very large increases in IFN, IFN-β mediated antiviral genes OAS1, Mx1, inflammatory cytokine/chemokine IP10 and MCP-1 (Figure 2). CpG DNA did not induce significantly increase of IFN-β and OAS1 in mouse BMDDC (Figure 2, P > 0.05).
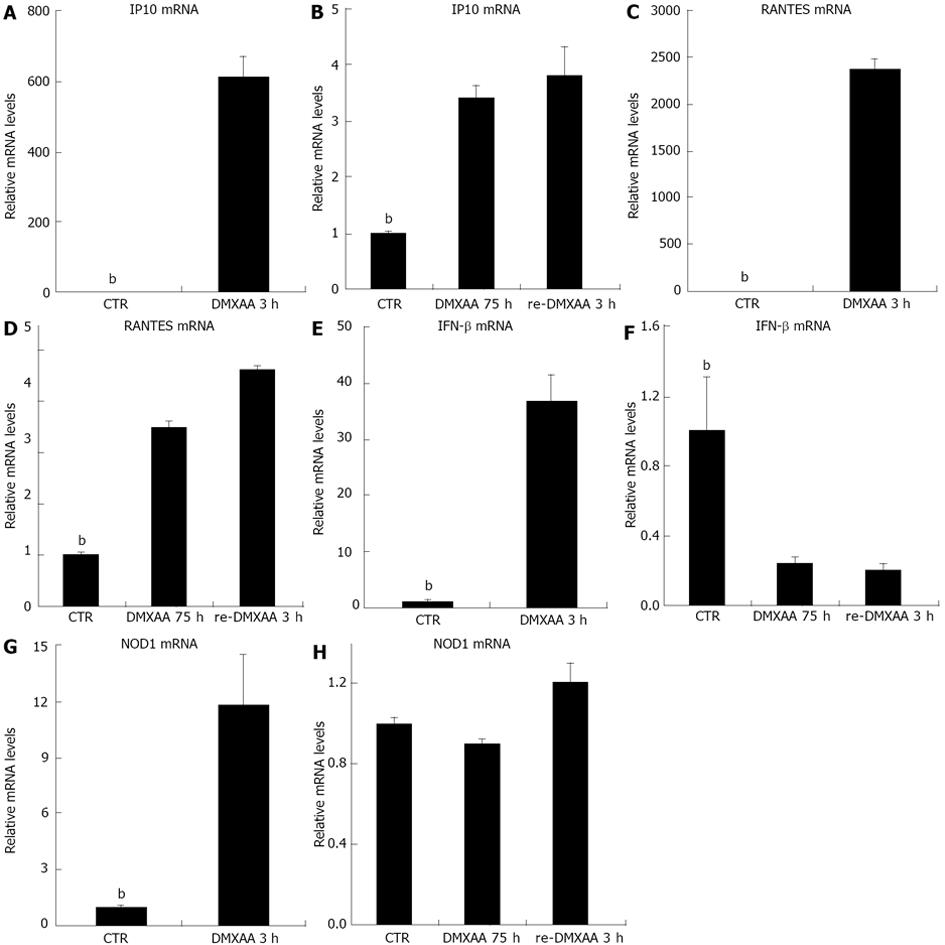
Given the activity that DMXAA activates IFN-β mediated antiviral signaling pathways in C10 cells[13], and DMXAA pretreatment of macrophage cell line induces a state of refractoriness to re-exposure DMXAA[11], the response of C10 cells to re-exposure DMXAA was examined. C10 cells were pretreated with DMXAA. After 72 h, cells were continued to incubate with DMXAA for 3 h (DMXAA 75 h), cells were washed and re-exposure to fresh DMXAA in the same condition for 3 h (re-DMXAA 3 h). C10 cells stimulated with DMXAA for 3 h were set up as positive control. Analysis of the response of cells to DMXAA revealed a strong increase in IP10 mRNA (Figure 3A) at 3 h. DMXAA upregulated IP10 mRNA declined at 75 h (Figure 3B), though the level of IP10 mRNA was still strongly and persistently elevated. DMXAA rechallenged for 3 h induced very little IP10 mRNA (Figure 3B, 4 fold over medium control, P < 0.01) vs DMXAA 3 h incubation (Figure 3A). Similar to IP10 mRNA level, DMXAA significantly induced RANTES mRNA (Figure 3C) at 3 h. DMXAA upregulated RANTES mRNA declined at 75 h (Figure 3D, 4 fold over medium control, P < 0.01). DMXAA rechallenged for 3 h induced some RANTES mRNA (Figure 3D, 5 fold over medium control, P < 0.01) vs DMXAA 3 h of incubation (Figure 3C). Moreover, DMXAA significantly upregulated IFN-β mRNA (Figure 3E) at 3 h of incubation. Quite striking and unexpected, DMXAA induced a diminished response in IFN-β mRNA expression, DMXAA upregulated IFN-β mRNA declined at 75 h of incubation (Figure 3F). DMXAA rechallenged for 3 h downregulated IFN-β mRNA level (Figure 3F) vs DMXAA 3 h of incubation (Figure 3E). DMXAA rechallenged for 3 h stimulated a diminished response