Published online Nov 26, 2012. doi: 10.4331/wjbc.v3.i11.184
Revised: October 10, 2012
Accepted: October 30, 2012
Published online: November 26, 2012
Processing time: 162 Days and 5.3 Hours
Transglutaminases (TGs; E.C. 2.3.2.13) are ubiquitous enzymes which catalyze post-translational modifications of proteins. TGs and TG-catalyzed post-translational modifications of proteins have been shown to be involved in the molecular mechanisms responsible for several human diseases. In particular, TG activity has been hypothesized to also be involved also in the molecular mechanisms responsible for human neurodegenerative diseases. In support of this hypothesis, Basso et al recently demonstrated that the TG inhibition protects against oxidative stress-induced neuronal death, suggesting that multiple TG isoforms participate in oxidative stress-induced cell death and that nonselective TG isoform inhibitors will be most effective in fighting oxidative death in neurological disorders. In this commentary, we discuss the possible molecular mechanisms by which TG activity could be involved in the pathogenesis of neurological diseases, with particular reference to neurodegenerative diseases, and the possible involvement of multiple TG isoforms expressed simultaneously in the nervous system in these diseases. Moreover, therapeutic strategies based on the use of selective or nonselective TG inhibitors for the amelioration of the symptoms of patients with neurological diseases, characterized by aberrant TG activity, are also discussed.
- Citation: Iannaccone M, Stefanile A, Vivo GD, Martin A, Serretiello E, Gentile V. Transglutaminase inhibition: A therapy to protect cells from death in neurodegeneration? World J Biol Chem 2012; 3(11): 184-186
- URL: https://www.wjgnet.com/1949-8454/full/v3/i11/184.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v3.i11.184
It was with great interest that we read the recent article by Basso et al[1], which demonstrates that the transglutaminase (TG) inhibition protects against oxidative stress-induced neuronal death and suggests that multiple TG isoforms participate in oxidative stress-induced cell death.
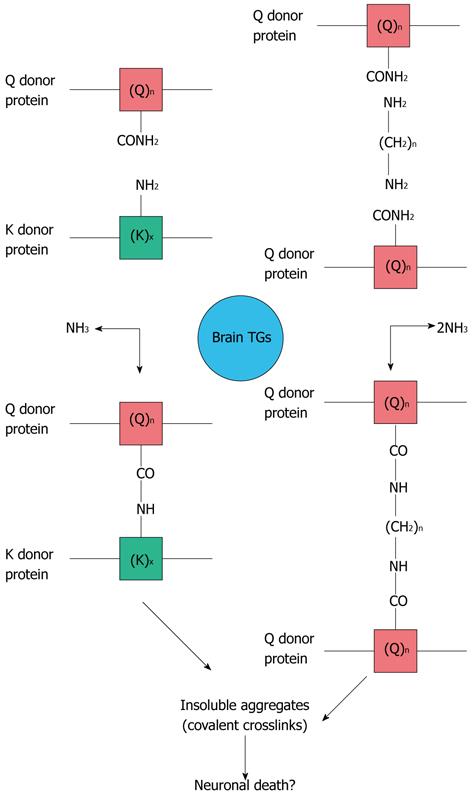
TGs are enzymes which catalyze irreversible post-translational modifications of proteins. Examples of TG-catalyzed reactions include: (1) acyl transfer between the γ-carboxamide group of a protein/polypeptide glutaminyl residue and the ε-amino group of a protein/polypeptide lysyl residue; (2) attachment of a polyamine to the γ-carboxamide of a glutaminyl residue; and (3) deamidation of the γ-carboxamide group of a protein/polypeptide glutaminyl residue[2-7]. To date, at least eight distinct differentially distributed TGs have been identified in the human body. Several forms of TGs are simultaneously expressed in the Nervous System[8-10]. Moreover, several alternative splice variants of TGs, mostly in the 3’-end region, have been identified. In particular TG2, which is the best-studied enzyme of the TG family, shows at least five splice variants[11-14]. Some of these splice variants, interestingly, are aberrantly expressed in neurological pathologies, such as Alzheimer’s disease (AD)[14].
Numerous scientific reports have suggested that TG activity is involved in the molecular mechanisms responsible for the pathogenesis of neurodegenerative diseases (Figure 1), but, to date, definitive experimental findings regarding the role of these enzymes in the development of these human diseases have not yet been obtained. Protein aggregates in affected brain regions are histopathological hallmarks of AD and many other neurodegenerative diseases[15], and more than 20 years ago, Selkoe et al[16] first suggested that TG activity might contribute to the formation of protein aggregates in the AD brain. Since then, however, although many studies suggested the possible involvement of the TGs in the formation of deposits of protein aggregates in neurodegenerative diseases, they do not indicate whether aberrant TG activity is directly responsible for the disease’s progression.
In this interesting study, Basso et al[1] found that in addition to TG2, the TG1 gene expression level is significantly induced following stroke in vivo or due to oxidative stress in vitro. Moreover, structurally diverse inhibitors, used at concentrations that inhibit TG1 and TG2 simultaneously, are neuroprotective. Together, these studies suggest that multiple TG isoforms, not only TG2, participate in oxidative stress-induced cell death signalling, and that isoform nonselective inhibitors of TG will be most effective in combating oxidative death in neurological disorders. This is an interesting and worthwhile study, and we agree with the suggestion that multiple TG isoform(s) can participate in neuronal death processes. However, we believe that, to minimize the possible side effects, selective inhibitors of the TGs should be required in the future for therapeutical approaches. In light of a lack of long-term effective treatments for human neurodegenerative diseases, the possibility that selective TG inhibitors may be of clinical benefit has been seriously considered. In this respect, some encouraging results have already been obtained with TG inhibitors in preliminary studies with different biological models of polyglutamine(CAG)-expansion diseases. For example, cystamine, which is already in phase II studies in humans with Huntington disease (HD), is a potent in vitro inhibitor of enzymes that require an unmodified cysteine at the active site[17]. It is important to note, however, that nausea, motor impairment and dosing schedule are some side effects reported as reasons for non-adherence during studies in humans[18,19]. Inasmuch as TGs contain a crucial active-site cysteine, cystamine has the potential to inhibit these enzymes by disulfide interchange reactions. A disulfide interchange reaction results in the formation of cysteamine and a cysteamine-cysteine mixed disulfide residue at the active site. Recent studies have shown that cystamine decreases the number of protein inclusions in transfected cells expressing the atrophin protein containing a pathological-length polyglutamine domain, responsible for the Dentato-Rubro-Pallido-Luysian Atrophy[20]. In other studies, cystamine administration to HD-transgenic mice resulted in an increase in life expectancy and amelioration of neurological symptoms[21,22]. Neuronal inclusions were decreased in one of these studies[22]. Although all these scientific reports seem to support the hypothesis of a direct role of TG activity in the pathogenesis of the polyglutamine diseases, cystamine is also found to act in the HD-transgenic mice by mechanisms other than the inhibition of TGs, such as the inhibition of Caspases[23], suggesting that this compound can have an additive effect in the therapy of HD. However, in support to the efficacy of this compound as a TG inhibitor, a recent scientific report showed that cystamine reduces aggregate formation in a mouse model of oculopharyngeal muscular dystrophy (OMPD), in which also the TG2 knockdown is capable of suppressing the aggregation and the toxicity of the mutant protein PABPN1[24], suggesting this compound as a possible therapeutic for OMPD.
In conclusion, a critical problem in the use of TG inhibitors in treating neurological diseases relates to the fact that, as previously reported, the human brain contains at least four TGs, including TG1, 2, 3[9] and possibly TG6[25,26], and a strong non-selective inhibitor of TGs might also inhibit plasma TG Factor XIIIa, causing a bleeding disorder. Therefore, from a number of standpoints it would seem that a selective inhibitor, which discriminates between TGs, would be preferable to an indiscriminate TG inhibitor.
Peer reviewers: Dr. Xiaotian Zhong, Department Health Policy and Management, School of Public Health, Harvard University, Boston, MA 02115, United States; Wayne Grant Carter, PhD, School of Biomedical Sciences, University of Nottingham, Queen’s Medical Centre, Nottingham NG7 2UH, United Kingdom; Steven G Gray, PhD, Translational Cancer Research Group, Department of Clinical Medicine, Trinity Centre for Health Sciences, Rm 2.103, Institute of Molecular Medicine, St James's Hospital, Dublin 8, Ireland; Gualtiero Alvisi, PhD, Department of Infectious Diseases Heidelberg University, INF345, 69121 Heidelberg, Germany; Chunbin Zou, MD, PhD, Assistant Professor of Medicine, Acute Lung Injury Center of Excellence, University of Pittsburgh Medical Center, BST E1103, 200 Lothrop Street, Pittsburgh, PA 15213, United States
S- Editor Song XX L- Editor A E- Editor Zhang DN
| 1. | Basso M, Berlin J, Xia L, Sleiman SF, Ko B, Haskew-Layton R, Kim E, Antonyak MA, Cerione RA, Iismaa SE. Transglutaminase inhibition protects against oxidative stress-induced neuronal death downstream of pathological ERK activation. J Neurosci. 2012;32:6561-6569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Folk JE. Mechanism and basis for specificity of transglutaminase-catalyzed epsilon-(gamma-glutamyl) lysine bond formation. Adv Enzymol Relat Areas Mol Biol. 1983;54:1-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Lorand L, Conrad SM. Transglutaminases. Mol Cell Biochem. 1984;58:9-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 542] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 4. | Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol. 2003;4:140-156. [PubMed] |
| 5. | Iismaa SE, Mearns BM, Lorand L, Graham RM. Transglutaminases and disease: lessons from genetically engineered mouse models and inherited disorders. Physiol Rev. 2009;89:991-1023. [PubMed] |
| 6. | Ricotta M, Iannuzzi M, Vivo GD, Gentile V. Physio-pathological roles of transglutaminase-catalyzed reactions. World J Biol Chem. 2010;1:181-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Belkin AM. Extracellular TG2: emerging functions and regulation. FEBS J. 2011;278:4704-4716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 155] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 8. | Bailey CD, Johnson GV. Developmental regulation of tissue transglutaminase in the mouse forebrain. J Neurochem. 2004;91:1369-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Kim SY, Grant P, Lee JH, Pant HC, Steinert PM. Differential expression of multiple transglutaminases in human brain. Increased expression and cross-linking by transglutaminases 1 and 2 in Alzheimer's disease. J Biol Chem. 1999;274:30715-30721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 171] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Grenard P, Bates MK, Aeschlimann D. Evolution of transglutaminase genes: identification of a transglutaminase gene cluster on human chromosome 15q15. Structure of the gene encoding transglutaminase X and a novel gene family member, transglutaminase Z. J Biol Chem. 2001;276:33066-33078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 147] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Gentile V, Saydak M, Chiocca EA, Akande O, Birckbichler PJ, Lee KN, Stein JP, Davies PJ. Isolation and characterization of cDNA clones to mouse macrophage and human endothelial cell tissue transglutaminases. J Biol Chem. 1991;266:478-483. [PubMed] |
| 12. | Monsonego A, Shani Y, Friedmann I, Paas Y, Eizenberg O, Schwartz M. Expression of GTP-dependent and GTP-independent tissue-type transglutaminase in cytokine-treated rat brain astrocytes. J Biol Chem. 1997;272:3724-3732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Lai TS, Liu Y, Li W, Greenberg CS. Identification of two GTP-independent alternatively spliced forms of tissue transglutaminase in human leukocytes, vascular smooth muscle, and endothelial cells. FASEB J. 2007;21:4131-4143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Citron BA, SantaCruz KS, Davies PJ, Festoff BW. Intron-exon swapping of transglutaminase mRNA and neuronal Tau aggregation in Alzheimer's disease. J Biol Chem. 2001;276:3295-3301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Adams RD, Victor M. Principles of Neurology. New York: McGraw-Hill 2009; . |
| 16. | Selkoe DJ, Ihara Y, Salazar FJ. Alzheimer's disease: insolubility of partially purified paired helical filaments in sodium dodecyl sulfate and urea. Science. 1982;215:1243-1245. [PubMed] |
| 17. | Griffith OW, Larsson A, Meister A. Inhibition of gamma-glutamylcysteine synthetase by cystamine: an approach to a therapy of 5-oxoprolinuria (pyroglutamic aciduria). Biochem Biophys Res Commun. 1977;79:919-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Dubinsky R, Gray C. CYTE-I-HD: phase I dose finding and tolerability study of cysteamine (Cystagon) in Huntington's disease. Mov Disord. 2006;21:530-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Langman CB, Greenbaum LA, Sarwal M, Grimm P, Niaudet P, Deschênes G, Cornelissen E, Morin D, Cochat P, Matossian D. A randomized controlled crossover trial with delayed-release cysteamine bitartrate in nephropathic cystinosis: effectiveness on white blood cell cystine levels and comparison of safety. Clin J Am Soc Nephrol. 2012;7:1112-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Igarashi S, Koide R, Shimohata T, Yamada M, Hayashi Y, Takano H, Date H, Oyake M, Sato T, Sato A. Suppression of aggregate formation and apoptosis by transglutaminase inhibitors in cells expressing truncated DRPLA protein with an expanded polyglutamine stretch. Nat Genet. 1998;18:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 242] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 21. | Karpuj MV, Becher MW, Springer JE, Chabas D, Youssef S, Pedotti R, Mitchell D, Steinman L. Prolonged survival and decreased abnormal movements in transgenic model of Huntington disease, with administration of the transglutaminase inhibitor cystamine. Nat Med. 2002;8:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 281] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 22. | Dedeoglu A, Kubilus JK, Jeitner TM, Matson SA, Bogdanov M, Kowall NW, Matson WR, Cooper AJ, Ratan RR, Beal MF. Therapeutic effects of cystamine in a murine model of Huntington's disease. J Neurosci. 2002;22:8942-8950. [PubMed] |
| 23. | Lesort M, Lee M, Tucholski J, Johnson GV. Cystamine inhibits caspase activity. Implications for the treatment of polyglutamine disorders. J Biol Chem. 2003;278:3825-3830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 120] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | Davies JE, Rose C, Sarkar S, Rubinsztein DC. Cystamine suppresses polyalanine toxicity in a mouse model of oculopharyngeal muscular dystrophy. Sci Transl Med. 2010;2:34ra40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Hadjivassiliou M, Aeschlimann P, Strigun A, Sanders DS, Woodroofe N, Aeschlimann D. Autoantibodies in gluten ataxia recognize a novel neuronal transglutaminase. Ann Neurol. 2008;64:332-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 177] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 26. | Thomas H, Beck K, Adamczyk M, Aeschlimann P, Langley M, Oita RC, Thiebach L, Hils M, Aeschlimann D. Transglutaminase 6: a protein associated with central nervous system development and motor function. Amino Acids. 2011;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |