Published online Sep 26, 2012. doi: 10.4331/wjbc.v3.i9.175
Revised: August 22, 2012
Accepted: August 29, 2012
Published online: September 26, 2012
The WNT/β-catenin and phosphoinositide 3-kinase (PI3K/AKT) signaling cascades both have been implicated in the formation and progression of colorectal cancer. Oncogenic PI3K/AKT signaling suppresses the activity of forkhead box O3a (FOXO3a) transcription factor through phosphorylation leading to its nuclear exclusion. Inhibition of the PI3K/AKT signaling by PI3K or AKT inhibitors results in the translocation of FOXO3a to the nucleus, and is considered to be a promising therapeutic strategy for many cancers including colon cancer. Now, however, a new study in Nature Medicine has revealed a nuclear interaction of β-catenin with FOXO3a as a promoter of metastatic progression in colon cancer. The work has important implications for the treatment of colon cancers, suggests a companion biomarker strategy to enable a personalized medicine approach, and offers an alternative therapeutic strategy to overcome resistance to PI3K and AKT inhibitors.
- Citation: Link W. Nuclear accumulation of β-catenin and forkhead box O3a in colon cancer: Dangerous liaison. World J Biol Chem 2012; 3(9): 175-179
- URL: https://www.wjgnet.com/1949-8454/full/v3/i9/175.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v3.i9.175
Colon cancer is a leading cause of cancer mortality in western countries[1]. Early detection allows the tumor to be removed by surgery which, along with the appropriate adjuvant chemotherapy, eliminates the disease in a high percentage of cases. However, despite recent progress in colon cancer screening and treatment, in the advanced stages, colon tumors are resistant to a broad spectrum of antitumor drugs, added to which the cancerous cells are capable of dispersing throughout the body, giving rise to metastasis. At present, there are no effective treatments for slowing down the progression of colon cancer in these late stages, and most patients die as a result of disease progression. In recent years new drugs have been designed that are targeted at blocking the activity of certain molecules responsible for promoting the growth and dissemination of tumor cells. Some of these drugs, which are currently in the clinical trial stage, are showing very good results in certain patients, while others show no improvement at all.
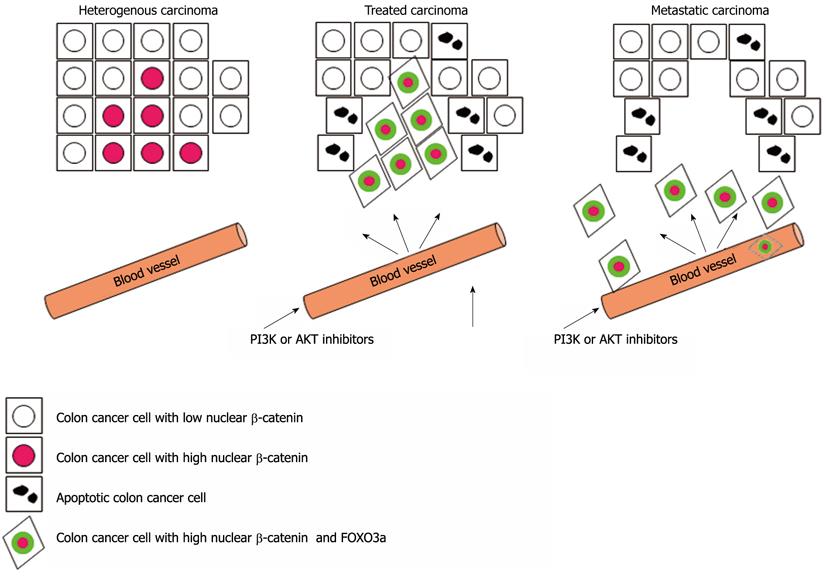
Now, a report in Nature Medicine has identified the molecular mechanisms that determine patients’ response to certain drugs used in the treatment of colon cancer[2]. The authors took a top-down approach based on their clinical observation that the coincidence of nuclear β-catenin and forkhead box O3a (FOXO3a) in samples from patients with colon cancer correlated with shorter survival time and metastasis stage (Figure 1). Most cases of colon cancer are initiated by nuclear accumulation of β-catenin protein due to its own mutation, or inactivation of the adenomatous polyposis coli (APC) tumor suppressor that controls the stability of the β-catenin protein[3-8]. β-catenin, the mammalian homolog of Drosophila Armadillo is a multifunctional oncogenic protein that is found at the plasma membrane of epithelial cells where it is implicated in the formation of adherens junctions[9-11]. β-catenin has been shown to be a key component of the Wnt signaling pathway[12]. β-catenin is phosphorylated by the glycogen synthase kinase 3β: adenomatous polyposis coli [glycogen synthase kinase (GSK)-3β:APC] complex leading to its ubiquitination and proteosome-mediated degradation. Upon binding of Wnt to its receptor, frizzled (Fz), disheveled (Dsh) is recruited to the membrane and inactivates GSK-3β. Thus, increased Wnt signaling results in diminished phosphorylation and reduced degradation of β-catenin. Stabilization and nuclear translocation of β-catenin allows its association with several transcriptional regulators such as T cell factor (TCF), lymphoid enhancer and transcription factors that promote the perpetual activation of Wnt target genes even in the absence of any extracellular signals. In addition, several alternative interaction partners of β-catenin have been reported including the androgen receptor[13], vitamin D receptor[14], the homeodomain factor Prop1[15] and FOXO transcription factors[16]. Many Wnt/β-catenin target genes have been shown to be involved in oncogenic growth and cellular transformation[17].
Conversely, members of the mammalian FOXO family of proteins have emerged as tumor suppressors[18,19]. FOXO factors are evolutionarily conserved proteins implicated in several fundamental cellular processes[18-20]. The mammalian members of FOXO subclass of forkhead transcription factors FOXO1, FOXO3A, FOXO4 and FOXO6 function as transcriptional regulators in the cell nucleus[21]. FOXO transcription factors bind as monomers to their consensus DNA binding sequence TTGTTTAC and activate or repress multiple genes such as Bim and FasL involved in apoptosis[22,23], p27kip[24] and cyclin D[25] in cell cycle regulation, GADD45a in DNA damage repair[22,23,26,27], manganese superoxide dismutase (MnSOD) in stress response[28], Foxp3 in T-cell regulation[29,30], atrogin 1 in skeletal muscle atrophy[31], and glycogenolytic gene glucose-6-phosphatase (G6pc) in metabolism[32]. Recent studies also reveal the importance of FOXOs in preserving the self-renewal capacity of hematopoietic stem cells[33,34] and pluripotency of human embryonic stem cells[35]. FOXO factors can undergo AKT mediated phosphorylation, which promotes binding to 14-3-3, nuclear export through the export receptor CRM1 and cytoplasmic sequestration. Under stress conditions or in the absence of growth or survival factors, when the PI3K/AKT pathway is inhibited, FOXO proteins translocate to the cell nucleus, where their transcriptional functions can be executed. FOXO proteins are inactivated via cytoplasmic mislocalization by oncogenic signaling in a broad variety of human cancers including colon cancer[18]. Accordingly, reactivation of FOXO factors based on their tumor suppressor properties is considered as a very attractive anticancer strategy.