in IFN-β mRNA expression indicated that some refractoriness was induced. It could be explained by hypo-response of the signal pathway. Interestingly, DMXAA significantly upregulated NOD1 mRNA (Figure 3G) at 3 h of incubation. DMXAA increased NOD1 mRNA declined at 75 h of incubation (Figure 3H). DMXAA rechallenged for 3 h induced some refractoriness in NOD1 mRNA expression (Figure 3H) vs medium control. It is hypothesized that refractoriness of NOD1 mRNA could explain IFN-β refractory signal both induced by 75 h of incubation and rechallenged for 3 h of DMXAA.
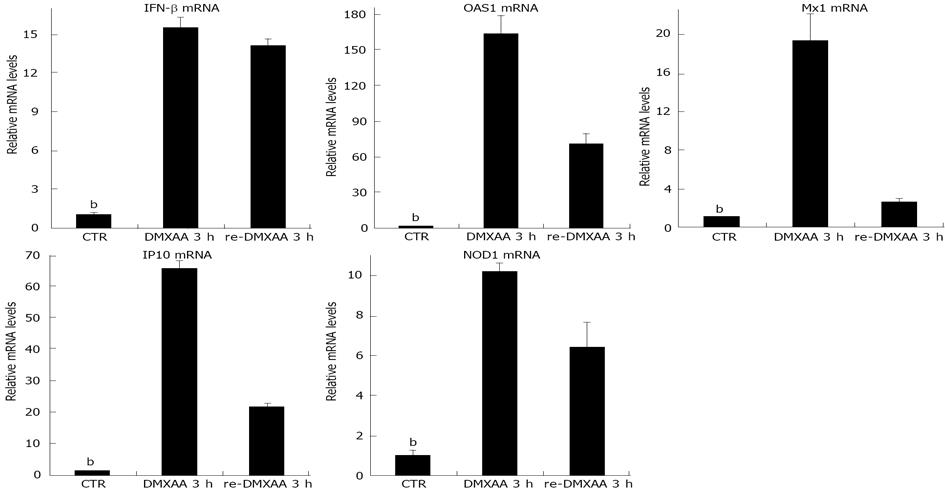
To examine whether refractoriness of IFN-β signaling occurs in vivo, mice were given DMXAA intranasally. After 7 d, mice were rechallenged with same dose of DMXAA intranasally for 3 h (re-DMXAA 3 h group) or mice were continued to wait for 3 h (DMXAA 7 d + 3 h group). Another group of naïve mice administrated DMXAA intranasally for 3 h was set up as positive control (DMXAA 3 h group). Mice were sacrificed and nasal epithelia were removed[13]. Mice pretreatment with DMXAA for 7 d still had strong response to secondary DMXAA administration for 3 h with significant 14.5 fold increase in IFN-β mR expression, similar to mice administrated one dose DMXAA for 3 h with strong increase in IFN-β mR level (Figure 4) DMXAA significantly upregulated OAS1, Mx1, IP10 and NOD1 mRNA levels in DMXAA 3 h group of one dose administration. DMXAA rechallenged 3 h group still had strong OAS1, Mx1, IP10 and NOD1 mRNA expression (Figure 4), but significately declined compared to that of DMXAA 3 h group with one dose administration. No significant differences were observed in mRNA levels of IFN-β, OAS1, Mx1, IP10 and NOD1 between DMXAA rechallenged 3 h group and DMXAA 7 d + 3 h group (data not shown). The results indicated that pretreatment with DMXAA for 7 d, did not induce strong refractoriness to secondary DMXAA administration in vivo (Figure 4).
