Published online Nov 26, 2011. doi: 10.4331/wjbc.v2.i11.246
Revised: October 12, 2011
Accepted: October 19, 2011
Published online: November 26, 2011
AIM: To determine if the cytotail of the principal sheddase tumor necrosis factor-α converting enzyme (TACE; ADAM17) controls protein ectodomain shedding.
METHODS: Site-directed mutagenesis was performed to derive TACE variants. The resulting TACE expression plasmids with amino acid substitutions in the extracellular, cysteine-rich disintegrin domain (CRD) and/or deleted cytotail, along with an expression vector for the enhanced green fluorescence protein were transfected into shedding-defective M1 mutants stably expressing transmembrane L-selectin or transforming growth factor (TGF)-α. The expression levels of the TACE substrates at the cell surface were determined by flow cytometry.
RESULTS: Consistent with published data, a single point mutation (C600Y) in the CRD led to shedding deficiency. However, removal of the cytotail from the C600Y TACE variant partially restored ectodomain cleavage of TGF-α and L-selectin. Cytotail-deleted mutants with any other substituting amino acid residues in place of Cys600 displayed similar function compared with tail-less C600Y TACE.
CONCLUSION: The cytotail plays an inhibitory role, which becomes evident when it is removed from an enzyme with another mutation that affects the enzyme function.
- Citation: Li X, Pérez L, Fan H. Inhibitory role of TACE/ADAM17 cytotail in protein ectodomain shedding. World J Biol Chem 2011; 2(11): 246-251
- URL: https://www.wjgnet.com/1949-8454/full/v2/i11/246.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v2.i11.246
Protein ectodomain shedding serves as an important means for regulating the function of cell surface proteins required for a variety of physiological processes[1]. For example, generation of freely diffusible epidermal growth factor receptor (EGFR) ligands including transforming growth factor (TGF)-α from their transmembrane precursors is essential for the development of multiple organs[2-4]. Ectodomain shedding is also crucial for pathogenesis. Thus, overproduction of soluble EGFR ligands causes cellular transformation[5]; generation of the circulating cytokine tumor necrosis factor (TNF)-α from its transmembrane precursor is responsible for cachexia, septic shock and other inflammatory conditions[6-8].
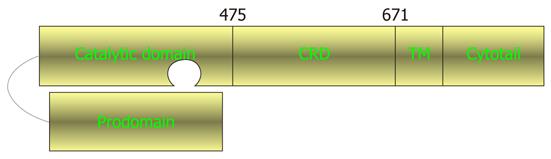
TNF-α converting enzyme (TACE) is a principal sheddase that cleaves not only transmembrane TNF-α, but also a large number of other membrane proteins[9]. As a member of the large family of a disintegrin and metalloprotease domains (ADAM), TACE or ADAM17 is a membrane-anchored metalloprotease (Figure 1)[10,11]. The protease is biosynthesized as a zymogen, in which the catalytic domain is led by an amino-terminal prodomain, and followed in succession by a cysteine-rich disintegrin domain (CRD), a transmembrane segment and a carboxyl terminal cytotail. The TACE zymogen is enzymatically inactive because the prodomain interacts with the active site, causing inaccessibility to protein substrates.
Ectodomain shedding by TACE is a tightly regulated process. Accordingly, a variety of stimuli including growth factors, inflammatory mediators, ionophores, carcinogens and tumor promoters can induce shedding[12-14]. However, the cellular and molecular mechanism that controls TACE-mediated shedding remains unclear. Although prodomain removal is a prerequisite for TACE to gain catalytic activity[15-17], it does not seem sufficient for shedding activation, because an increase in prodomain removal is not observed following stimulation[11]. Therefore, activation of shedding appears to be through modulation of mature TACE.
It has been established that signaling pathways involving two mitogen-activated protein kinases (MAPKs), Erk and p38, mediate the activation of shedding in response to various stimuli[12,13,18]. The serine- and threonine-rich TACE cytotail is suspected to play a role in the regulation of TACE function. In particular, both MAPKs have been shown to phosphorylate directly the TACE cytotail at Thr-735[18-20]. Erk activity-dependent phosphorylation at Ser-819 has also been demonstrated. Furthermore, Ser-791 is phosphorylated in resting cells, and undergoes dephosphorylation in response to growth factor stimulation[21]. However, mutation of these phosphorylation sites individually or in combination, and even removal of the entire cytotail have no detectable effects on shedding[21-24]. Thus, the function of the TACE cytotail remains illusive.
There is also evidence suggesting a role for the CRD in TACE-mediated shedding[9,24-26]. We have previously demonstrated that a substitution (C600Y) within the CRD results in enzyme inactivity[9]. Interestingly, in an attempt to examine the function of other cysteines in the CRD, we found that deletion of the cytotail partially restores the shedding activity in the C600Y TACE variant. This finding suggests an inhibitory role for the cytotail in ectodomain shedding and resolves a long mystery with regarding the function of the TACE cytotail, which becomes apparent only when there is another defect in the enzyme.
Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum (FBS), bovine serum albumin (BSA), penicillin, streptomycin, 1,10-phenanthroline, EDTA, paraformaldehyde and inorganic salts were purchased from Sigma-Aldrich (St. Louis, MO). Mouse monoclonal antibodies recognizing the ectodomain of L-selectin and TGF-α have been described previously[12,21,27]. Phycoerythrin (PE)-conjugated goat anti-mouse IgG (whole molecule) was purchased from Jackson ImunoResearch Laboratories (West Grove, PA, United States). Cell Lifters were purchased from Corning Inc. (Corning, NY, United States).
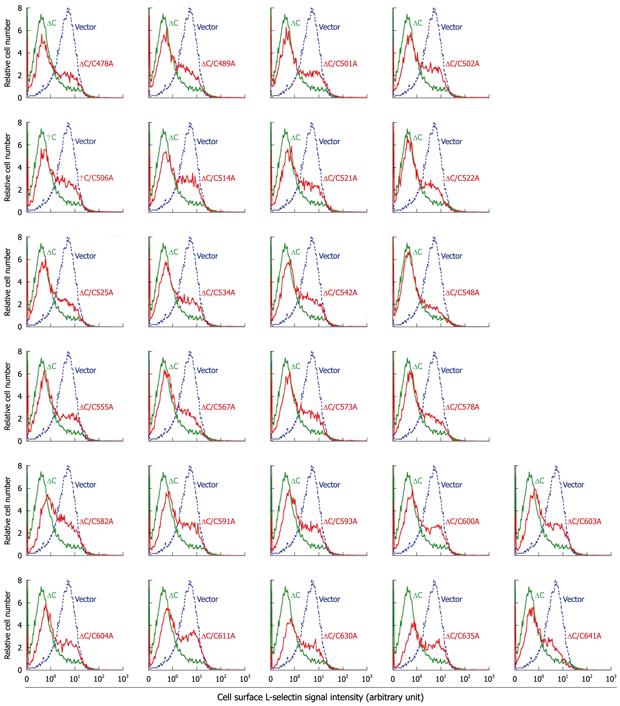
pRK5-based plasmids for expressing wild-type murine TACE, C600Y TACE, cytotail-truncated TACE (ΔC) and the ΔC derivatives containing C600Y and C600A mutations (i.e. ΔC/C600Y and ΔC/C600A, respectively) have been described previously[9]. Additional ΔC constructs carrying an alanine in place of each of the remaining 25 cysteines in the CRD (i.e. ΔC/C478A, ΔC/C489A, ΔC/C501A, ΔC/C502A, ΔC/C506A, ΔC/C514A, ΔC/C521A, ΔC/C522A, ΔC/C525A, ΔC/C534A, ΔC/C542A, ΔC/C548A, ΔC/C555A, ΔC/C567A, ΔC/C573A, ΔC/C578A, ΔC/C582A, ΔC/C591A, ΔC/C593A, ΔC/C603A, ΔC/C604A, ΔC/C611A, ΔC/C630A, ΔC/C635A and ΔC/C641A) were constructed for this work by polymerase chain reactions using the high fidelity Pfu DNA polymerase (Stratagene, La Jolla, CA, United States). Sequence authenticity of the inserts in the final expression vectors was confirmed by automated DNA sequencing performed by the UMDNJ-Robert Wood Johnson Medical School DNA Core Facility.
The shedding-defective M1-L-selectin and M1-TGF-α cells, which overexpress transmembrane L-selectin and TGF-α, respectively, have been described previously[22]. The cell lines were maintained as adherent cultures using DMEM supplemented with 8% FBS and the antibiotics penicillin and streptomycin.
The enzyme activity of wild-type or mutant TACE was determined with cell-based shedding assays using two-color flow cytometry as described previously[28]. M1-L-selectin and M1-TGF-α cells were transiently cotransfected with a TACE expression vector (or the control RK5 vector) and a mammalian expression plasmid for enhanced green fluorescence protein (GFP) at a ratio of 3:1. Twenty hours after transfection, cell culture plates were placed on ice and the medium was replaced with cold PBS, supplemented with 10 mmol/L 1, 10-phenanthroline (a metalloproteases inhibitor), 5 mmol/L EDTA, 2% BSA and 0.1% NaN3 (PEB). Cells were scraped off the plates with a Cell Lifter, collected and centrifuged at 1000 r/min at 4 °C for 5 min. Following the removal of the supernatant, cells were resuspended in 50 μL PEB containing 500 ng DREG56 (anti-L-selectin) or 200 ng anti-TGF-α, incubated on ice for 30 min with gentle agitation, washed twice with PEB and reacted with 50 μL PE-conjugated goat anti-mouse IgG diluted in PEB (1:200) for 30 min. After another two washes with PEB, cells were fixed in 500 μL of 1% paraformaldehyde (prepared in PEB) and were immediately analyzed by flow cytometry using a Coulter Epics XL.MCL flow cytometer (Beckman Coulter, Miami, FL, United States). GFP and PE signals were simultaneously detected through the FL1 and FL2 channels, respectively. For each analysis, unstained stable cells (M1-L-selectin or M1-TGF-α) cotransfected with GFP and the control pRK5 vector without an insert, and immunostained stable cells transfected with pRK5 only (i.e. no GFP), were used to set up the instrument to obtain optimal GFP-PE signal compensation. Mock-stained parental M1 cells were used to verify the final compensatory conditions under which about 98% of the cells were recognized as GFP- and PE-doubly negative cells.
Previously, we have identified a TACE variant carrying a C600Y substitution within the CRD from a shedding-defective cell line. Cell-based shedding assays confirmed that the variant sheddase lacks the capacity to cleave substrates[9]. On the basis of the tertiary structure of the CRD of ADAM10, which share a high level of sequence homology to that of TACE[9], Cys-600 forms a disulfide bridge with Cys-593; also, there may be additional 12 disulfide bridges[29]. To determine if other cysteines and/or disulfide bridges are also important for TACE function, we replaced each of the remaining cysteine in the CRD with alanine. For the convenience of vector construction, the C→A mutants were constructed on the ΔC TACE backbone because previous studies have shown that deletion of the cytotail does not affect TACE function[21,23,24]. The enzyme activities of the resulting ΔC/C→A variants were determined in the M1-L-selectin cell line, which overexpresses transmembrane L-selectin but is defective in ectodomain shedding, due to the loss of one TACE allele and a mutation in the other allele resulting in an M435I substitution at the active site[9,28].
Contrary to our prediction, all the ΔC/C→A variants were largely active in L-selectin shedding (Figure 2). Particularly surprising was the L-selectin shedding activity exhibited by the ΔC/C600A variant, because we have previously shown that ΔC/C600A and ΔC/C600Y have no detectable enzyme activity, similar to the full-length C600Y TACE[9]. In that study, the inactivity of C600Y TACE was demonstrated in all three TACE-defective cell lines, with multiple substrates, and using different detective methods; however, the ΔC/C600A and ΔC/C600Y constructs were tested only with one TACE substrate using one-color flow cytometry, which cannot discriminate TACE-expressing cells from untransfected cells[9]. Therefore, we believe that the data showing enzymatic inactivity in the ΔC/C600A and ΔC/C600Y constructs were erroneous. In contrast, the shedding-proficiency in the ΔC/C600A mutant, as demonstrated using two-color flow cytometry in which eGFP served as a surrogate marker for TACE expression (Figure 2), ought to be considered reliable.
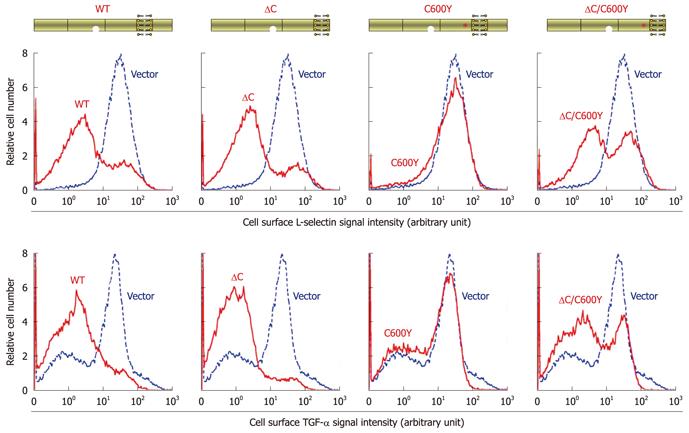
We next compared the enzyme activities of the full-length C600Y TACE, the ΔC/C600Y construct, and their respective parental forms (i.e. wild-type TACE and ΔC TACE). Consistent with published data, ΔC TACE demonstrated levels of enzyme activity that were similar to those in wild-type TACE, in the cleavage of transmembrane L-selectin and TGF-α, whereas full-length C600Y TACE completely failed to cleave these substrates (Figure 3). Evidently, the ΔC/C600Y construct partially regained L-selectin and TGF-α shedding activities, as compared to the C600Y variant (Figure 3). These results indicate that the cytotail plays an inhibitory role in ectodomain shedding, which became detectable when there was another defect in the enzyme.
TACE is responsible for ectodomain shedding of numerous membrane proteins and it is required for a variety of physiological processes, but how its activity is regulated remains unsatisfactorily defined. It was thought, immediately after the sequencing of the TACE cDNA, that the cytotail of the sheddase plays a critical role in the enzyme activity[11]. However, this hypothesis has been questioned because removal of the entire cytotail has demonstrated no detectable effect on TACE-dependent shedding[21-23,28]. The lack of an apparent effect of the cytotail deletion (in wild-type TACE) was again reproduced in this work. Nevertheless, removal of the cytotail from the shedding-defective C600Y TACE variant clearly led to a substantial restoration of enzyme activity. Thus, the cytotail plays an inhibitory role, which becomes noticeable only in the presence of another defect in the enzyme.
How the cytotail exerts an inhibitory activity in shedding has yet to be defined. It is known that the cytotail is phosphorylated in response to activators of ectodomain shedding[11,18-21]. We speculate that the phosphorylation alleviates the inhibitory activity of the cytotail, which is also achievable by the deletion of the cytotail.
Cys-600 has been predicted to participate in the formation of a disulfide bridge[29]. Interestingly, recent studies have indicated that agents that affect the redox potential regulate TACE activity[24,25]. Furthermore, protein disulfide isomerase has been shown to inhibit TACE activity through modifying the conformation of the CRD[25]. The fact that the inhibitory role of the cytotail was discovered in the context of Cys-600 mutations suggests that the cytotail of TACE regulates disulfide bridging and thus the conformation of the CRD.
In conclusion, our study, aimed at furthering the understanding of how the CRD regulates the function of TACE, has led to the recognition of a new, inhibitory role for the cytotail in ectodomain shedding. However, the original question about which, if any, cysteines other than Cys-600 in the CRD are critical for the enzyme activity of TACE has yet to be answered using full-length constructs.
This work was presented to the Gordon Research Conference on Regulated Proteolysis of Cell Surface Proteins held in Davidson College, NC, United States in July, 2011.
Tumor necrosis factor-α converting enzyme (TACE) is responsible for the cleavage of numerous membrane proteins at the cell surface. The enzyme activity of TACE is tightly regulated by intracellular signaling pathways. The carboxyl-terminal cytotail has long been thought to function as a signaling transducer in the regulation of TACE function. However, deletion of the cytotail so far has not shown a detectable effect on protein ectodomain shedding.
In addition to normal development, TACE is required for the pathogenesis of diseases including inflammation and autoimmunity. Identification of new domain function in TACE has implications for molecular cell physiology and pathophysiology, and also may help development of new therapeutic agents.
The present study identifies an inhibitory role for the TACE cytotail. This new function is detectable when the cytotail is removed from a TACE construct with a mutation in the cysteine-rich disintegrin domain, which adversely affects the enzyme function. Previous studies have failed to recognize this important role because the cytotail-truncation mutants used in those studies did not have an additional mutation.
Despite a limited number of data presented by the authors, the newly identified function of the cytotail is both interesting and of potential significance. The introduction was somewhat unnecessarily detailed, and a few places could be revised for enhanced clarification.
Peer reviewer: Shiyou Chen, PhD, Assistant Professor, University of Georgia, 501 DW Brooks Drive, Athens, GA 30602, United States
S- Editor Cheng JX L- Editor Kerr C E- Editor Zheng XM
| 1. | Becherer JD, Blobel CP. Biochemical properties and functions of membrane-anchored metalloprotease-disintegrin proteins (ADAMs). Curr Top Dev Biol. 2003;54:101-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol. 2005;6:32-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 886] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 3. | Jackson LF, Qiu TH, Sunnarborg SW, Chang A, Zhang C, Patterson C, Lee DC. Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. EMBO J. 2003;22:2704-2716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 317] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 4. | Zhao J, Chen H, Peschon JJ, Shi W, Zhang Y, Frank SJ, Warburton D. Pulmonary hypoplasia in mice lacking tumor necrosis factor-alpha converting enzyme indicates an indispensable role for cell surface protein shedding during embryonic lung branching morphogenesis. Dev Biol. 2001;232:204-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Sandgren EP, Luetteke NC, Palmiter RD, Brinster RL, Lee DC. Overexpression of TGF alpha in transgenic mice: induction of epithelial hyperplasia, pancreatic metaplasia, and carcinoma of the breast. Cell. 1990;61:1121-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 511] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 6. | Tracey KJ, Morgello S, Koplin B, Fahey TJ, Fox J, Aledo A, Manogue KR, Cerami A. Metabolic effects of cachectin/tumor necrosis factor are modified by site of production. Cachectin/tumor necrosis factor-secreting tumor in skeletal muscle induces chronic cachexia, while implantation in brain induces predominantly acute anorexia. J Clin Invest. 1990;86:2014-2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 162] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Horiuchi K, Kimura T, Miyamoto T, Takaishi H, Okada Y, Toyama Y, Blobel CP. Cutting edge: TNF-alpha-converting enzyme (TACE/ADAM17) inactivation in mouse myeloid cells prevents lethality from endotoxin shock. J Immunol. 2007;179:2686-2689. [PubMed] |
| 8. | Conway JG, Andrews RC, Beaudet B, Bickett DM, Boncek V, Brodie TA, Clark RL, Crumrine RC, Leenitzer MA, McDougald DL. Inhibition of tumor necrosis factor-alpha (TNF-alpha) production and arthritis in the rat by GW3333, a dual inhibitor of TNF-alpha-converting enzyme and matrix metalloproteinases. J Pharmacol Exp Ther. 2001;298:900-908. [PubMed] |
| 9. | Li X, Fan H. Loss of ectodomain shedding due to mutations in the metalloprotease and cysteine-rich/disintegrin domains of the tumor necrosis factor-alpha converting enzyme (TACE). J Biol Chem. 2004;279:27365-27375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Moss ML, Jin SL, Milla ME, Bickett DM, Burkhart W, Carter HL, Chen WJ, Clay WC, Didsbury JR, Hassler D. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature. 1997;385:733-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1291] [Cited by in RCA: 1275] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 11. | Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2422] [Cited by in RCA: 2420] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 12. | Fan H, Derynck R. Ectodomain shedding of TGF-alpha and other transmembrane proteins is induced by receptor tyrosine kinase activation and MAP kinase signaling cascades. EMBO J. 1999;18:6962-6972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 205] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 13. | Gechtman Z, Alonso JL, Raab G, Ingber DE, Klagsbrun M. The shedding of membrane-anchored heparin-binding epidermal-like growth factor is regulated by the Raf/mitogen-activated protein kinase cascade and by cell adhesion and spreading. J Biol Chem. 1999;274:28828-28835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 143] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Pandiella A, Massagué J. Multiple signals activate cleavage of the membrane transforming growth factor-alpha precursor. J Biol Chem. 1991;266:5769-5773. [PubMed] |
| 15. | Gonzales PE, Solomon A, Miller AB, Leesnitzer MA, Sagi I, Milla ME. Inhibition of the tumor necrosis factor-alpha-converting enzyme by its pro domain. J Biol Chem. 2004;279:31638-31645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Milla ME, Leesnitzer MA, Moss ML, Clay WC, Carter HL, Miller AB, Su JL, Lambert MH, Willard DH, Sheeley DM. Specific sequence elements are required for the expression of functional tumor necrosis factor-alpha-converting enzyme (TACE). J Biol Chem. 1999;274:30563-30570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 130] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Schlöndorff J, Becherer JD, Blobel CP. Intracellular maturation and localization of the tumour necrosis factor alpha convertase (TACE). Biochem J. 2000;347 Pt 1:131-138. [PubMed] |
| 18. | Xu P, Derynck R. Direct activation of TACE-mediated ectodomain shedding by p38 MAP kinase regulates EGF receptor-dependent cell proliferation. Mol Cell. 2010;37:551-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 213] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 19. | Díaz-Rodríguez E, Montero JC, Esparís-Ogando A, Yuste L, Pandiella A. Extracellular signal-regulated kinase phosphorylates tumor necrosis factor alpha-converting enzyme at threonine 735: a potential role in regulated shedding. Mol Biol Cell. 2002;13:2031-2044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 249] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 20. | Soond SM, Everson B, Riches DW, Murphy G. ERK-mediated phosphorylation of Thr735 in TNFalpha-converting enzyme and its potential role in TACE protein trafficking. J Cell Sci. 2005;118:2371-2380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 201] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 21. | Fan H, Turck CW, Derynck R. Characterization of growth factor-induced serine phosphorylation of tumor necrosis factor-alpha converting enzyme and of an alternatively translated polypeptide. J Biol Chem. 2003;278:18617-18627. [PubMed] |
| 22. | Li X, Pérez L, Pan Z, Fan H. The transmembrane domain of TACE regulates protein ectodomain shedding. Cell Res. 2007;17:985-998. [PubMed] |
| 23. | Reddy P, Slack JL, Davis R, Cerretti DP, Kozlosky CJ, Blanton RA, Shows D, Peschon JJ, Black RA. Functional analysis of the domain structure of tumor necrosis factor-alpha converting enzyme. J Biol Chem. 2000;275:14608-14614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 400] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 24. | Wang Y, Herrera AH, Li Y, Belani KK, Walcheck B. Regulation of mature ADAM17 by redox agents for L-selectin shedding. J Immunol. 2009;182:2449-2457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 25. | Willems SH, Tape CJ, Stanley PL, Taylor NA, Mills IG, Neal DE, McCafferty J, Murphy G. Thiol isomerases negatively regulate the cellular shedding activity of ADAM17. Biochem J. 2010;428:439-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 26. | Amour A, Slocombe PM, Webster A, Butler M, Knight CG, Smith BJ, Stephens PE, Shelley C, Hutton M, Knäuper V. TNF-alpha converting enzyme (TACE) is inhibited by TIMP-3. FEBS Lett. 1998;435:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 476] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 27. | Kahn J, Walcheck B, Migaki GI, Jutila MA, Kishimoto TK. Calmodulin regulates L-selectin adhesion molecule expression and function through a protease-dependent mechanism. Cell. 1998;92:809-818. [RCA] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 160] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 28. | Pérez L, Kerrigan JE, Li X, Fan H. Substitution of methionine 435 with leucine, isoleucine, and serine in tumor necrosis factor alpha converting enzyme inactivates ectodomain shedding activity. Biochem Cell Biol. 2007;85:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Janes PW, Saha N, Barton WA, Kolev MV, Wimmer-Kleikamp SH, Nievergall E, Blobel CP, Himanen JP, Lackmann M, Nikolov DB. Adam meets Eph: an ADAM substrate recognition module acts as a molecular switch for ephrin cleavage in trans. Cell. 2005;123:291-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 344] [Article Influence: 17.2] [Reference Citation Analysis (0)] |