AN OVERVIEW OF THE BIOLOGICAL ROLES OF GALECTIN-1
Galectin-1 is a 14.5-kDa β-galactoside-binding protein that belongs to a 15-member protein family[1-4], which are all evolutionarily well conserved[5], expressed by many different cell types[1-4] with major roles exerted in the immune system[6-9] and involved in the progression of various cancer types[2,3,10], including melanomas[7,11-14] and gliomas[15-20].
Galectin-1 belongs to the prototype galectins, which are characterized by one carbohydrate recognition domain (CRD) that can occur as a monomer or as a non-covalent homodimer consisting of subunits of a single CRD (galectin-1, about 29 kDa)[1-4]. Galectin-1 is functionally present intracellularly (cytosol, nuclei and intracellular plasma membrane) and extracellularly (extracellular cell membrane and extracellular matrix)[4]. Although galectins as a whole do not have the signal sequence required for protein secretion, galectin-1 is secreted by a yet unidentified export mechanism that bypasses the classical endoplasmic reticulum/Golgi apparatus-dependent secretory pathway that appears to balance tightly between intra- and extracellular galectin-1[21,22].
In addition to its carbohydrate-binding ability, galectin-1 is able to perform protein–protein interactions[23,24]. As a result, it participates in a variety of oncogenic processes including cell transformation[2,3,4,10], cell proliferation vs cell death[25-28], cell migration[2,3,4,18,29-31], metastasis[2,3,4,10] and angiogenesis[32-35]. As detailed below, galectin-1 also exerts subtle biochemical controls including modulations of the unfolded protein response (UPR)[33,36], following cytotoxic insults contributed by chemotherapy[11,36] and radiotherapy[37], as well as potential roles in mRNA splicing[38-40].
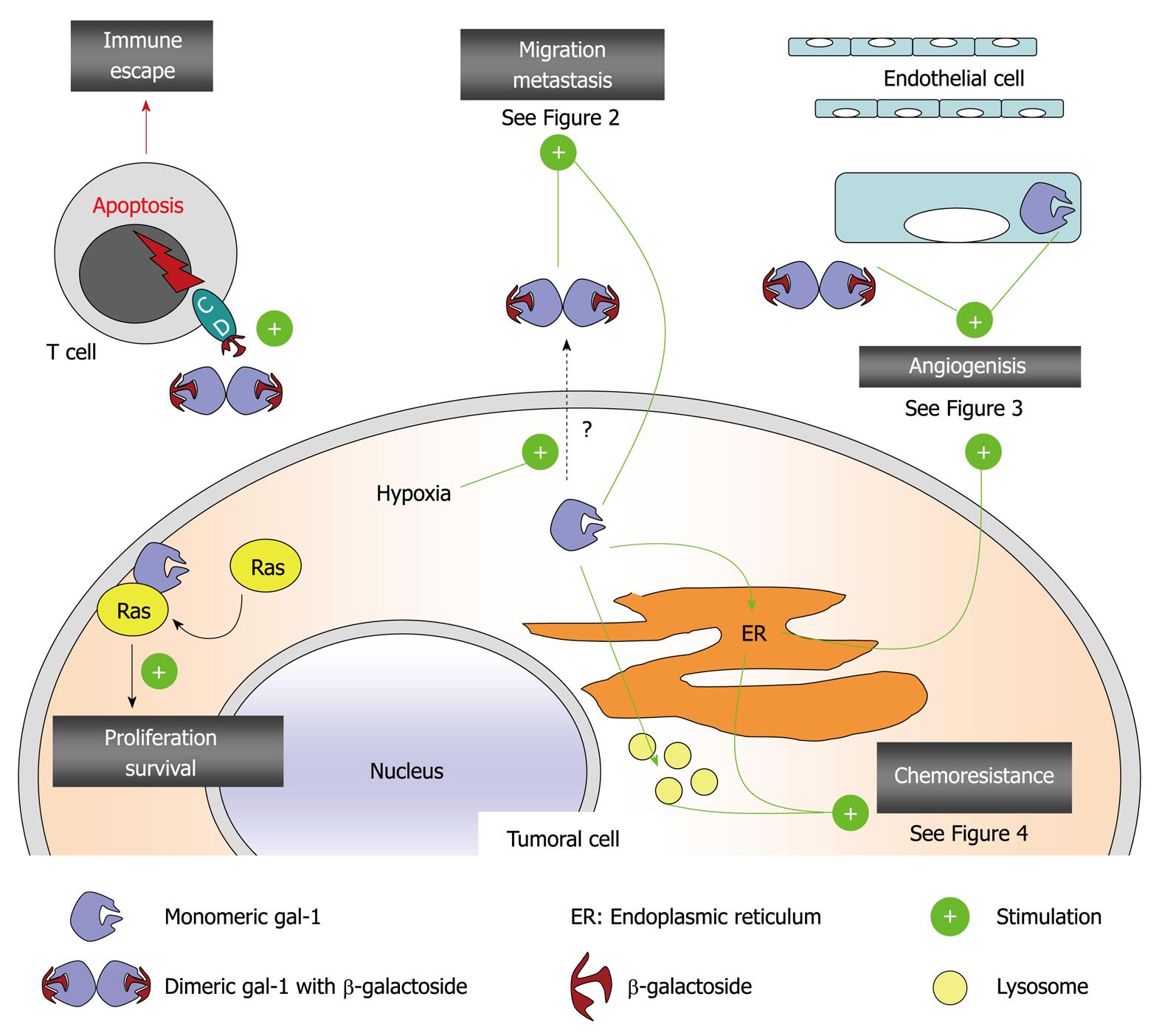
All these biological roles played by galectin-1 occur through the interactions with a myriad of extracellular and intracellular ligands and/or receptors including, but not limited to, laminin, fibronectin, vitronectin, integrins, CA-125, H-ras, CD45 and gemin-4[1-4,18,19]. Galectin-1 also plays major roles in tumor immune escape processes and in tuning the immune response. This aspect of galectin-1-related functions has been extensively reviewed[8,41-43] and is not further discussed here. Figure 1 illustrates the overall picture of galectin-1-related major roles in tumor cell biology.
Figure 1 Major roles of galectin-1 in tumor biology.
CD: Cluster of differentiation antigen, could be CD2, CD3, CD7, CD29, CD43 and/or CD45 that are expressed at the cell surface of activated T cell and that display typical β-galactosides recognized by galectin-1.
GALECTIN-1 AND MELANOMAS
Although melanomas account for only 4% of all dermatological cancers, they are responsible for 80% of deaths from skin cancer[44,45]. In fact, the incidence of melanoma is increasing worldwide, and the prognosis for patients with high-risk or advanced metastatic melanoma remains poor despite advances in the field[44]. Patients who progress to stage IV metastatic melanoma have a median survival of < 1 year[44]. Only 14% of patients with metastatic melanoma survive for 5 years[46]. Melanomas display both intrinsic and acquired resistance to proapoptotic stimuli[47], while most chemotherapeutic agents still used to combat melanoma are proapoptotic agents[48,49].
Great hope has been placed in vaccines, but only limited successes have been observed to date[44]. Indeed, clinical trials of melanoma vaccines have yielded inconclusive data on whether a positive melanoma-specific immune response predicts treatment benefit[45]. In fact, melanoma cells are able to escape natural, endogenous or therapeutically induced immune attacks[50]. The Rabinovich group[7,8] has elegantly demonstrated that melanoma-secreted galectin-1 induces apoptosis of activated T cells through recognition of glycosylated CD3, CD7 and CD45, thereby constituting an important mechanism of tumor escape in experimental melanomas. The blockade of this inhibitory signal can allow for and potentiate effective immune responses against melanoma cells with profound implications for cancer immunotherapy. Decreasing galectin-1 expression in melanoma could be achieved through the use of anti-galectin-1 siRNA, which we have developed[51] and aim to use for vaccine therapy in glioma patients[19] as detailed below.
Melanomas are associated with marked angiogenesis[52], and hypoxia favors melanoma progression[53]. Galectin-1 is a hypoxia-inducible protein[54,55] with marked proangiogenic effects[32-35] that have been demonstrated in experimental melanomas[11]. Great hope has also been placed in antiangiogenic therapies to combat melanomas[56,57], and decreasing galectin-1 expression in melanomas would be one strategy to accomplish this.
The PubMed database already includes about 2800 publications on galectins as of September 2011 with 920 of them related to galectin-1, but only 17 publications cross-reference melanoma and galectin-1, and they often do so indirectly. In other words, little information is available about the exact roles of galectin-1 in melanoma biology. The first report was published in 1995 by van den Brûle et al[58], who have shown that galectin-1 modulates human melanoma cell adhesion to laminin. Subsequently, galectin-1 has been demonstrated to participate in the aggregation of human melanoma cells through binding to the 90K/MAC-2BP glycoprotein[59]. As mentioned above, Rabinovich and his group have demonstrated the roles of galectin-1 in immune tumor escape processes using an experimental melanoma model[7]. We have used the same model to demonstrate the protective roles of galectin-1 against chemotherapy-induced cytotoxic insults in melanoma cells[11]. Although Rondepierre et al[14] have demonstrated a direct role for galectin-1 in the biological aggressiveness of experimental melanomas, Bolander et al[12] have failed to observe any correlation between the levels of galectin-1 expression in melanoma and patient survival. Nevertheless, our recent clinical data have indicated that galectin-1 is particularly highly expressed in advanced melanoma lesions, notably in comparison to galectin-3 and -9 (submitted manuscript).
Altogether, these data that are already available in the literature strongly suggest that galectin-1 could be implicated in various biological processes linked to melanoma progression, such as tumor immune escape, tumor angiogenesis and chemoresistance. However, most of these data are from experimental models, and clinical confirmation of these findings is warranted.
GALECTIN-1 AND GLIOMAS
There are 19 publications available in the PubMed database with cross references about galectin-1 and gliomas and 17 with cross references about galectin-1 and melanomas (as of July 2011). However, much more information is available about how galectin-1 controls glioma cell biology than melanoma cell biology.
Gliomas are the most common primary brain tumors, among which glioblastoma (GBM) is the most malignant form. Malignant gliomas, especially GBMs, are characterized by the diffuse invasion of distant brain tissue by a myriad of single migrating cells with reduced levels of apoptosis and consequent resistance to the cytotoxic insults of proapoptotic drugs[60-62]. In contrast, GBM cells are less resistant to cell death induced by sustained proautophagic processes[62]. Current clinical recommendations for treating malignant glioma patients, and more specifically GBM patients, include maximum surgical resection followed by concurrent radiation and chemotherapy with temozolomide[62-64]. This clinical protocol is now the standard of care for treating GBM patients, and the overall survival rates have increased from 11% to 27% at 2 years, from 4% to 16% at 3 years, from 3% to 12% at 4 years, and from 2% to 10% at 5 years, compared to surgery and radiotherapy alone[63,64].
As detailed below, galectin-1 significantly affects glioma progression. Experimental data have already been validated, at least partly, by clinical data. By using computer-assisted microscopy, we have quantitatively characterized the levels of expression of galectins-1, 3 and 8 in 116 human astrocytic tumors of grades I-IV by immunohistochemistry. The data have indicated that the levels of galectin-1 and 3 expression significantly changes during the progression of malignancy in human astrocytic tumors, whereas galectin-8 remains unchanged[16]. We have extended our analyses to include a quantitative immunohistochemical determination of galectin-1 expression in 220 gliomas, including 151 astrocytic, 38 oligodendroglial and 31 ependymal tumors obtained from surgical resections[65]. We have also xenografted three human glioblastoma cell lines (H4, U87 and U373 models) into the brains of nude mice to characterize the in vivo galectin-1 expression pattern following subsequent invasion into the normal brain parenchyma[65]. In addition, we have characterized in vitro the role of galectin-1 in U373 tumor astrocyte migration and kinetics. Our data have revealed expression of galectin-1 in all human glioma types, with no striking differences between astrocytic, oligodendroglial and ependymal tumors[65]. The level of galectin-1 expression is correlated only with grade of astrocytic tumors[65]. Furthermore, immunopositivity of high-grade astrocytic tumors from patients with short-term survival periods is stronger than tumors from patients with long-term survival[65]. In human glioblastoma xenografts, galectin-1 is preferentially expressed in the more invasive parts of the xenografts[65]. In vitro experiments have revealed that galectin-1 stimulates the migration of U373 astrocytes[65]. Most of these data that we have produced[16,65] along with Yamaoka et al[15], have been validated by Jung et al[17].
We recently have reviewed the general roles of galectins, in particular galectin-1, in gliomas[18]. We therefore focus our attention below on the biochemical pathways in which galectin-1 is implicated when controlling various processes related to glioma progression and to a lesser extent, melanoma progression.
As mentioned earlier, we do not review in detail the marked roles exerted by galectin-1 when tuning the immune system response, and those associated with the tumor immune escape phenomenon, because all these aspects have been reviewed in depth by others[8,41-43]. Nevertheless, we highlight galectin-1 as a major target to combat gliomas when vaccine therapy is proposed for gliomas[66] and melanomas. Active specific immunotherapy based on dendritic cell vaccination is indeed considered to be a new promising concept aimed at generating an antitumoral immune response in malignant gliomas and melanomas that still have dismal prognosis despite multimodal treatments[19,66]. However, it is now widely accepted that the success of immunotherapeutic strategies to promote tumor regression will rely not only on enhancing the effector arm of the immune response, but also on the downregulation of the counteracting tolerogenic signals[19,66]. We recently reviewed why galectin-1 should be actively targeted to lower glioma-mediated immune escape during vaccine therapy[19].
GALECTIN-1 CONTROLS GLIOMA CELL MIGRATION
Although cell migration is the net result of adhesion, motility and invasion[60], galectin-1 modifies each of these three cell-migration-related processes in glioma cells.
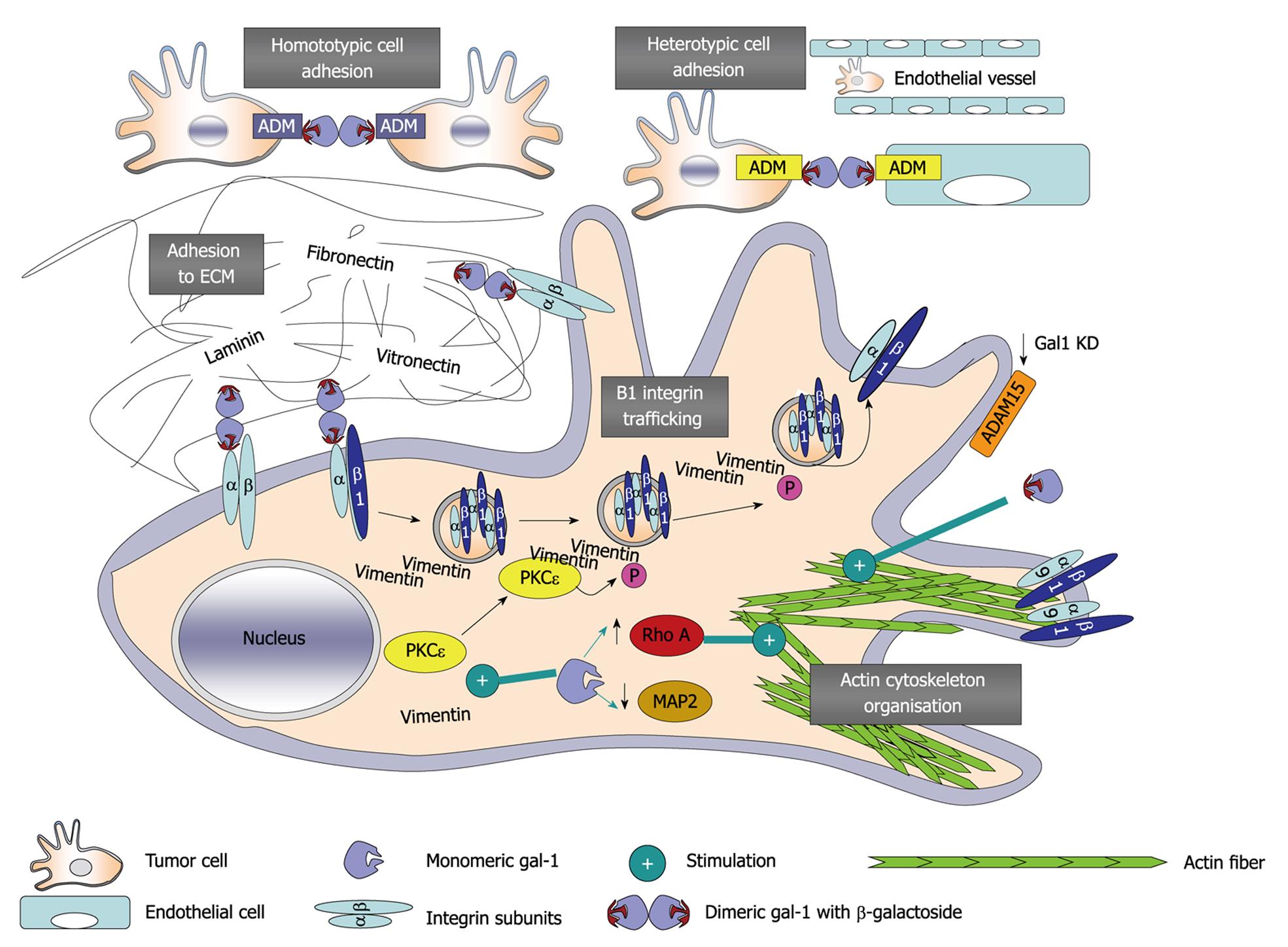
Immunohistochemical analysis of galectin-1 expression in human U87 and U373 glioblastoma xenografts from the brains of immunodeficient mice has revealed a higher level of galectin-1 expression in invasive areas compared with non-invasive areas of the xenografts[16,65,67]. Immunodeficient mice intracranially grafted with U87 or U373 cells that constitutively express low levels of galectin-1 (by stable transfection of an expression vector containing the antisense mRNA of galectin-1) have longer survival periods than those grafted with U87 or U373 cells that express normal levels of galectin-1,[67], and galectin-1 added to the culture medium markedly increases the motility of human neoplastic astrocytes[16,65,67]. We have demonstrated that these effects are at least partly related to marked modifications in the organization of the actin cytoskeleton and increases in small GTPase RhoA expression[67]. We have also investigated stable knockdown of galectin-1 in human U87 glioblastoma cells, and observed major alterations in gene expression when we used cDNA microarray analysis. Among the 631 genes tested that are potentially involved in cancer, the expression of 86 genes was increased at least twofold[68], including ADAM-15 (disintegrin and metalloproteinase domain-containing protein 15) and microtubule-associated protein (MAP) 2, and expression of these proteins was confirmed by immunocytochemistry[68]. The major differences in the patterns of the actin stress fiber organization have also been observed[68], as previously reported for other glioma models[67], and U87 glioma cells that are stably deficient for galectin-1 expression are significantly less motile than control cells[68], as previously observed[67].
Genes whose patterns of expression markedly change during galectin-1 knockdown include α-7/β-1 and α-9/β-1 integrins[68]. These data must be analyzed in parallel with those we report above with respect to galectin-1-mediated modifications in ADAM-15 expression because the ADAM family of membrane-anchorage glycoproteins encompass a catalytically active matrix metalloproteinase domain and a disintegrin domain, and may also be involved in the proteolytic cleavage of cell-surface proteins and in integrin-mediated cell adhesion (including α-9/β-1 integrin/ADAM-15 interactions) via RGD-dependent and -independent binding[68]. Using immunofluorescence approaches, we observed that the depletion of galectin-1 through both stable knockdown and transient-targeted siRNA treatments induced an intracellular accumulation of β-1 integrin, along with a decrease in the expression of this integrin at points of adhesion on the cell membrane[69]. Galectin-1 depletion does not alter the gene expression level of β-1 integrin[69]. Transient galectin-1 depletion induces perinuclear accumulation of protein kinase C (PKC)ε and intermediate filament vimentin, both of which have been shown to mediate integrin recycling in motile cells. These data emphasize the involvement of galectin-1 in the PKCε/vimentin-controlled trafficking of β-1 integrin in glioma cells. Figure 2 illustrates the gross picture of galectin-1-related major roles in glioma cell migration.
Figure 2 Processes and pathways mediated by galectin-1 in cancer cell migration.
ADM: Adhesion molecules; ADAM-15: Disintegrin and metalloproteinase domain-containing protein 15; ECM: Extracellular matrix; MAP2: Microtubule-associated protein 2; P: Phosphorylation; PKCε: Protein kinase C ε; RhoA: Ras homolog protein, member A GTPase.
GALECTIN-1 CONTROLS NEOANGIOGENESIS IN GLIOMAS AND MELANOMAS
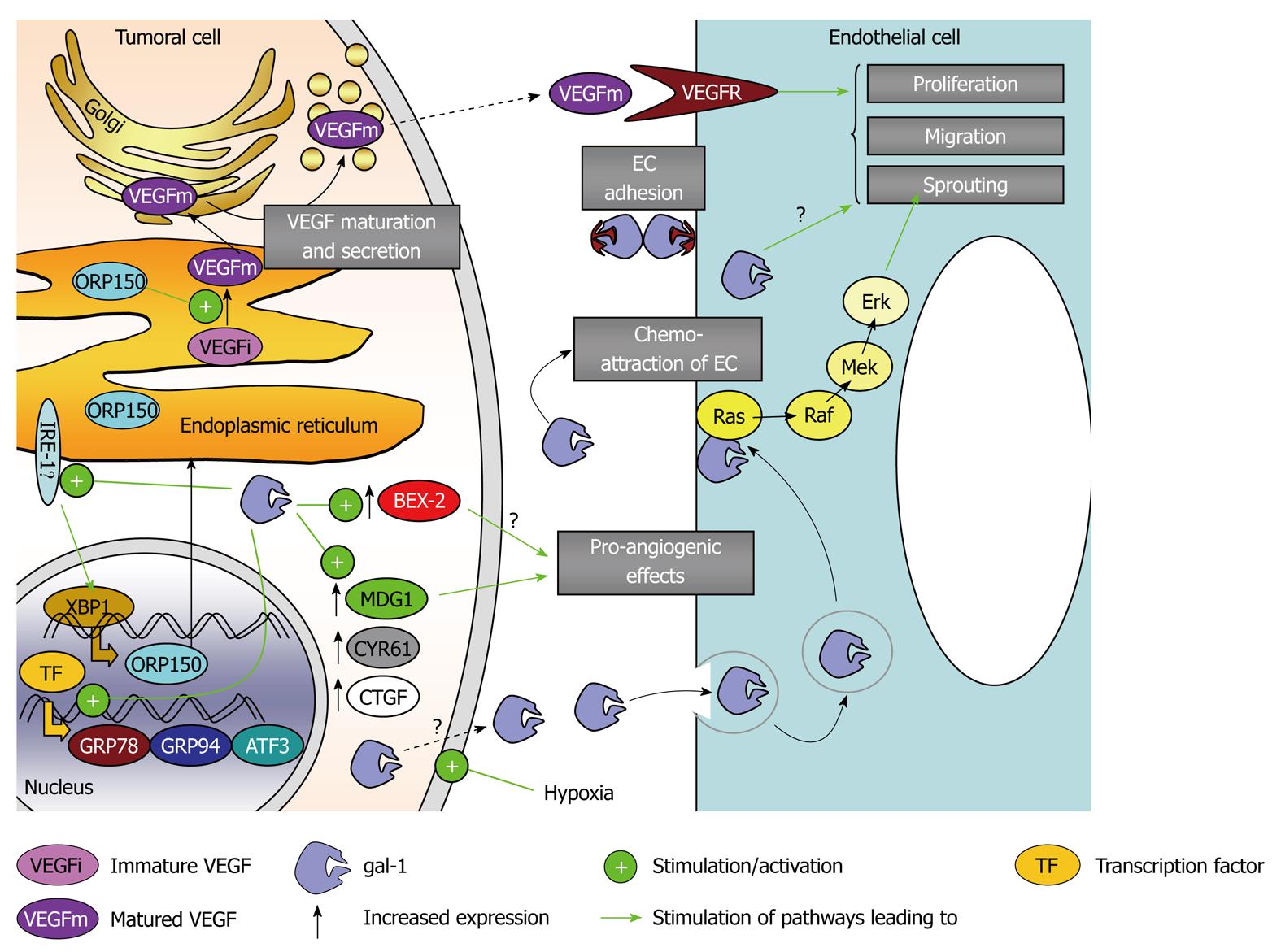
As mentioned earlier, galectin-1 is a hypoxia-regulated protein[54,55] that has been shown to have major roles in angiogenesis[32,35] of gliomas[33] and melanomas[11]. We first highlighted that galectin-1 depleted melanoma tumors in vivo display lower angiogenesis levels in close association with marked necrotic processes[11]. We have recently begun to decipher the molecular and biochemical pathways through which galectin-1 controls angiogenesis. In gliomas, we have demonstrated that galectin-1 signals through the IRE-1α (endoplasmic reticulum transmembrane kinase/ribonuclease inositol-requiring 1α), which regulates the expression of oxygen-regulated protein 150 (ORP150) that in turn controls vascular endothelial growth factor (VEGF) maturation[33]. Thus, galectin-1 controls glioma angiogenesis through ORP150-mediated VEGF maturation[33]. Similarly, galectin-1 depletion is associated with decreased ORP150 expression level in melanoma cells (unpublished data). Galectin-1 also modulates the expression of several other hypoxia-related genes (e.g. CTGF, ATF3, PPP1R15A, HSPA5, TRA1 and CYR61) in the Hs683 glioma model, which are known to display various roles in angiogenesis, which we have demonstrated in galectin-1-dependent angiogenesis in glioma[33].
We have observed a marked decrease in the expression of the brain-expressed X-linked gene, BEX2, with decreased galectin-1 expression in glioma cells through targeted anti-galectin-1 siRNA[34]. We have thus focused on BEX2, and observed that decreasing BEX2 expression in human Hs683 glioma cells increased the survival of Hs683 orthotopic xenograft-bearing immunodeficient mice, whose tumors displayed decreased angiogenic levels[34]. Furthermore, this decrease in BEX2 expression impaired vasculogenic mimicry channel formation in vitro, as observed when depleting galectin-1 in both glioma and melanoma cells. Thus, BEX2 is a second target, in addition to ORP150, through which galectin-1 controls angiogenesis in gliomas.
BEX2 also modulates glioma cell migration at both adhesion and invasion levels through the modification of several genes previously reported to play a role in cancer cell migration, including MAP2, plexin C1, SWAP70, and β-6 integrin[34].
Galectin-1 controls key angiogenic factors/ pathways in tumoral cells. In addition, galectin-1 has been shown to regulate directly the biological properties of endothelial cells, such as proliferation, activation and in vitro tubular network formation[32,35]. We have observed earlier that galectin-3 also participates in glioma angiogenesis[70].
GALECTIN-1 AND ITS MODULATING ROLES IN CHEMOTHERAPY AND RADIOTHERAPY IN THE SPECIFIC CONTEXT OF MELANOMAS AND GLIOMAS
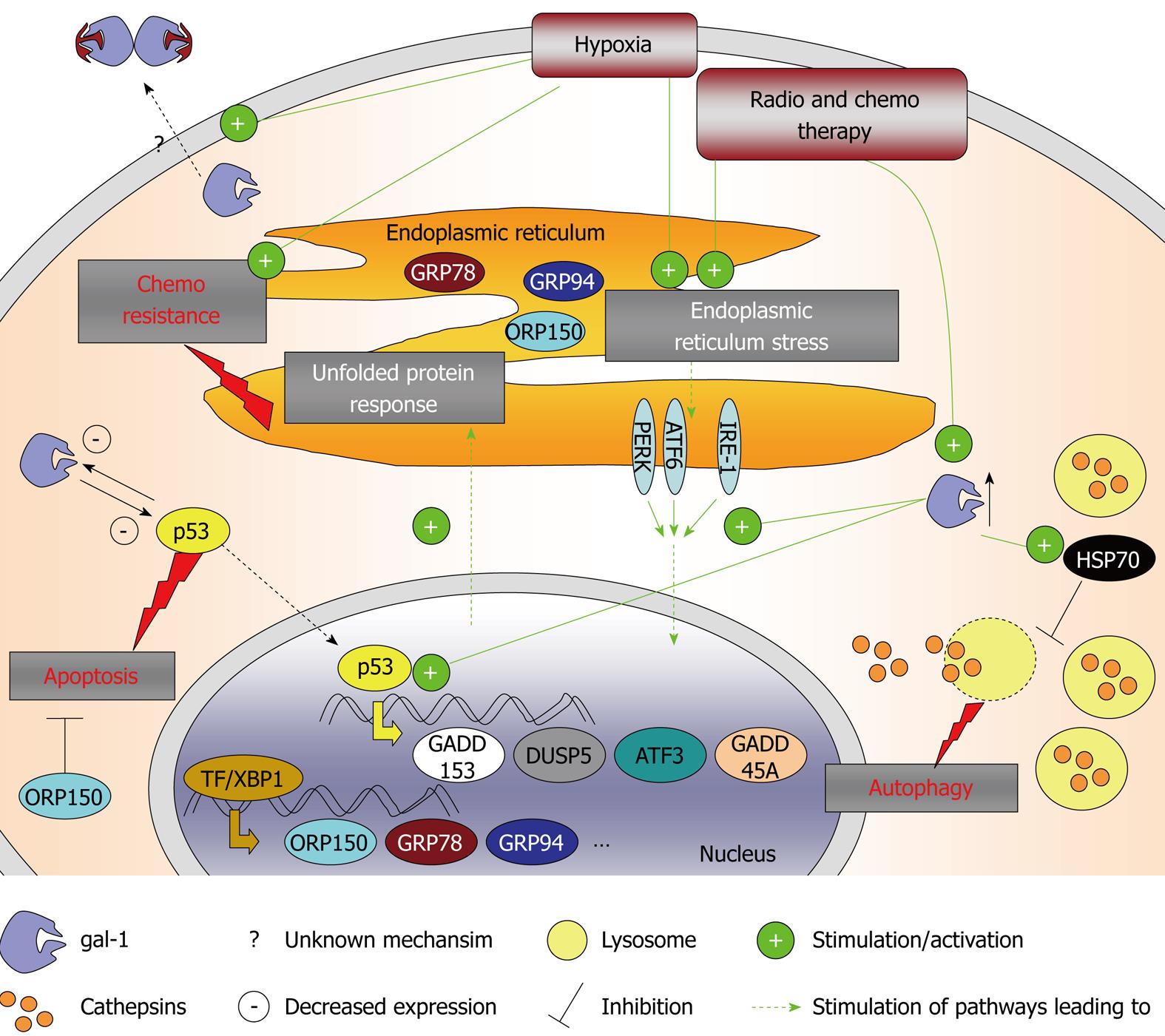
Intratumoral hypoxia causes genetic changes in cancers that produce a microenvironment that selects for cells with a more aggressive phenotype[71]. Hypoxia can initiate cell demise by apoptosis/necrosis but can also prevent cell death by provoking adaptive responses that facilitate cell proliferation or angiogenesis, thus contributing to tumor malignant progression[71], and in particular, gliomas[72] and melanomas[53]. Hypoxia is also known to modulate the UPR, a coordinated program that promotes cell survival under conditions of endoplasmic reticulum (ER) stress, which is known to contribute to tumor malignant progression and drug resistance of solid tumors[73]. Considering that galectin-1 is a hypoxia-regulated protein[54,55], we have investigated whether it can interfere with ER stress responses and chemosensitivity. We have examined whether decreasing galectin-1 expression (by means of a siRNA approach) in human Hs683 GBM cells and B16F10 melanoma cells could increase their sensitivity to pro-autophagic or proapoptotic drugs. These data have revealed that temozolomide, the standard treatment for glioma patients, increases galectin-1 expression in Hs683 cells both in vitro[36] and in vivo[33]. In contrast, reducing galectin-1 expression in these Hs683 glioma cells using siRNA increases the antitumor effects of various chemotherapeutic agents, in particular temozolomide, both in vitro and in vivo[33,36], which is a feature that we also have observed in experimental melanomas[11]. This decrease in galectin-1 expression in Hs683 glioma cells does not induce apoptotic or autophagic features, but is found to modulate p53 transcriptional activity and decrease p53-targeted gene expression including DDIT3/GADD153/CHOP, DUSP5, ATF3 and GADD45A[36]. Puchades et al[74] have demonstrated that galectin-1 expression is negatively regulated by transfection with TP53 in glioma cells. We have further observed that the decrease in galectin-1 expression in glioma cells also impairs the expression levels of seven other genes implicated in chemoresistance: ORP150, HERP, GRP78/Bip, TRA1, BNIP3L, GADD45B and CYR61; some of which are located in the ER and whose expression is also known to be modified by hypoxia[36]. In the case of the B16F10 mouse melanoma model, decreasing galectin-1 expression in vitro by means of an anti-galectin-1 siRNA approach does not modify their sensitivity to apoptosis or autophagy[11]. However, it does induce heat-shock-protein-70-mediated lysosomal membrane permeabilization, a process associated with cathepsin B release into the cytosol, which in turn is believed to sensitize the cells to the pro-autophagic effects of temozolomide when grafted in vivo[11].
Galectin-1 expression is also upregulated by ionizing irradiation in glioma cell lines[37]. Therefore galectin-1 appears to be induced in cases of various cellular stress stimuli and could promote cell survival through the various mechanisms described above and illustrated in Figure 3.
Figure 3 Galectin-1-mediated control of angiogenesis.
ATF3: Activating transcription factor 3; BEX2: Brain-expressed X-linked 2; CTGF: Connective tissue growth factor; CYR61: Cysteine-rich angiogenic inducer 61; Erk: Extracellular signal-regulated kinase; GRP78 and GRP94: Glucose-regulated protein 78 and 94; IRE1-α: Endoplasmic reticulum to nucleus signaling 1 α; MDG1: Microvascular endothelial differentiation gene 1; Mek: Mitogen-activated protein kinase kinase; ORP150: Oxygen-related protein 150; Raf: Homolog to murine v-raf leukemia oncogene serine/threonine kinase; Ras: Rat sarcoma virus oncogene protein; TF: Transcription factor; VEGF: Vascular endothelial growth factor; VEGFR: VEGF receptor; XBP1: X-box-binding protein 1. EC: Endothelial cell.
CONCLUSION
Gliomas and melanomas are associated with dismal prognosis because of their marked intrinsic resistance to proapoptotic stimuli such as conventional chemotherapy and radiotherapy, as well as their capability to escape immune cell attacks. In addition, gliomas and melanomas display pronounced neoangiogenesis. Galectin-1 is a hypoxia-sensitive protein that is abundantly secreted by glioma and melanoma cells, displays marked proangiogenic effects, and provides immunotolerogenic environments to melanoma and glioma cells through the killing of activated T cells that attack these tumor cells. Galectin-1 also protects glioma and melanoma cells against cytotoxic insults (chemotherapy and radiotherapy) through a direct role in the UPR. Altogether, these facts clearly point to galectin-1 as an important target in gliomas and melanomas, to weaken the defenses of these two types of cancers against radiotherapy, chemotherapy and immunotherapy/vaccine therapy, and to reinforce antiangiogenic therapies Figure 4.
Figure 4 How galectin-1 is involved in chemoresistance of cancer cells.
ATF3 and ATF6: Activating transcription factor 3 and 6; DUSP5: Dual specificity protein phosphatase 5; GADD153 and GADD45A: Growth arrest and DNA damage inducible protein 153 and 45 α; GRP78 and GRP94: Glucose-regulated protein 78 and 94; HSP70: Heat shock protein 70; IRE1-α: Endoplasmic reticulum to nucleus signaling 1 α; ORP150: Oxygen-related protein 150; PERK: Protein kinase RNA-like endoplasmic reticulum kinase; TF: Transcription factor; XBP1: X-box-binding protein 1.
Peer reviewers: Ming Zhang, PhD, Associate Professor, Department of Molecular Pharmacology and Biological Chemistry, Feinberg School of Medicine Northwestern University, 303 E. Superior St., Chicago, IL 60611, United States; Shile Huang, PhD, Associate Professor, Department of Biochemistry and Molecular Biology, Louisiana State University Health Sciences Center, Shreveport, LA 71130-3932, United States; Tatjana Abaffy, Dr, Molecular and Cellular Pharmacology, University of Miami, Miller School of Medicine, 1600 NW 10 Ave, Miami, FL 33136, United States
S- Editor Cheng JX L- Editor Kerr C E- Editor Zheng XM