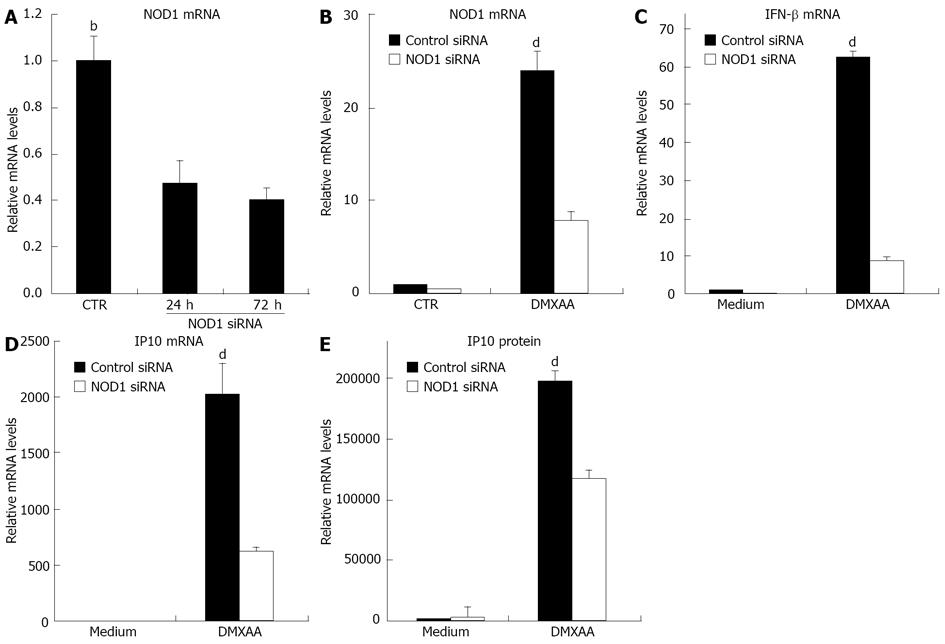
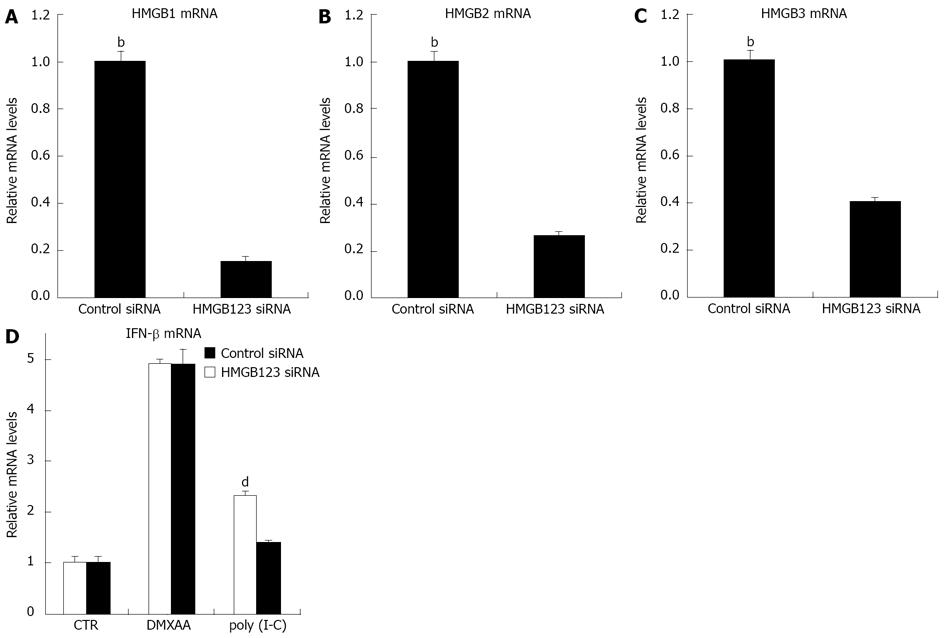
We wanted to demonstrate that DMXAA could activate IFN-β production via the NOD1 signal. C10 cells were transfected with NOD1 siRNA. NOD1 siRNA was highly effective in down regulating baseline levels of NOD1 mRNA at 24 and 72 h time points (Figure 5A). C10 cells were thus treated with NOD1 siRNA or control siRNA and then exposed to DMXAA[12]. Interestingly, DMXAA markedly upregulated NOD1 mRNA, however the NOD1 siRNA clearly blunted this increase in NOD1 message (Figure 5B). Importantly, knockdown of NOD1 mRNA significantly inhibited the response of C10 cells to DMXAA, mRNA levels of IFN-β and IP10 were significantly reduced (Figure 5C and D) and secreted level of IP10 protein was reduced by 40% after exposure to DMXAA (Figure 5E). This partial response could be due to incomplete knockdown of NOD1 activity, or DMXAA stimulation of other pathways. The results indicated that NOD1 is required for IFN-β and IP10 signals in response to DMXAA induction. HMGB functions as an universal sentinel in type I IFN signaling and inflammatory cytokine induction by DNA or RNA targeted to activate the cytosolic nucleic-acid sensing receptors. We therefore wanted to demonstrate whether DMXAA activates IFN-β signal via the HMGB pathway. C10 cells were transfected with HMGB1/2/3 siRNA. HMGB1/2/3 siRNA was highly effective in down regulating baseline levels of HMGB1 (Figure 6A), HMGB2 (Figure 6B) and HMGB3 (Figure 6C), respectively at 48 h. Cells were then treated with HMGB1/2/3 siRNA or control siRNA for 48 h, and furthermore exposed to DMXAA