Copyright
©2012 Baishideng Publishing Group Co.
World J Biol Chem. Apr 26, 2012; 3(4): 61-72
Published online Apr 26, 2012. doi: 10.4331/wjbc.v3.i4.61
Published online Apr 26, 2012. doi: 10.4331/wjbc.v3.i4.61
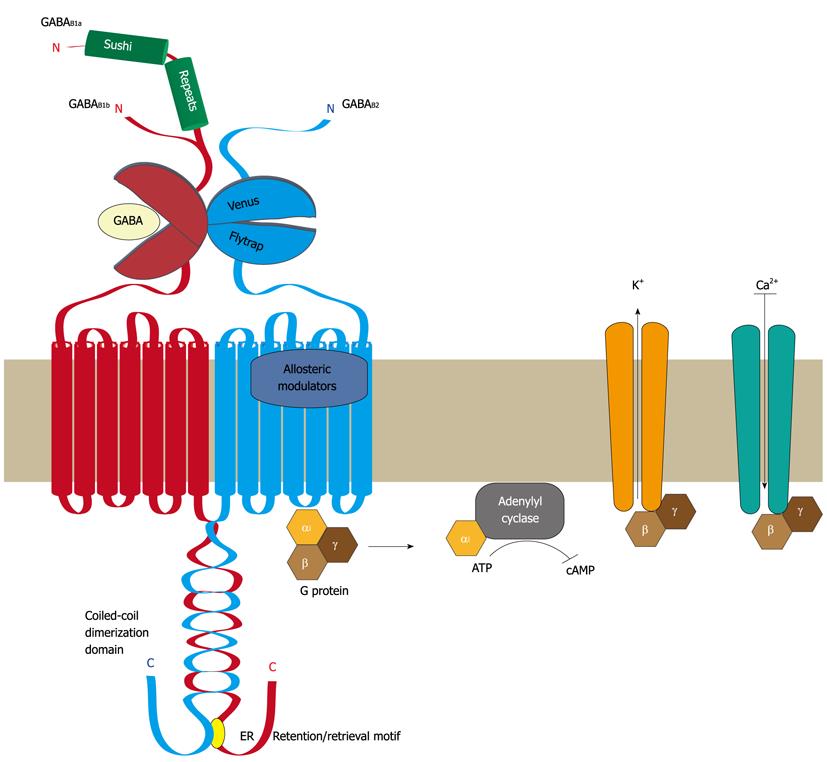
Figure 1 Structural organization of GABAB receptors.
Functional GABAB receptors are heterodimers composed of the two subunits GABAB1 and GABAB2. Both subunits are heptahelical membrane proteins with a large extracellular located N-terminal domain containing a “Venus flytrap” module and a large intracellular C-terminal domain containing a coiled-coil protein-protein interaction module. GABAB1 and GABAB2 heterodimerize via their “Venus flytrap” and coiled-coiled domains. An endoplasmic reticulum (ER) retention/retrieval signal is present distal to the coiled-coil domain in GABAB1 and prevents ER exit of GABAB1 unless it is masked by heterodimerization with GABAB2. The “Venus flytrap” module of GABAB1 constitutes the GABA binding site, whereas that of GABAB2 is inactive and not involved in ligand binding. Instead, the heptahelical domain of GABAB2 contains a binding site for allosteric modulators, which affects the affinity of ligands binding to the GABA site. Binding of GABA results in the recruitment and activation of Gαi/o proteins via GABAB2. The activated Gαi/o subunit inhibits the adenylyl cyclase, resulting in lowered cAMP levels, while the Gβγ dimer activates K+ channels and inhibits Ca2+ channels, leading in either case to neuronal inhibition. There exist two isoforms of GABAB1, named GABAB1a and GABAB1b, which are generated by alternative promoter usage. They only differ by the additional presence of two so-called “sushi repeats” (protein-protein interaction modules) in the N-terminal domain of GABAB1a. GABA: γ-Aminobutyric acid; ATP: Adenosine-5'-triphosphate; cAMP: 3'-5'-cyclic adenosine monophosphate.
- Citation: Benke D, Zemoura K, Maier PJ. Modulation of cell surface GABAB receptors by desensitization, trafficking and regulated degradation. World J Biol Chem 2012; 3(4): 61-72
- URL: https://www.wjgnet.com/1949-8454/full/v3/i4/61.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v3.i4.61