IKAROS AS A TUMOR SUPPRESSOR AND REGULATOR OF HEMATOPOIESIS
The Ikaros gene encodes a zinc finger, DNA-binding protein that acts as regulator of gene transcription and chromatin remodeling[1]. Studies of Ikaros mutant mice have established Ikaros as a master regulator of hematopoiesis[1]. Partial arrest or defects in normal hematopoiesis often lead to aberrant cellular proliferation and leukemia/lymphoma, therefore, it is not surprising that Ikaros knockout mice that lack one copy of Ikaros develop T cell leukemia[2]. The remarkable observation is that these mice developed T cell leukemia with 100% penetrance, and in each case, the leukemic clones arose from cells that had lost the single wild-type Ikaros allele[2]. This suggests an essential role for Ikaros as a tumor suppressor in T cell differentiation. In humans, defects in the Ikaros gene (90% of observed defects involved deletions of one allele, while the remainder involved nonsense or functionally inactivating mutations of a single allele) can result in the production of dominant negative (DN) Ikaros isoforms that act to suppress the function of full-length Ikaros. Ikaros defects have been associated with the development of a variety of hematopoietic malignancies. These include childhood acute lymphoblastic leukemia (ALL)[3,4] infant T-cell ALL[5], adult B cell ALL[6], myelodysplastic syndrome[7], acute myeloid leukemia[8], and adult and juvenile chronic myeloid leukemia[9]. Ikaros defects leading to a loss of Ikaros activity have been detected in 30% of pediatric B-cell ALL, in > 80% of BCR-ABL1 ALL, and approximately 5% of T-cell ALL[10,11]. In addition, defective Ikaros has been identified as a poor prognostic marker for childhood ALL[4,12-14]. A noteworthy observation is that, in almost all primary human leukemia cells in which an Ikaros defect is observed, one wild-type Ikaros copy is retained. These data not only show a strong association between the loss of Ikaros function and the development of human leukemia, but also suggest that even a moderate alteration of Ikaros function (e.g. haploinsufficiency) is sufficient to promote malignant transformation. The aberrant expression of small DN Ikaros isoforms has also been associated with the development of human pituitary adenoma[15]. The current hypothesis is that small Ikaros isoforms act as DN mutants in human cells and their overexpression promotes malignant transformation, while the full-length Ikaros acts as a tumor suppressor.
Several crucial questions remain unanswered. (1) Is the loss of Ikaros activity an essential step in the malignant transformation of hematopoietic cells? (2) How is the function of Ikaros regulated in normal and leukemia cells? (3) Can alterations in the regulation of Ikaros function contribute to the development of leukemia?
A partial answer to the first question came when the T leukemia cells derived from Ikaros-deficient mice were transduced with retrovirus to express wild-type Ikaros. The introduction of wild-type Ikaros at physiological levels led to cessation of growth, induction of T-cell differentiation, and cell cycle arrest in Ikaros-deficient T-leukemia cells[16]. These results suggest that the presence of functional wild-type Ikaros, at physiological levels, is sufficient to arrest the aberrant proliferation of malignant cells. This experiment involved a single leukemia cell line that completely lacked Ikaros expression, therefore, this does not fully answer the question of whether the loss of Ikaros function is an essential step in leukemogenesis, although it does underscore the importance of functional Ikaros in tumor suppression. To address these issues regarding the importance of the regulation of Ikaros activity in the development of leukemia, the first step will be to identify the mechanisms that regulate Ikaros activity in normal and malignant hematopoiesis, and to dissect their role in regulating the function of Ikaros.
IKAROS IS PHOSPHORYLATED AT MULTIPLE SITES
The function of many proteins is regulated by their phosphorylation status. Protein phosphorylation is a reversible, dynamic process. The balance between phosphorylation states of a protein regulates its overall function. The in vivo phosphopeptide mapping of Ikaros provided the first evidence that Ikaros is phosphorylated at multiple sites[17]. The observation that phosphorylated amino acids within Ikaros are evolutionarily conserved suggests that phosphorylation is an important mechanism regulating Ikaros function. Further phosphopeptide mapping demonstrated that Ikaros phosphorylation sites are very similar in primary thymocytes, in leukemia cells, and in the HEK 293T embryonic kidney carcinoma cell line following transduction or transfection to express Ikaros[17,18]. This suggests that phosphorylation of Ikaros occurs by kinases that are present in multiple tissues and that phosphorylation is an integral feature of Ikaros regulation (Figure 1).
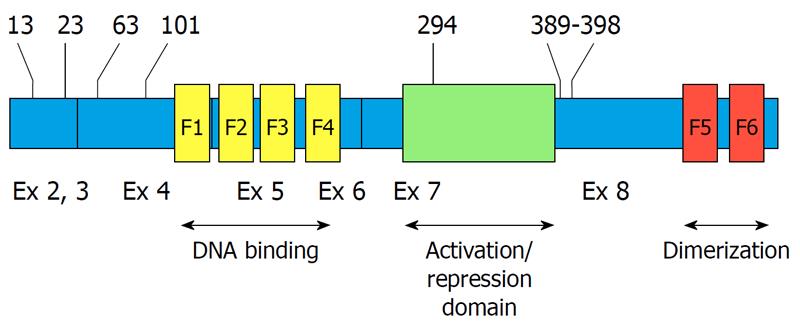
Figure 1 Location of casein kinase 2 phosphorylation sites on Ikaros.
The phosphorylated amino acids are indicated by numbers at the top. The location of zinc fingers is indicated by yellow (F1-F4) and red bands (F5-F6). Exons (Ex) are indicated at the bottom. Exon 1 (untranslated) is not shown.
CELL-CYCLE-SPECIFIC PHOSPHORYLATION OF IKAROS
The first study to examine the role of phosphorylation in regulating Ikaros function focused on the cell-cycle-specific phosphorylation of Ikaros. In vivo phosphopeptide mapping of Ikaros at different stages of the cell cycle has revealed that during mitosis, Ikaros undergoes hyperphosphorylation[17]. Point mutation analysis has demonstrated that the cell-cycle-specific phosphorylation of Ikaros occurs at an evolutionarily conserved linker sequence that connects DNA-binding zinc finger motifs. Mutational analysis of phosphomimetic and phosphoresistant Ikaros mutants has shown that the cell-cycle-specific phosphorylation of Ikaros regulates its DNA-binding ability and nuclear localization during mitosis[17]. The linker sequence that connects the zinc finger motifs is preserved in all Kruppel-like zinc finger proteins[19], therefore, this mitosis-specific phosphorylation is not unique to Ikaros, but rather it appears to serve as a global control mechanism of cell cycle progression during mitosis. The kinase that is responsible for the mitosis-specific phosphorylation of Ikaros has not been identified and is considered to be a different kinase from the one responsible for Ikaros phosphorylation during G1 and S phases.
PHOSPHORYLATION OF IKAROS BY CASEIN KINASE 2 AND CELL CYCLE PROGRESSION
The studies described above have established that phosphorylation can control the function of Ikaros in a cell-cycle-specific manner, but have not identified the signal transduction pathway that regulates the function of Ikaros during G1 and S phases of the cell cycle. Studies by Georgopoulos and colleagues have identified several amino acids in Ikaros that are phosphorylated by casein kinase 2 (CK2) (Figure 1). Substitution analysis has revealed that phosphorylation of Ikaros by CK2 at its C-terminal region regulates its ability to control G1/S cell cycle progression[20]. These results have identified CK2 as the enzyme that directly controls Ikaros function, and have demonstrated that CK2-mediated phosphorylation can regulate cell cycle progression.
CK2-MEDIATED PHOSPHORYLATION REGULATES IKAROS FUNCTION IN TRANSCRIPTIONAL REGULATION AND DIFFERENTIATION
A subsequent study has identified four novel evolutionarily conserved CK2 phosphorylation sites located at the N-terminal end of Ikaros (Figure 1)[18]. Functional analysis of Ikaros phosphomimetic mutants (where phospho-sites are mutated to aspartate to mimic phosphorylation) and phosphoresistant mutants (where phospho-sites are mutated to alanine to mimic the dephosphorylated state) has revealed that phosphorylation by CK2 affects Ikaros function at many different levels. Phosphorylation of amino acids 13 and 294 results in decreased Ikaros DNA binding affinity for probes that are derived from pericentromeric heterochromatin (PC-HC). In vivo, phosphorylation of the same amino acids causes Ikaros to lose its ability to localize into PC-HC, resulting in diffuse nuclear distribution of Ikaros. These results have provided the first evidence that CK2-mediated phosphorylation regulates not only the ability of Ikaros to bind DNA, but also its subcellular localization and function in chromatin remodeling[18]. We want to emphasize that the cell-cycle-specific phosphorylation of Ikaros that occurs during mitosis is not due to the activity of CK2 because: (1) CK2 is active during G1 and S phases of the cell cycle while this phosphorylation is mitosis-specific; and (2) the CK2 consensus site is well established, and the mitosis-specific phosphorylation of Ikaros occurs at a consensus linker sequence that shows no resemblance to the consensus recognition motif of CK2.
This study also examined the role of CK2-mediated phosphorylation in regulating the ability of Ikaros to bind the upstream regulatory element of the Ikaros target gene, TdT (dntt). Results have shown that phosphoresistant Ikaros mutants have much higher DNA-binding affinity toward the TdT regulatory elements when compared to wild-type Ikaros in thymocytes or in HEK 293T cells. Further analysis has revealed that phosphorylation of Ikaros changes during T-cell differentiation. Following the induction of thymocyte differentiation with phorbol myristate acetate, Ikaros undergoes dephosphorylation at amino acids 13 and 294. This results in increased Ikaros binding to the TdT regulatory element and repression of TdT transcription[18]. These data demonstrate that CK2-mediated phosphorylation of Ikaros regulates expression of a key gene in T-cell development - TdT. Thus, CK2-mediated phosphorylation of Ikaros is one of the regulatory mechanisms that govern Ikaros function in normal hematopoiesis.
PROTEIN PHOSPHATASE 1 DEPHOSPHORYLATES IKAROS AND REGULATES ITS ACTIVITY
The studies described above were limited in that they examined only the phosphorylation of Ikaros amino acids that are detected in vivo. Phosphopeptide mapping suggests the presence of more phosphorylated amino acids on Ikaros and thus the potential for additional phosphorylation-regulated functions of Ikaros. The discovery that Ikaros is dephosphorylated in vivo by protein phosphatase 1 (PP1), and identification of the PP1-Ikaros interaction site, has provided an opportunity for further studies of the role of phosphorylation in regulating Ikaros function[21]. Ikaros with a mutated PP1 interaction site cannot be dephosphorylated by this enzyme. When this mutant is transfected into HEK 293T cells, its protein binds DNA very poorly compared to wild-type Ikaros. This indicates that a very large percentage of Ikaros undergoes phosphorylation in vivo and that dephosphorylation of Ikaros is essential for its activity. In addition, this mutant is unable to localize to PC-HC, which confirms that phosphorylation controls the subcellular localization of Ikaros. When phosphoresistant mutations of the CK2 phosphorylation sites are introduced into the PP1-nonbinding Ikaros mutant, DNA-binding ability and PC-HC localization is restored to a level similar to that observed for wild-type Ikaros. These data have established that CK2-mediated phosphorylation is the major regulator of Ikaros function as a DNA-binding protein and in chromatin remodeling/PC-HC localization[21]. They also suggest that PP1 has the opposite effect on Ikaros function, and that these two signaling pathways are the major regulators of Ikaros activity.
One striking feature of the PP1-nonbinding Ikaros mutant is its low level of expression, as compared to wild-type Ikaros, when transfected into HEK 293T cells. That difference is prominent at the protein level, whereas the mRNA level of both constructs is similar. A possible explanation is that Ikaros that is unable to interact with PP1 undergoes increased degradation compared to wild-type Ikaros. This hypothesis is supported by the observation that Ikaros contains two very strong PEST sequences. The presence of PEST sequences is associated with increased degradation of protein following phosphorylation at amino acids [proline (P), glutamic acid (E), serine (S), and threonine (T)] that are located within the PEST sequence. Analysis of the known CK2 phosphorylation sites that have been identified by us and by the Georgopoulos group has revealed that PEST sequences contain multiple phospho-sites that are phosphorylated in vivo by CK2. This suggests that CK2-mediated phosphorylation might promote degradation of Ikaros. In vivo degradation assays have demonstrated that the Ikaros mutant that does not interact with PP1 has a severely decreased half-life compared to wild-type Ikaros. The introduction of phosphoresistant mutations at the known CK2-mediated phosphorylation sites prolongs the half-life of the PP1-nonbinding mutant[21]. These results have identified another important role of CK2: to regulate the protein stability and turnover of Ikaros. These results have demonstrated that the hyperphosphorylation of Ikaros leads not only to the loss of its function, but also to a reduction in Ikaros levels due to increased degradation. Subsequent experiments have demonstrated that Ikaros is polyubiquitinated, which has provided evidence that Ikaros degradation occurs via the ubiquitin pathway. Overall, the studies of CK2-mediated phosphorylation and PP1-mediated dephosphorylation of Ikaros illustrate the importance of these signal transduction pathways and the role of phosphorylation in regulating Ikaros activity in cells.
ADDITIONAL IKAROS PHOSPHORYLATION SITES
An additional study to identify and analyze the phosphorylation of Ikaros has been performed by the Smale group[22]. This has involved a comprehensive approach using LC-MS/MS analysis of Ikaros phosphorylation in the murine VL3-3M2 T leukemia cell line. This analysis has identified several additional phosphorylation sites in Ikaros, including several threonine and serine residues, as well as two tyrosines. The functional significance of one of these novel phosphorylation sites (amino acid 441) has been studied using phosphomimetic and phosphoresistant mutants, but no alteration of Ikaros function has been observed in a transient transfection assay. The functional significance of the additional phospho-sites identified in this study has not been elucidated. These data illustrate the potential for additional mechanisms of phosphorylation-mediated regulation of Ikaros function.
REGULATION OF CK2 AND PP1 ACTIVITY
CK2 and PP1 both have numerous substrates and their activity involves a complex network of different metabolites. The focus of this review is the regulation of Ikaros function by phosphorylation, therefore, we briefly mention the major regulators of CK2 and PP1 activity that are known to affect cellular proliferation. Phosphorylation of PP1 by cdc2 kinase results in PP1 inactivation in a cycle-dependent manner[23]. Three known tumor suppressors directly bind and inhibit CK2 activity: (1) it has been demonstrated that the tumor suppressor p53 inhibits CK2 by binding to its regulatory β subunit[24]; (2) similarly, another tumor suppressor p21WAF1 binds to the β regulatory subunit of CK2 and inhibits its activity[25]; (3) adenomatous polyposis coli protein also inhibits CK2 by interacting with its CK2 α-subunit[26]. Activators of CK2 include stimulators of cellular proliferation such as: polyamines[27] and fibroblast growth factor-2[28]. These findings suggest that Ikaros acts as a part of multiple signal transduction networks that regulate cellular proliferation and malignant transformation.
CONCLUSION
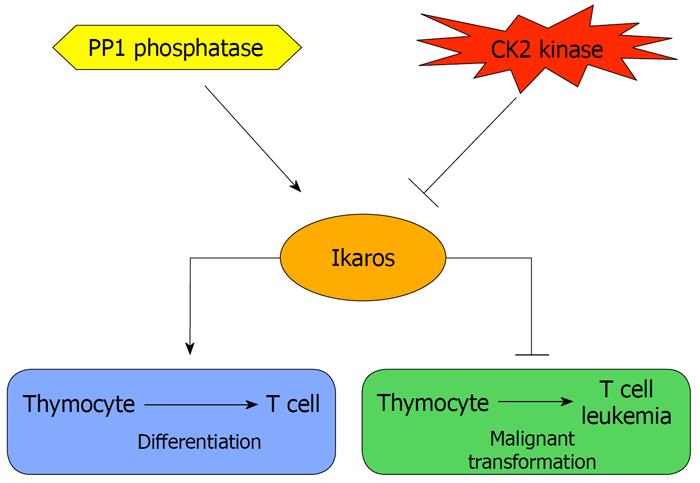
Studies of Ikaros phosphorylation have provided a partial answer to the question concerning the regulation of Ikaros function in normal and leukemia cells, as well as how alteration in the regulation of Ikaros might contribute to leukemia. The studies described above have established phosphorylation/dephosphorylation as one of the major mechanisms that controls Ikaros activity. These data strongly suggest that CK2 is the principal regulator of Ikaros function. CK2 is an extensively studied regulator of cellular proliferation and a tumor-promoter protein[29]. Overexpression of CK2 has been associated with the development of various types of tumors, including mammary gland, prostate, lung, and kidney cancers[30]. Forced expression of the catalytic subunit of CK2 in transgenic mice leads to the development of T-cell leukemia and lymphoma[31-34], similar to that observed in mice with impaired Ikaros function[2,16,35,36]. Thus, we hypothesize that Ikaros function is controlled by its phosphorylation status and that overexpression of CK2 leads to hyperphosphorylation of Ikaros, which results in its loss of tumor suppressor activity and the subsequent development of leukemia (Figure 2). Additional studies utilizing Ikaros phosphomimetic and phosphoresistant mutants in mouse models and in primary human and murine cells are necessary to confirm and/or refine this hypothesis. The abundance of Ikaros phosphorylation sites, as well as the lack of a complete understanding of Ikaros function in chromatin remodeling and tumor suppression, suggests that many additional signal transduction pathways that regulate Ikaros function remain to be discovered.
Figure 2 A model for the role of casein kinase 2 and protein phosphatase 1 in regulating Ikaros activity in T-cell differentiation and tumor suppression.
CK2: Casein kinase 2.
Peer reviewers: Ugo Moens, PhD, Professor, Institute of Medical Biology, Faculty of Health Sciences, University of Tromsø, N-9037 Tromsø, Norway; Joaquin Arino, Professor, Institut de Biotecnologia i Biomedicina, Department Bioquimica i Biology Molecular, Universitat Autónoma Barcelona, 08193, Cerdanyola del Valles, Barcelona, Spain
S- Editor Cheng JX L- Editor Kerr C E- Editor Zheng XM