or TLR3 ligand Poly I-C (positive control) for 6 h. Both DMXAA and Poly I-C markedly upregulated IFN-β mRNA, however, the HMGB1/2/3 siRNA did not blunted this increase of DMXAA induced IFN mRNA. Importantly, knockdown of HMGB1/2/3 significantly inhibited the response of cells to Poly I-C induced IFN mRNA (Figure 6D, P < 0.05). The knockdown expression profiles suggest an involvement of HMGB signal in induction of IFN-β mRNA by Poly I-C, but not by DMXAA. DMXAA induced IFN-β mRNA through stimulation of other pathways, such as NOD.
Initial studies suggested that DMXAA, directly activate innate immune system, can be used to protect from influenza pneumonia[13]. Desensitization of IFN-β signal in mouse macrophage cell line on re-exposure to DMXAA has been demonstrated years ago[11]. Up to now, it is little to know whether or to what degree of IFN-β signaling refractoriness is induced by DMXAA re-administration in mouse respiratory epithelial cells. The studies presented here thus identify DMXAA has ability to activate IFN-β dependent antiviral gene and other chemokine/cytokine expressions in mouse thioglycollate-elicited peritoneal macrophages, BMDM and BMDDC (Figures 1 and 2). Pretreatment with DMXAA induces IFN-β siganling refractoriness in respiratory epithelial cells in vitro and in vivo. NOD1 but not HMGB1/2/3 involved in IFN-β signal refractoriness induced by DMXAA re-exposure in mouse respiratory epithelial cells (Figures 3-6). The data indicated that IFN-β signal refractoriness may also happen in clinical administration of DMXAA and reduce the therapeutic efficacy, but no direct clinical observations.
Viral infection activates a variety of pattern recognition-receptors (PRR), such as Toll-like receptors (TLRs) and NOD-like receptors (NLRs) that induce the production of IFN regulatory factor 3 (IRF3), nuclear factor-κB (NF-κB), and other pathways[15,16]. Virally-induced secretion of IFN-β largely mediated through IRF3 is especially important, as this molecule then acts in a paracrine fashion on neighboring cells to upregulate a group of key antiviral proteins (such as OAS1, Mx1 and protein kinase R) that prevent subsequent infection and spread of the virus. Most viruses have developed sophisticated and different mechanisms to try prevention IFN pathway activation[13,17,18]. An alternative strategy to harness the innate immune system is to use agents that mimic viral infection and activate the innate immunity through PRR. Type I IFNs are essential for host defenses against viruses. As early as the 1960s, type I IFNs were reported to be antivirals[19-23]. Refractoriness to IFN within h and continuous up to 3 d in cultured human fibroblasts was observed[24,25]. Four known TLRs (TLRs 3, 7, 8 and 9) recognize different forms of “foreign” nucleic acids, and induce IFN to activate antiviral genes[26]. Compared to using recombinant IFN protein, agonists that activate specific TLRs have been shown to be less toxic, are easier to administer and more effective[27]. TLR3 agonist Poly I-C has been studied for the treatment of influenza infection[28]. TLR4, TLR7 and TLR9 ligands, including LPS, single-stranded RNA and CpG-rich DNA, respectively, triggered IFN induction in cultured cells[29]. Studies reported that TLR ligands can be used against influenza infection but with some major limitations as antiviral agents.
In this study, we showed that DMXAA stimulated IFN-β production and IFN-β-dependent antiviral gene OAS1, Mx1 and chemokine/cytokine IP10, MCP1 and IL-6 expressions in mouse thioglycollate-elicited peritoneal macrophages, BMDM and BMDDC. To our knowledge, this is the first report that DMXAA can stimulate IFN-β dependent antiviral gene OAS1 and Mx1 expressions in mouse macrophages and dendritic cells. Macrophages and dendritic cells function as major sensors of invading pathogens[30]. It has been shown that TLRs are not involved in activation of dendritic cells of DMXAA induction[9].
To determine whether there is any induction of refractoriness by DMXAA on IFN relative genes, C10 cells were stimulated with DMXAA (Figure 3). DMXAA re-exposure induced refractoriness that was characterized by an almost complete inhibition of IFN-β expression (Figure 3B, D, F, and H). We next showed that the recovery of refractoriness of IFN mRNA is essentially complete about 7 d in vivo, a little different profiles from that of OAS1, Mx1 and IP10 mRNA levels (Figure 4).
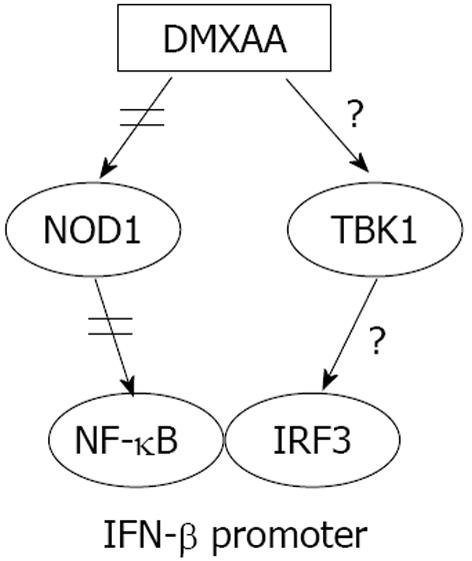
NOD1, a member of the NLR family which are intracellular cytoplasmic sensors, is widely expressed in mammals but seems to be especially important in epithelial and mesothelial cells[12]. DMXAA activates NOD rip-like interacting caspase-like apoptosis-regulatory protein kinase (RICK) pathway[12]. We wanted to evaluate whether DMXAA-induced NOD activation involved in refractoriness of IFN-β signaling. It was able to show inhibition of DMXAA-induced IFN-β, IP10 mRNA and/or protein using siRNA to NOD1 (Figure 5B-E), but not DMXAA-induced IFN-β mRNA with siRNA to HMGB1/2/3 (Figure 6D). Activation of IFN-β by DMXAA involved in NOD1 but not HMGB1/2/3 signal pathway (Figures 4-6). We thus believe that DMXAA re-exposure induced refractoriness of IFN-β signal through NOD1 pathway (Figure 7). NOD1 mediates NF-κB activation required for IFN-β expression, but not IRF3[31]. This suggests that in pharmacological condition of DMXAA administration, there is another PRR functioning in tandem with NOD1 that involves in activation of IRF3 pathway[11,13,31]. It is not known for certain how important the NOD1 pathway vs other potential activating pathway in refractoriness of IFN-β signal. As one of the main cellular segregation and degradation systems, autophagy has recently caught the high attention in the fields of innate immunity. NOD1 and NOD2 as the link between intracellular bacterial sensing and induction of autophagy was reported[32]. It was unclear whether autophagy machinery involved in the effect of DMXAA. Determining where and how DMXAA induces autophagy in protection against virus infection and the relationship of autophagy and refractoriness of type I IFN signaling is an area of great interest[12,13].
There are a number of implications of this study. DMXAA shares virus-triggered innate immune pathway, at least including IFN-β, NOD/RICK and NF-κB signals. DMXAA had a relatively window of function and induced refractoriness of IFN-β signal. This suggests that daily administration of such compounds like DMXAA, may not be effective as dosing intervals shorter than the period of refractoriness would strongly reduce the efficacy of the administration, that targeted signal pathways still in refractoriness.
In summary, DMXAA that activates multiple antiviral pathways including IFN-β pathway is an attractive strategy in antiviral therapies. Development of analogues of xanthone-like DMXAA, small molecular IFN-β inducing drugs without or less refractoriness of IFN-β signaling, to target specific IFN-β mediated antiviral pathways is much promising at treating particular virus.
We thanks Dr. Steven M. Albelda for generous supports.
Drugs that can rapidly inhibit respiratory infection from influenza or other respiratory pathogens are needed. We recently demonstrated that a small, cell-permeable compound, 5,6-di-methylxan-thenone-4-acetic acid (DMXAA), was able to induce production of interferon (IFN)-γ and IFN-β-dependent proteins and protect epithelial cells in vitro from virally-induced cell death and to protect mice from a lethal dose of H1N1 influenza A virus. However, within hours of incubation, type I IFN signaling in mouse macrophage cell line was desensitization to DMXAA. In this study, authors assessed the possibility that DMXAA induced refractoriness of interferon-signaling involved in nucleotide oligomerization domain 1 (NOD1) pathway.
One of the most important protections against viral infections is the release of a variety of immunostimulatory cytokines and chemokines by epithelial cells, macrophages and neutrophils. Viral infection activates a variety of pattern recognition-receptors, such as Toll-like recepters and NOD-like receptors, NOD-like receptors (NLRs). NOD1, a member of the NLR family which are intracellular cytoplasmic sensors, is widely expressed in mammals cells. DMXAA activates NOD/rip-like interacting caspase-like apoptosis-regulatory protein kinase pathway. The authors evaluated whether DMXAA-induced NOD activation involved in refractoriness of IFN-β signaling.
DMXAA takes advantage of the primary innate immune defense against viral infection by directly activating the interferon pathway. In this study, DMXAA activates IFN-β pathway with high level of IFN-β dependent antiviral genes in mouse thioglycollate-elicited peritoneal macrophages, bone marrow derived macrophages and bone marrow derived dendritic cells. Activation of IFN-β by DMXAA involved in NOD1 but not high-mobility group box 1/2/3 signal. NOD1 pathway plays an important role in refractoriness of IFN-β signaling induced by DMXAA in mouse C10 respiratory epithelial cells and BALB/c mice nasal epithelia. These data indicate that DMXAA is not well adapted to the intrinsic properties of IFN-β signaling.
Approaches to restore sensitivity of IFN-β signaling by find other xanthone compounds may function similarly, could enhance the efficacy of protection from influenza pneumonia and potentially in other respiratory viral infections.
This manuscript is very good for publication with minor revision. The drug refractoriness of anti-viral drugs is a very important topic, and the findings presented in this manuscript are generally interesting.
P- Reviewers Clements JE, Ding WX S- Editor Gou SX L- Editor A E- Editor Lu YJ
| 1. | Ahmed R, Oldstone MB, Palese P. Protective immunity and susceptibility to infectious diseases: lessons from the 1918 influenza pandemic. Nat Immunol. 2007;8:1188-1193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 166] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 2. | Sugrue RJ, Tan BH, Yeo DS, Sutejo R. Antiviral drugs for the control of pandemic influenza virus. Ann Acad Med Singapore. 2008;37:518-524. [PubMed] |
| 3. | Baz M, Abed Y, Papenburg J, Bouhy X, Hamelin ME, Boivin G. Emergence of oseltamivir-resistant pandemic H1N1 virus during prophylaxis. N Engl J Med. 2009;361:2296-2297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 172] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 4. | Shahangian A, Chow EK, Tian X, Kang JR, Ghaffari A, Liu SY, Belperio JA, Cheng G, Deng JC. Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice. J Clin Invest. 2009;119:1910-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 406] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 5. | Garofalo R, Mei F, Espejo R, Ye G, Haeberle H, Baron S, Ogra PL, Reyes VE. Respiratory syncytial virus infection of human respiratory epithelial cells up-regulates class I MHC expression through the induction of IFN-beta and IL-1 alpha. J Immunol. 1996;157:2506-2513. [PubMed] |
| 6. | Kuenzel S, Till A, Winkler M, Häsler R, Lipinski S, Jung S, Grötzinger J, Fickenscher H, Schreiber S, Rosenstiel P. The nucleotide-binding oligomerization domain-like receptor NLRC5 is involved in IFN-dependent antiviral immune responses. J Immunol. 2010;184:1990-2000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 168] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 7. | Wang J, Oberley-Deegan R, Wang S, Nikrad M, Funk CJ, Hartshorn KL, Mason RJ. Differentiated human alveolar type II cells secrete antiviral IL-29 (IFN-lambda 1) in response to influenza A infection. J Immunol. 2009;182:1296-1304. [PubMed] |
| 8. | Jassar AS, Suzuki E, Kapoor V, Sun J, Silverberg MB, Cheung L, Burdick MD, Strieter RM, Ching LM, Kaiser LR. Activation of tumor-associated macrophages by the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid induces an effective CD8+ T-cell-mediated antitumor immune response in murine models of lung cancer and mesothelioma. Cancer Res. 2005;65:11752-11761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Wallace A, LaRosa DF, Kapoor V, Sun J, Cheng G, Jassar A, Blouin A, Ching LM, Albelda SM. The vascular disrupting agent, DMXAA, directly activates dendritic cells through a MyD88-independent mechanism and generates antitumor cytotoxic T lymphocytes. Cancer Res. 2007;67:7011-7019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Wang LC, Woon ST, Baguley BC, Ching LM. Inhibition of DMXAA-induced tumor necrosis factor production in murine splenocyte cultures by NF-kappaB inhibitors. Oncol Res. 2006;16:1-14. [PubMed] |
| 11. | Roberts ZJ, Goutagny N, Perera PY, Kato H, Kumar H, Kawai T, Akira S, Savan R, van Echo D, Fitzgerald KA. The chemotherapeutic agent DMXAA potently and specifically activates the TBK1-IRF-3 signaling axis. J Exp Med. 2007;204:1559-1569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Cheng G, Sun J, Fridlender ZG, Wang LC, Ching LM, Albelda SM. Activation of the nucleotide oligomerization domain signaling pathway by the non-bacterially derived xanthone drug 5’6-dimethylxanthenone-4-acetic acid (Vadimezan). J Biol Chem. 2010;285:10553-10562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Cheng G, Wang LC, Fridlender ZG, Cheng GS, Chen B, Mangalmurti NS, Saloura V, Yu Z, Kapoor V, Mozdzanowska K. Pharmacologic activation of the innate immune system to prevent respiratory viral infections. Am J Respir Cell Mol Biol. 2011;45:480-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Albelda SM, Lau KC, Chien P, Huang ZY, Arguiris E, Bohen A, Sun J, Billet JA, Christofidou-Solomidou M, Indik ZK. Role for platelet-endothelial cell adhesion molecule-1 in macrophage Fcgamma receptor function. Am J Respir Cell Mol Biol. 2004;31:246-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Perry AK, Chen G, Zheng D, Tang H, Cheng G. The host type I interferon response to viral and bacterial infections. Cell Res. 2005;15:407-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 269] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 16. | Evans SE, Xu Y, Tuvim MJ, Dickey BF. Inducible innate resistance of lung epithelium to infection. Annu Rev Physiol. 2010;72:413-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Sherry B. Rotavirus and reovirus modulation of the interferon response. J Interferon Cytokine Res. 2009;29:559-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | von Kobbe C JP, Sitterlin D, Bachi A, Wu X, Wilm M, Carmo-Fonseca M, Izaurralde E. Vesicular stomatitis virus matrix protein inhibits host cell gene expression by targeting the nucleoporin Nup98. Mol Cell. 2000;6:1243-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 216] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 19. | Friedman RM. Role of interferon in viral interference. Nature. 1964;22:848-849. [RCA] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Flammer JR, Dobrovolna J, Kennedy MA, Chinenov Y, Glass CK, Ivashkiv LB, Rogatsky I. The type I interferon signaling pathway is a target for glucocorticoid inhibition. Mol Cell Biol. 2010;30:4564-4574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 21. | Sarasin-Filipowicz M, Oakeley EJ, Duong FH, Christen V, Terracciano L, Filipowicz W, Heim MH. Interferon signaling and treatment outcome in chronic hepatitis C. Proc Natl Acad Sci USA. 2008;105:7034-7039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 544] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 22. | Thacker SG, Berthier CC, Mattinzoli D, Rastaldi MP, Kretzler M, Kaplan MJ. The detrimental effects of IFN-α on vasculogenesis in lupus are mediated by repression of IL-1 pathways: potential role in atherogenesis and renal vascular rarefaction. J Immunol. 2010;185:4457-4469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 23. | Cilloniz C, Pantin-Jackwood MJ, Ni C, Goodman AG, Peng X, Proll SC, Carter VS, Rosenzweig ER, Szretter KJ, Katz JM. Lethal dissemination of H5N1 influenza virus is associated with dysregulation of inflammation and lipoxin signaling in a mouse model of infection. J Virol. 2010;84:7613-7624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 24. | Larner AC, Chaudhuri A, Darnell JE. Transcriptional induction by interferon. New protein(s) determine the extent and length of the induction. J Biol Chem. 1986;261:453-459. [PubMed] |
| 25. | Sarasin-Filipowicz M, Wang X, Yan M, Duong FH, Poli V, Hilton DJ, Zhang DE, Heim MH. Alpha interferon induces long-lasting refractoriness of JAK-STAT signaling in the mouse liver through induction of USP18/UBP43. Mol Cell Biol. 2009;29:4841-4851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 145] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 26. | Clarke CJ, Trapani JA, Johnstone RW. Mechanisms of interferon mediated anti-viral resistance. Curr Drug Targets Immune Endocr Metabol Disord. 2001;1:117-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Urosevic M, Fujii K, Calmels B, Laine E, Kobert N, Acres B, Dummer R. Type I IFN innate immune response to adenovirus-mediated IFN-gamma gene transfer contributes to the regression of cutaneous lymphomas. J Clin Invest. 2007;117:2834-2846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Wong JP, Christopher ME, Viswanathan S, Karpoff N, Dai X, Das D, Sun LQ, Wang M, Salazar AM. Activation of toll-like receptor signaling pathway for protection against influenza virus infection. Vaccine. 2009;27:3481-3483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 29. | Asselin-Paturel C, Trinchieri G. Production of type I interferons: plasmacytoid dendritic cells and beyond. J Exp Med. 2005;202:461-465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 232] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 30. | Wang RF, Miyahara Y, Wang HY. Toll-like receptors and immune regulation: implications for cancer therapy. Oncogene. 2008;27:181-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 31. | Prantner D, Darville T, Nagarajan UM. Stimulator of IFN gene is critical for induction of IFN-beta during Chlamydia muridarum infection. J Immunol. 2010;184:2551-2560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 32. | Travassos LH, Carneiro LA, Ramjeet M, Hussey S, Kim YG, Magalhães JG, Yuan L, Soares F, Chea E, Le Bourhis L. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat Immunol. 2010;11:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 916] [Cited by in RCA: 1010] [Article Influence: 63.1] [Reference Citation Analysis (0)] |