The current work by Tenbaum et al[2] however establishes FOXO3a as a Janus-faced protein that, dependent on the nuclear β-catenin status, can reduce cell proliferation via inducing apoptosis or cell cycle arrest, or promote cell scattering and metastasis[36]. The work provides physiological relevance to the previous observation that β-catenin can bind to FOXO proteins, thereby enhancing FOXO-dependent and inhibiting TCF transcriptional activity[16,37]. In this context, the present work provides some intriguing evidence. The authors have shown that FOXO3a and β-catenin co-regulate metastasis-relevant genes including genes that are involved in cytoskeleton remodeling and cell shape and motility. These findings raise the question whether the expression of metastatic genes is part of an intrinsic FOXO3a-driven transcriptional program that is over-ridden by the predominant expression of proapoptotic target genes in the absence of β-catenin, or whether β-catenin drives the recruitment of FOXO3a specifically to promoters of metastasis-relevant genes. The authors reveal IQGAP2 as a new target gene of the FOXO3a/β-catenin complex that is required for destabilizing E-cadherin-containing adherens junctions. Given that specific inhibition of the PI3K/AKT signaling pathway has become one of the most sought after goals of pharmaceutical applications, the implications of this work for targeted cancer therapy are extremely important. At least 16 class I PI3K and > 12 AKT inhibitors are in clinical development aimed at the inhibition of the PI3K/AKT signaling, thereby restoring nuclear localization of FOXO3a. Using patient-derived sphere cultures and xenograft models, the authors present striking evidence that the treatment of colon cancer cells harboring high level expression of nuclear β-catenin with the small molecule AKT inhibitor API-2 known to relocate FOXO3a to the nucleus promoted cell scattering in vitro and metastasis in vivo. Hence, therapeutic inhibition of PI3K/AKT signaling in colon cancer might have deleterious long-term effects because β-catenin renders the cells resistant to FOXO3a-mediated apoptosis and converts FOXO3a into a metastasis-promoting factor. This scenario clearly illustrates the need to select carefully a biomarker-defined population that will benefit from treatment with PI3K/AKT inhibitors, or to identify other therapeutic solutions for patients who will not respond well to the treatment and avoid the risk of administering ineffective drugs. In the context of a personalized medicine setting, patients with low nuclear β-catenin levels would probably best respond to therapeutic inhibition of oncogenic PI3K/AKT signaling. Most importantly, this study demonstrates that β-catenin is not only a predictive biomarker that correlates with the response to treatment with PI3K or AKT inhibitors, but is intimately involved in the molecular mechanism that renders colon cancer cells resistant to these agents. Accordingly, the study further reveals that tankyrase inhibition by the small molecule compound XAV-939, which increases degradation of β-catenin and reduces its nuclear concentration[38], sensitizes resistant cells specifically to PI3K and AKT inhibitors. Hence, the combined use of agents that target the Wnt signaling pathway together with PI3K or AKT inhibitors may be effective in colon cancer patients with high nuclear β-catenin and oncogenic PI3K/AKT signaling. XAV-939 has failed to be active in vivo, therefore, clinical proof-of-concept has to await the development of a new generation of tankyrase inhibitors with potent in vivo activity.
Given that FOXO3a has been shown to be the target of a broad variety of post-translational modifications that fine-tune its intracellular localization, it is not surprising that many different agents (tool compounds and approved drugs) capable of inducing the nuclear accumulation of FOXO3a have been reported. The growing list of FOXO3a regulators includes Ca2+/calmodulin inhibitors, nuclear export inhibitors, MEK1/2 inhibitors, IKK inhibitors, and a diverse spectrum of anticancer drugs, such as paclitaxel, doxorubicin, lapatinib, gefitinib, imatinib and cisplatin[21,39-41]. According to the data presented by Tenbaum et al[2], those agents should be “red flagged” for the treatment of colon cancer and carefully assessed for their metastasis-promoting properties in the presence of high nuclear β-catenin concentrations. It remains to be established whether the spatial coincidence of the two proteins in the cell nucleus is sufficient to assemble the FOXO3a/β-catenin complex or whether this interaction is regulated by post-translational modifications that might be blocked pharmacologically. The results of the study by Tenbaum et al[2] are in accordance with the notion that the tumor suppressor functions of FOXO proteins are context dependent[42]. FOXO factors regulate a broad variety of cellular functions, some of which seemingly oppose their therapeutic activation or inhibition, which may lead to undesirable clinical outcomes. Therapeutic interference with FOXO functions might have both beneficial effects in one disease setting while having deleterious effects in another[43]. A fascinating aspect of this work is the use of a model system based on the sequential inoculation of patient-derived tumor cells that are capable of regenerating the disease with the same distinctive characteristics as in the individual patient, retaining its original genetic, clinical and pathological alterations. This “xenopatient” system might prove to be an excellent tool to predict individual response to experimental drugs before subjecting the patient to new treatments. It might also be useful to pinpoint the specific molecular effectors that mediate the resistance to apoptosis and the metastatic phenotype triggered by the FOXO3a/β-catenin complex and thereby provide potential drug targets against metastatic progression of colon cancer. An exhaustive survey of clinical samples of patients with different cancers is required to establish a causal relationship between FOXO3a activation and metastasis for non-colon cancers. Future work might reveal that β-catenin is not the only binding partner capable of undermining the tumor suppressor functions of FOXO3a.
Peer reviewer: Dr. Sung H Kim, Professor, Cancer Preventive Material Research Center, Kyunghee University, 1 Hoegidong Dongdaemungu, Seoul 130-701, South Korea
S- Editor Cheng JX L- Editor Kerr C E- Editor Zhang DN
| 1. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11831] [Article Influence: 845.1] [Reference Citation Analysis (4)] |
| 2. | Tenbaum SP, Ordóñez-Morán P, Puig I, Chicote I, Arqués O, Landolfi S, Fernández Y, Herance JR, Gispert JD, Mendizabal L. β-catenin confers resistance to PI3K and AKT inhibitors and subverts FOXO3a to promote metastasis in colon cancer. Nat Med. 2012;18:892-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 320] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 3. | Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hedge P, McKechnie D. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1636] [Cited by in RCA: 1551] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 4. | Miyoshi Y, Nagase H, Ando H, Horii A, Ichii S, Nakatsuru S, Aoki T, Miki Y, Mori T, Nakamura Y. Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene. Hum Mol Genet. 1992;1:229-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 643] [Cited by in RCA: 631] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 5. | Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787-1790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2986] [Cited by in RCA: 3096] [Article Influence: 110.6] [Reference Citation Analysis (0)] |
| 6. | Powell SM, Zilz N, Beazer-Barclay Y, Bryan TM, Hamilton SR, Thibodeau SN, Vogelstein B, Kinzler KW. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359:235-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1308] [Cited by in RCA: 1301] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 7. | Sparks AB, Morin PJ, Vogelstein B, Kinzler KW. Mutational analysis of the APC/beta-catenin/Tcf pathway in colorectal cancer. Cancer Res. 1998;58:1130-1134. [PubMed] |
| 8. | Wagenaar RA, Crawford HC, Matrisian LM. Stabilized beta-catenin immortalizes colonic epithelial cells. Cancer Res. 2001;61:2097-2104. [PubMed] |
| 9. | Conacci-Sorrell M, Zhurinsky J, Ben-Ze'ev A. The cadherin-catenin adhesion system in signaling and cancer. J Clin Invest. 2002;109:987-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Hayashida Y, Honda K, Idogawa M, Ino Y, Ono M, Tsuchida A, Aoki T, Hirohashi S, Yamada T. E-cadherin regulates the association between beta-catenin and actinin-4. Cancer Res. 2005;65:8836-8845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653:1-24. [PubMed] |
| 13. | Mulholland DJ, Dedhar S, Coetzee GA, Nelson CC. Interaction of nuclear receptors with the Wnt/beta-catenin/Tcf signaling axis: Wnt you like to know? Endocr Rev. 2005;26:898-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 303] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 14. | Pálmer HG, Anjos-Afonso F, Carmeliet G, Takeda H, Watt FM. The vitamin D receptor is a Wnt effector that controls hair follicle differentiation and specifies tumor type in adult epidermis. PLoS One. 2008;3:e1483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Olson LE, Tollkuhn J, Scafoglio C, Krones A, Zhang J, Ohgi KA, Wu W, Taketo MM, Kemler R, Grosschedl R. Homeodomain-mediated beta-catenin-dependent switching events dictate cell-lineage determination. Cell. 2006;125:593-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 218] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 16. | Essers MA, de Vries-Smits LM, Barker N, Polderman PE, Burgering BM, Korswagen HC. Functional interaction between beta-catenin and FOXO in oxidative stress signaling. Science. 2005;308:1181-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 550] [Cited by in RCA: 586] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 17. | Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4088] [Cited by in RCA: 4442] [Article Influence: 233.8] [Reference Citation Analysis (0)] |
| 18. | Dansen TB, Burgering BM. Unravelling the tumor-suppressive functions of FOXO proteins. Trends Cell Biol. 2008;18:421-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 217] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 19. | Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 788] [Cited by in RCA: 867] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 20. | Zanella F, Link W, Carnero A. Understanding FOXO, new views on old transcription factors. Curr Cancer Drug Targets. 2010;10:135-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Yang JY, Hung MC. Deciphering the role of forkhead transcription factors in cancer therapy. Curr Drug Targets. 2011;12:1284-1290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Finnberg N, El-Deiry WS. Activating FOXO3a, NF-kappaB and p53 by targeting IKKs: an effective multi-faceted targeting of the tumor-cell phenotype? Cancer Biol Ther. 2004;3:614-616. [PubMed] |
| 23. | Tran H, Brunet A, Griffith EC, Greenberg ME. The many forks in FOXO's road. Sci STKE. 2003;2003:RE5. [PubMed] |
| 24. | Dijkers PF, Medema RH, Pals C, Banerji L, Thomas NS, Lam EW, Burgering BM, Raaijmakers JA, Lammers JW, Koenderman L. Forkhead transcription factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27(KIP1). Mol Cell Biol. 2000;20:9138-9148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 519] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 25. | Schmidt M, Fernandez de Mattos S, van der Horst A, Klompmaker R, Kops GJ, Lam EW, Burgering BM, Medema RH. Cell cycle inhibition by FoxO forkhead transcription factors involves downregulation of cyclin D. Mol Cell Biol. 2002;22:7842-7852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 447] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 26. | Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410-7425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 935] [Cited by in RCA: 1027] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 27. | Yang JY, Xia W, Hu MC. Ionizing radiation activates expression of FOXO3a, Fas ligand, and Bim, and induces cell apoptosis. Int J Oncol. 2006;29:643-648. [PubMed] |
| 28. | Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1187] [Cited by in RCA: 1249] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 29. | Harada Y, Harada Y, Elly C, Ying G, Paik JH, DePinho RA, Liu YC. Transcription factors Foxo3a and Foxo1 couple the E3 ligase Cbl-b to the induction of Foxp3 expression in induced regulatory T cells. J Exp Med. 2010;207:1381-1391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 228] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 30. | Ouyang W, Beckett O, Ma Q, Paik JH, DePinho RA, Li MO. Foxo proteins cooperatively control the differentiation of Foxp3+ regulatory T cells. Nat Immunol. 2010;11:618-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 391] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 31. | Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399-412. [PubMed] |
| 32. | Onuma H, Vander Kooi BT, Boustead JN, Oeser JK, O'Brien RM. Correlation between FOXO1a (FKHR) and FOXO3a (FKHRL1) binding and the inhibition of basal glucose-6-phosphatase catalytic subunit gene transcription by insulin. Mol Endocrinol. 2006;20:2831-2847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Miyamoto K, Araki KY, Naka K, Arai F, Takubo K, Yamazaki S, Matsuoka S, Miyamoto T, Ito K, Ohmura M. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 677] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 34. | Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1294] [Cited by in RCA: 1218] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 35. | Zhang X, Yalcin S, Lee DF, Yeh TY, Lee SM, Su J, Mungamuri SK, Rimmelé P, Kennedy M, Sellers R. FOXO1 is an essential regulator of pluripotency in human embryonic stem cells. Nat Cell Biol. 2011;13:1092-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 216] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 36. | Yan Y, Lackner MR. FOXO3a and β-catenin co-localization: double trouble in colon cancer? Nat Med. 2012;18:854-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Hoogeboom D, Essers MA, Polderman PE, Voets E, Smits LM, Burgering BM. Interaction of FOXO with beta-catenin inhibits beta-catenin/T cell factor activity. J Biol Chem. 2008;283:9224-9230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 224] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 38. | Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1480] [Cited by in RCA: 1659] [Article Influence: 103.7] [Reference Citation Analysis (0)] |
| 39. | Wilson MS, Brosens JJ, Schwenen HD, Lam EW. FOXO and FOXM1 in cancer: the FOXO-FOXM1 axis shapes the outcome of cancer chemotherapy. Curr Drug Targets. 2011;12:1256-1266. [PubMed] |
| 40. | Zanella F, Rosado A, García B, Carnero A, Link W. Chemical genetic analysis of FOXO nuclear-cytoplasmic shuttling by using image-based cell screening. Chembiochem. 2008;9:2229-2237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 41. | Zanella F, Rosado A, Garcia B, Carnero A, Link W. Using multiplexed regulation of luciferase activity and GFP translocation to screen for FOXO modulators. BMC Cell Biol. 2009;10:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Link W. Context-dependent therapeutic potential of FOXO proteins in oral squamous cell carcinoma. Oral Oncol. 2011;47:229-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 43. | Link W. The therapeutic potential of FOXO proteins. Curr Drug Targets. 2011;12:1232-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |