Published online Mar 27, 2022. doi: 10.4331/wjbc.v13.i2.35
Peer-review started: March 27, 2021
First decision: July 27, 2021
Revised: September 6, 2021
Accepted: March 4, 2022
Article in press: March 4, 2022
Published online: March 27, 2022
Processing time: 360 Days and 20 Hours
An RNA-binding protein, LIN28A was initially discovered in nematodes Caenorhabditis elegans and regulated stem cell differentiation and proliferation. With the aid of mouse models and cancer stem cells models, LIN28A demon
Core Tip: The overexpression of LIN28A has been correlated with a number of tumours and a higher risk of relapse in cancer patients. Therefore, LIN28A could be developed as a prognostic indicator. With an increasing understanding of its roles in the pathological context, LIN28A has also become a promising therapeutic target for cancer treatment and regenerative therapy for neuropathies.
- Citation: Wu K, Ahmad T, Eri R. LIN28A: A multifunctional versatile molecule with future therapeutic potential . World J Biol Chem 2022; 13(2): 35-46
- URL: https://www.wjgnet.com/1949-8454/full/v13/i2/35.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v13.i2.35
First discovered in nematodes Caenorhabditis elegans (C. elegans) in 1997, the LIN28A (commonly referred to as LIN28 in some literature) protein is predominantly localised in the cytoplasm but can travel back and forth between the nucleus and cytoplasm. It was noted to be highly expressed during embryogenesis but gradually diminished to absent expression in adulthood in nematodes[1]. In humans, the absent expression was found to be only true in lung epithelium but it remains expressed during adulthood in certain cell or tissue types such as erythrocytes, renal epithelia of the loop of Henle and collecting ducts, cardiac muscle, and skeletal muscle[2]. Nonetheless, examination of LIN28 homologues in humans, mice, and Drosophila demonstrated its typical expression in undifferentiated and pluripotent cells, especially in embryonic stems cells (ESCs), then it is downregulated in response to development and differentiation. These findings suggested that LIN28A was involved in cellular development and differentiation. In fact, increased LIN28 expression has been associated with less differentiated and more aggressive tumours[2].
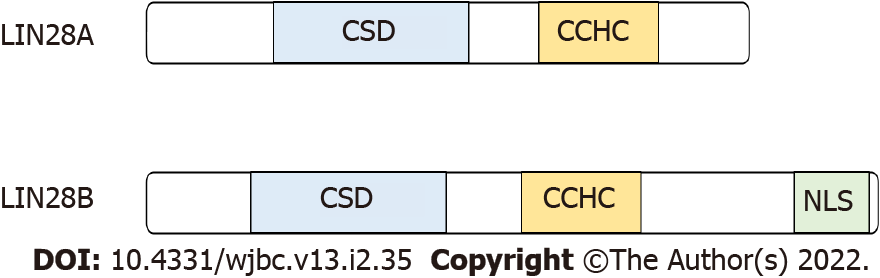
Invertebrates such as C. elegans and D. melanogaster possess a single LIN28 gene whereas all vertebrates possess two LIN28 paralogs. LIN28’s structure includes two RNA-binding domains, a N-terminal cold-shock domains (CSD), and a cysteine cysteine histidine cysteine (CCHC) zinc knuckle domains (ZKD) (Figure 1). LIN28 is regarded as the sole animal protein to possess the unique combination of a CSD and a C-terminal ZKD and has been implicated in promoting self-renewal and delaying differentiation, that resulted in proliferation of stem cells. Contrarily, its loss-of-function resulted in increased stem cell differentiation[2]. Its role in mammalian stem cells was elucidated in the early 2000s through studies such as human LIN28 being used to reprogram somatic fibroblasts into pluripotent stem cells[1]. These studies eventually demonstrated that LIN28A could bind to let-7 gene to repress its expression in regulating translation. This binding was observed in a similar mechanism in C. elegans[1]. Thus, the findings had validated LIN28A’s conserved role in stem cell self-renewal and differentiation.
In recent years, other roles of LIN28A have been linked to wound healing and tissue repair, cell growth and metabolism, and carcinogenesis while the signalling pathways have become more complex. In this review, we will elaborate on recent findings regarding the mechanisms and roles of the LIN28A protein in physiological functions and pathological processes. Subsequently, we will examine how these findings have translated into RNA-targeted therapies and drugs targeting protein interactions involving LIN28A in the treatment of cancer and other diseases.
In vertebrates, an important intrinsic signal in downregulating LIN28A would be the microRNA-125a (miRNA-125a)[3]. In contrast, pluripotency factors such as Sox2, Nanog, and Tcf3 can promote LIN28A expression. Among these factors, Sox2 is regarded the most essential in this promotion of expression based on a Bayesian probabilistic network modelling of single-cell gene expression[3]. In addition, inhibition of Dot1L H3K79 histone methyltransferase indirectly upregulates LIN28A[3]. Extrinsic signalling has been demonstrated in C. elegans: Nuclear receptor daf-12 transmits signals from steroid hormones to LIN28A, and in vitro: Homologous retinoic acid and oestrogen receptors downregulate LIN28A[3]. However, it is unclear if similar extrinsic signalling occurs in mammals.
Another signalling pathway involves brain-derived neurotrophic factor (BDNF) that initially activates extracellular signal-regulated protein kinase (Erk), which in turn mediates mitogen-activated protein kinase (MAPK) phosphorylation of transactivation response element RNA-binding protein (TRBP), an RNA-binding cofactor of the Dicer enzyme[4]. The act of phosphorylation decreased Merlin binding, which impedes polyubiquitination and proteasomal degradation of TRBP. Subsequently, BDNF can stabilise and elevate levels of LIN28A via co-association with TRBP. An interesting finding was this MAPK-mediated TRBP phosphorylation and induction only targeted LIN28A but not its paralog, LIN28B. Hence, the BDNF-MAPK pathway induces LIN28A for physiological functions connected to dendritic spine growth and peritoneal macrophage survival as part of the trophic responses[4].
LIN28A has been found to be involved in several feedback loops. Firstly, it has been ascertained that LIN28A inhibits let-7 expression while let-7 itself binds to mRNA of LIN28A to downregulate LIN28A expression, which establishes a double negative feedback loop[3]. Secondly, LIN28A can derepress c-Myc via let-7 inhibition, then c-Myc can upregulate LIN28A expression, which establishes a positive feedback loop. Thirdly, an initial inflammatory signal activates nuclear factor kappa-light-chain-enhancer (NF-κB) that elevates interleukin-6 (IL-6) levels that is also elevated by LIN28A’s inhibition of let-7. The increased IL-6 levels activate NF-κB, which completes the positive feedback loop[1].
In C. elegans, the mechanisms in regulating its four developmental stages can be divided into let-7 dependent and let-7 independent pathways[2]. The former involves LIN28 promoting the expression of lin-41 by repressing let-7, which in turn reduces binding of let-7 to LIN28A mRNA. Therefore, either LIIN28A or let-7 can suppress one another in forming a bistable switch. The latter involves lin-4 targeting LIN28 which upregulates hunchback-like protein-1 (hbl-1) that inhibits let-7[2]. Interestingly, the latter may be utilised in mammalian systems which would further corroborate the conserved role of LIN28 but there does not appear to be any concrete findings.
In this review, these two divisions are adapted to elaborate on LIN28A mechanisms in humans.
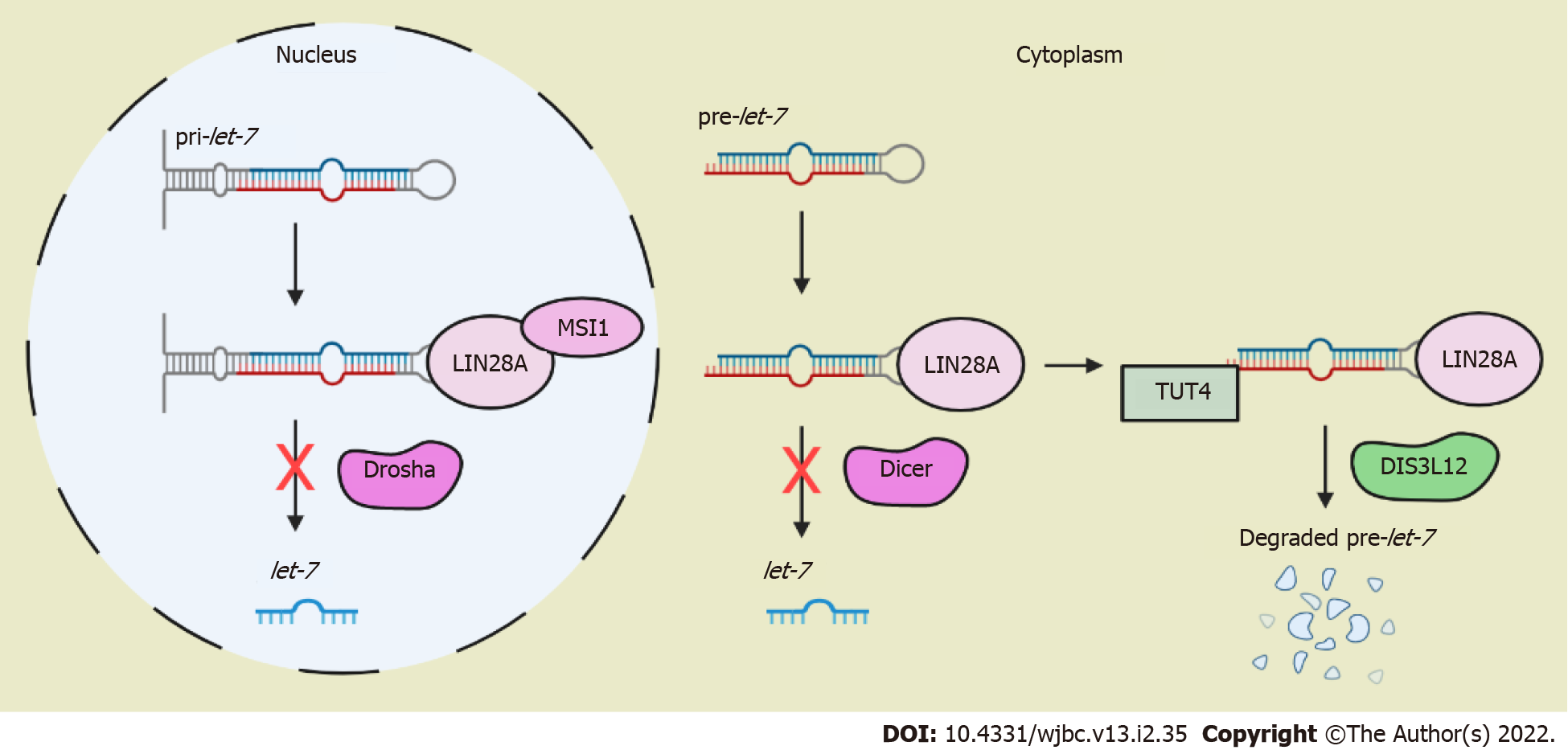
Let-7 dependent pathways: In the nucleus, LIN28A binds primary lethal-7 (pri-let-7) synergistically with RNA-binding protein musashi 1 (MSI1) to block let-7 processing via a miRNA-processing enzyme Drosha (Figure 1)[2]. In the cytoplasm, LIN28A binds precursor lethal-7 (pre-let-7) to competitively block the Dicer processing (another microRNA-processing enzyme) to prevent the formation of mature let-7. Then, LIN28A recruits terminal uridylyl transferase 4 (TUT4) for the oligo-uridylation of pre-let-7; this process prevents the cleavage of pre-let-7 by Dicer and acts as a signal for exonuclease DIS3L12 for its degradation (Figure 2)[2].
In terms of structural significance of LIN28, the pri-let-7 pathway appears not be as expounded as much as the pre-let-7 pathway. In the nucleus, it is hypothesised that the LIN28A’s CSDs bind with increased affinity to pri-let-7[5] to block cleavage by Drosha which prevents Drosha-mediated processing[6]. In a related study, LIN28 has demonstrated another mechanism similar to that of its counterpart, LIN28B: The histone H3K4 methyltransferase can mono-methylate LIN28A; which appears to enable its localisation in the nucleus and especially nucleolus, and increases its binding affinity to pri-let-7[7].
In the cytosol, LIN28’s ZKDs can recognise and bind to GGAG or GGAG-like motifs in pre-let-7’s terminal loop to compete with Dicer in inhibiting Dicer-mediated processing and TUTase would then be recruited[6]. Furthermore, TUT4 and TUT7 also possess CCHC Zn knuckles that are required in pre-let-7 oligouridylation[1]. While initial research only involved mechanisms surrounding binding of LIN28A to let-7 (let-7 dependent), subsequent research examined binding of LIN28A to specific mRNAs (let-7 independent).
Let-7 independent pathways: Several small-scale studies have isolated a number of potential LIN28 mRNA targets and most of them are mature mRNA and involved in cell cycle regulation, metabolism, or formation of ribonucleoprotein complexes (Table 1)[2,8]. In first understanding how LIN28 recognises these targets, three genome-wide studies determined rather divergent consensus sequences amongst the targets despite such large data sets[2].
| Cell cycle regulation | RNA-binding proteins | Histone components | Glucose metabolism | Early embryogenic genes |
| Cyclin A | hnRNP F | Histone H2A | IGF receptor | Sox2 |
| CDK4 | TDP-43 | Histone H4H | Insulin receptor | Sall4 |
| CCNB1 | TIA-1 | Linker histone H1FX | HMGA2 | Oct4 |
Furthermore, Cho et al[9] located their consensus sequence within the terminal loop of small hairpins[2]. Wilbert et al[10] noted their targets’ consensus sequences of interest were enriched in single-stranded RNA (ssRNA) within the hairpin and other loop structures whereas Qiu et al[11] found that their targets were enriched in LIN28-containing polysome fractions[2]. Then, Hafner et al[8] discovered LIN28 preferentially binds to ssRNA containing a uridine-rich element and guanosines when embedded in secondary structure[8]. However, the presence of this secondary structure may not be significant due to variation in stem length and loop size and lack of formal statistical analysis.
Despite the divergent consensus sequences, similarities such as the ssRNAs and loop structures indicate that the recognition mechanisms for LIN28 to these RNAs is gradually being uncovered. With an improved understanding of how LIN28 binds to its targets, researchers also looked at the significance of its pathways in humans.
LIN28A is most prominently known as a regulator of cell renewal and differentiation. Multiple studies highlight this evolutionarily conserved role in ESCs and trophoblast stem cells. In fact, Li et al[12] reported that PpCSP1 (a homologue of LIN28) can revert differentiated leaf cells to stem cells in moss Physcomitrella patens; indicating that LIN28’s role involving cell differentiation extends beyond the animal kingdom[12]. In the classical let-7 dependent pathway, LIN28 can suppress let-7 biogenesis to promote ESC differentiation[13]. In the let-7 independent pathway, LIN28 can impede translation of mRNAs such as high mobility group AT-Hook 2 (HMGA2) to either prevent its disruption of ESC differentiation or its effect of uncontrolled cell proliferation and apoptosis when HMGA2 is allowed to accumulate[13].
Similarly, LIN28A utilises the classical pathway to regulate trophoblast differentiation and neural precursor cells (NPCs) proliferation. To retain pluripotency in trophoblast progenitors, LIN28A increases; suppressing let-7 whereas to initiate their differentiation, LIN28 decreases; enabling let-7 to mature[14]. Proliferation of NPCs and increase in brain size is promoted by Sox2 through LIN28A. Notably, LIN28 has been suggested to be sufficient in rescuing NPC proliferation and neurogenic deficits in the absence of Sox2[15]. In facilitating cell cycle progression, LIN28A can promote expression of cyclin D1 (CCND1) and cell division cycle 25 homolog A (CDC25A) by inhibiting miRNA biogenesis (let-7 dependent) and bind to cell cycle regulatory mRNAs such as cyclin-dependent kinase 2 (CDK2) to promote their translation (let-7 independent)[16].
Overexpression of LIN28A improves tissue repair such as digit repair, pinnal tissue repair, epidermal hair regrowth, and axon regeneration in both peripheral nervous system and central nervous system (CNS)[17,18]. The LIN28A/let-7 axis was implicated in these various tissues. Firstly, LIN28A enhances proliferation of connective tissue and bone for digit repair and mesenchymal tissue for pinnal tissue repair[17]. Secondly, it prolongs anagen phases (the active phase of the hair growth cycle) in hair follicles to promote hair regrowth[17]. Lastly, it represses glial let-7 miRNAs which can inhibit axon regeneration by targeting nerve growth factor in Schwann cells; the inhibitory effects of neuronal let-7 miRNAs on axon regeneration are uncertain[18].
In addition, LIN28 manipulates reprogramming factors such as Klf4, c-Myc, and Sox11 to enable mature CNS neurons to regain their ability to support axon regeneration, with the possible involvement of Akt-mTOR and GSK3β pathways[18]. Corroborating studies indicate that LIN28 can also functionally replace c-Myc, one of the Yamanaka factors responsible in reprogramming mature cells into induced pluripotent stem cells, and recruit Tet1 to regulate DNA methylation and gene expression. These properties enable LIN28 to influence epigenetic remodelling in facilitating axon regeneration.
On the other hand, the LIN28-mediated metabolic enhancements such as enhanced glycolysis could meet the higher energetic demands of anabolic biosynthesis and cell migration in accelerating tissue repair. However, it was observed that let-7 repression alone is insufficient to replicate this facet of LIN28’s role; indicating that the let-7independent pathway is imperative in tissue repair[17].
LIN28A is one of the elements affecting organismal mortality and growth including the onset of puberty. Constitutive loss or embryonic deficiency of LIN28A has been associated with perinatal lethality and dwarfism. While diminished organogenesis contributed to dwarfism, the exact cause of the LIN28-deficient perinatal lethality is currently unknown[19]. An interesting finding is LIN28A acts earlier on organismal growth compared to its paralog LIN28B, such that the impacts by the former’s deletion are primarily restricted to foetal or early postnatal tissues and already manifest in utero[19]. However, the prenatal impacts can have life-long consequences, as described in Barker hypothesis, which states that epigenetic memory of poor foetal or infantile environment can become an important determinant of risk for major chronic diseases such as cardiovascular disease and type 2 diabetes[19].
In terms of skeletal muscle development, overexpression of let-7 (due to decreased LIN28A) in skeletal muscle is capable of causing growth retardation[20]. Studies demonstrate that let-7 can impede cell proliferation by downregulating insulin-like growth factor 1, an essential hormone in growth and development, and initiate cell cycle arrest by downregulating cell cycle factors such as CKD6 and CDC34. As a result, the activation, proliferation, and maturation of myosatellite cells (precursors to skeletal myocytes) are hindered[20]. Conversely, overexpression of LIN28A is associated with increased body size and delayed onset of puberty. It is hypothesised that the LIN28A/let-7 axis influences the hypothalamic-pituitary gland axis through secretion of hormones such as growth hormone and gonadotropin releasing hormone, which are required for growth and initiating the puberty onset[21].
In both in vitro and in vivo models, LIN28A overexpression demonstrates elevated glucose uptake and glycolysis. This is achieved by LIN28 increasing the levels of hexokinase 2, the enzyme considered as the rate-limiting step and the first step in glycolysis[22]. Concurrently, LIN28 overexpression also increased PTEN-induced kinase 1 and mitofusin 2, which mediates mitochondrial recycling and thus, reduce oxygen consumption in these LIN28-overexpressed cells such as Hep3B cells[22]. However, LIN28-overexpressed MEF isolated from mice presented an increased oxygen consumption; intimating that LIN28A’s effect on oxygen consumption may depend on cell type. Nonetheless, LIN28 catalyses a shift from oxidative metabolism towards glycolytic metabolism. Now that LIN28A has been established as a regulator of glucose metabolism; increased LIN28A expression and thus, decreased expression of let-7 led to normal glucose tolerance and an insulin-sensitised state, which lowered the risk of obesity and diabetes[23].
LIN28A’s various physiological roles have linked it to various pathological processes. Its relevance in cancers, especially of breast, ovarian, and colon tissue are covered extensively in comparison to diseases such as Friedrich’s ataxia and Parkinson’s disease.
Being a regulator of cell proliferation and differentiation, LIN28 overexpression and reduced let-7 expression is often correlated with certain cancers. It has been proven that downregulation of let-7 miRNAs catalyses the derepression of oncogenes such as Ras and c-MYC, contributing to tumorigenesis or metastasis (Table 2)[24,25]. Notably, these oncogenes play a role in cell differentiation and proliferation; the former resulting in activation of the RAS-mitogen-activated protein kinases (RAS-MAPK) pathway which leads to uncontrolled cell proliferation while the latter influences a multitude of pathways involved in cell cycle progression, cell proliferation and differentiation, cell adhesion, and metabolism[26]. Alternatively, LIN28A was involved in oncogenesis through other pathways such as the direct interaction with mRNA.
| Tissue | Primary tumour | Clinical Relevance of LIN28A |
| Breast | HER2 + tumour | Overexpression correlated with HER2 + tumours |
| Colon | Primary adenocarcinoma | Expressed in approximately 30% tumours |
| Kidney | Primary Wilms’ tumour | Overexpressed in late stage |
| Lung | Small cell lung cancer | Loss increases let-7 levels; inhibits cell cycle |
| Oesophagus | Primary human tumour | Expression linked to metastasis and poor prognosis |
| Ovary | Primary ovarian tumour | Knockdown increases let-7 expression |
For example, LIN28A can bind to mRNA of bone morphogenetic proteins 4 (BMP4) to induce BMP4 overexpression, which stimulates cell proliferation and tumour growth in ovarian cancer[24]. Another example is LIN28 enhancing mRNA translation of human epidermal growth factor receptor 2 (HER2) and HMGA1, which promotes cell proliferation in breast cancer[24]. Therefore, LIN28A overexpression is typically associated with poor prognosis and a higher risk of relapse (Table 2)[25].
From a metabolic perspective, LIN28A promotes aerobic glycolysis in cancer cells by upregulating glycolysis-associated genes. Previously, it was thought that the insulin-Akt-mTor pathway was the primary cause but the pathway was not significantly impacted by LIN28A levels[27]. Further studies demonstrated LIN28A overexpression resulting in elevation of an important glycolytic enzyme, pyruvate dehydrogenase kinase 1 (PDK1). PDK1 inactivates pyruvate dehydrogenase, the enzyme that converts pyruvate to acetyl-coenzyme A in the Krebs cycle. This inhibits oxidative phosphorylation activity.
Consequently, cancer cells would undergo a metabolic switch from oxidative phosphorylation to aerobic glycolysis in normoxic conditions, i.e. Warburg effect, as part of cancer progression[27]. In addition, LIN28A has been demonstrated to bind with the mRNAs of sterol regulatory element-binding protein 1 (SREBP-1) and SREBP cleavage-activating protein (SCAP) to augment translation and maturation of SREBP-1. This promotes fatty acid synthesis, which in turn protects cancel cells from fatty acid-induced endoplasmic reticulum stress[28].
In understanding the pathophysiology of carcinogenesis, cancer stem cells (CSCs) in certain cancers such as breast, colon/colorectal, and ovarian cancers are examined. In colon cancer, LIN28A overexpression promotes proliferation of colon cancer cells by promoting the transition of cell cycle from S to G2/M phase[29] through upregulation of cell cycle factors such as cyclin A2[30]. In breast and ovarian cancer, LIN28A promoted the G0 or G1 transition instead through the let-7 suppression and increase in expression of cell cycle factors such as CCND1 and CDK34 for breast cancer or CDK2 for ovarian cancer[16,31]. These findings suggest that LIN28A’s regulatory control in the cell cycle differs from tissue to tissue.
There are a few studies stating that LIN28A stabilises RNA FBXL19-AS1 which sponges anti-oncogenic proteins such as miRNA-203 (in colorectal cancer) and WD Repeat Domain 66 (in breast cancer)[32,33]. Consequently, cell migration and invasion are no longer inhibited. Interestingly, a number of articles point towards its paralog LIN28B for being responsible in cell migration and invasion by decreasing let-7 expression and activating the Wingless Integration 1 (Wnt)/ β-catenin pathway; leading to a significant reduction of E-cadherin levels but an elevation of vimentin levels; causing disruption of the epithelium which enables the migration of cancer cells from the primary site[34,35]. Furthermore, abnormal β-catenin-E-cadherin complexes could diminish cellular adhesion and epithelial cell interstitialisation, which are required in limiting cell growth and cell migration primarily through contact inhibition[35]. Consequently, cell migration and invasion are facilitated.
For ovarian cancer, its malignancy is exacerbated by LIN28A hindering the activity of cleaved caspase-3, caspase-7, and caspase-9. Thus, the DNA damage repair enzyme, poly adenosine diphosphate-ribose polymerase (PARP) cannot be cleaved, which inhibits cell apoptosis of these cancer cells[36]. A unique mechanism in LIN28-expressing ovarian cancer cells is their secretion of exosomes, which contain miRNAs such as miRNA-200 and miRNA-17-92 that are taken up by non-tumour cells[37]. Subsequently, they induce EMT with miRNA-200 inhibiting zinc finger E-box binding homeobox 1 (ZEB1) to derepress E-cadherin as well as miRNA-17-92 regulating CYP7B1 and E-cadherin expression to ultimately promote migration and invasion[38].
In contrast with the previous three cancers, overexpression of LIN28A and hence, downregulation of let-7 expression impeded cell proliferation, migration, and cell cycle progression in gastric cancer cells[39]. In fact, LIN28A overexpression induced apoptosis of these cells. Neither the related study or current literature explain this inverse relationship and its mechanisms.
In hepatitis, LIN28A plays an active role in the balancing of EMT for fibrosis with mesenchymal-to-epithelial transition (MET) for liver regeneration; both processes are vital for liver repair[40]. Firstly, one of LIN28A targets include miRNA-200c which decreases expression of Fas-associated phosphatase 1 in order to increase expression of a proto-oncogene, tyrosine-protein kinase Src kinase (Src) and stimulate liver fibrosis[40]. In addition, another target, miRNA-107 can modulate components of the IL-6 receptor (IL-6R) complex in order to downregulate expression of chemokine (C-C motif) ligand 2 (CCL2), that is an inflammatory chemokine elevated in chronic liver diseases such as hepatitis C. Therefore, miRNA-107 can inhibit IL-6 signalling in regulating the inflammatory response and to an extent, fibrosis[40]. Secondly, LIN28A’s repression of let-7 removes the latter’s inhibitory effect on IL-6 so activation of SRC can now elevate IL-6 Levels to induce inflammation[40]. Consequently, LIN28A and its targets disrupt the balance of the inflammatory response and prolong fibrosis, which can have detrimental effects such as cirrhosis, portal hypertension, and liver failure.
Chronic cirrhosis can lead to oncogenic transformation into hepatocellular carcinoma (HCC), considered the most common primary liver tumour and leading cause of cancer death in the world[41]. As with most LIN28-linked cancers, increased LIN28 and decreased let-7 are involved in the unregulated cellular proliferation and enhanced metastatic ability[40,41].
Biliary diseases can refer to primary biliary cholangitis, an autoimmune destruction of small to medium-sized bile ducts are destroyed and primary sclerosing cholangitis, inflammation of bile ducts coupled with structuring and sclerosis[40]. Both conditions contribute to cholestasis but more importantly, both involve inflammation. Therefore, LIN28A is suggested to play a critical role in tissue repair and inflammation or progression to cholangiocarcinoma. This is supported by in vitro studies showing that let-7 can regulate inflammatory processes by modulating expression of lipopolysaccharide (LPS) toll-like receptor of cholangiocytes[40], and mice with cholangiocarcinoma presenting with decreased let-7 and increased LIN28B instead[42].
Other liver diseases include non-alcoholic fatty liver disease (NAFLD), which is characterised by steatosis due to excessive consumption of sugar and fats or certain medications; and polycystic liver disease (PCLD), which encompasses autosomal dominant or recessive disorders occurring in association with polycystic kidney diseases[40]. In NAFLD, a primary inflammatory regulator is the NF-κB which has been correlated with an elevation of LIN28 Levels, which would decrease let-7 levels[43]. In PCLD, LIN28’s influence is hypothesised through two findings: The cholangiocytes in PCLD possessing the ability to undergo EMT and majority of miRNAs that were part of the let-7 family exhibited reduced expression in cystic cholangiocytes[40]. However, current literature does not elucidate LIN28’s roles and mechanisms in PCLD.
Parkinson’s disease is characterised by degeneration of the dopamine neurons in the midbrain’s substantia nigra that results in tremors and stiffness. In vitro, loss of LIN28A yielded neural stem cells with absent dopamine neurogenic potential and diminished repair capacity as well as more vulnerable dopamine neurons when exposed to toxic environments. This indicates loss of LIN28A affected development of healthy and properly functional dopamine neurons[44]. Hence, this loss is correlated with the pathogenesis involving degeneration of neurons. Next, Rett syndrome is characterised by loss-of-function mutations in MECP2 that results in intellectual and motor impairments. Proteomic analyses revealed that LIN28 overexpression repressed astrocyte differentiation and decreased synapse formation. This leads to defects in glial differentiation and neuronal maturation, which in turn impairs neurodevelopment and hence, creates a dysfunctional nervous system[45].
Considering LIN28A’s various physiological functions and pathological roles, therapeutic approaches have been developed involving its pathways and pharmaceutical drugs. This review will focus on drugs or proteins that directly target LIN28A or those that target essential or related components in the LIN28/let-7 axis.
With regard to LIN28A inhibitors, studies have investigated compounds such as 1632, TPEN, and L171. Firstly, 1632 prevented LIN28 from interacting with let-7, which in turn enables let-7 levels to rise to avoid the occurrence of stem-like phenotype in cancer cells and it was found to diminish clonogenic activity, indicating decreased capacity for proliferation by tumour cells[46]. In vitro studies demonstrate 1632’s ability to reduce tumour sphere formation, which correlates with reduced in vivo tumour formation and metastasis. Most importantly, 1632 does not appear to have instantaneous cytotoxic effects but rather selectively inhibit tumour-specific characteristics of cells[46].
Secondly, tristetraprolin (TTP) binds to the three prime untranslated region (3’ UTR) of LIN28A mRNA that stimulates its decay. As a result, the increased mature let-7 levels lead to suppression of CDC34 expression, which prevents unregulated cell cycle progression to curb the growth of cancer cells. Furthermore, expression of TTP has an inverse correlation with LIN28A levels in ovarian adenocarcinoma and human cancer cell lines including breast adenocarcinoma, erythroleukaemia, HCC, and neuron-committed teratocarcinoma[47].
Thirdly, compounds such as TPEN inhibits LIN28A’s ZKDs by chelating zinc ions to catalyse apoptosis in LIN28A-expressing stem cells while L171 directly binds to LIN28A’s CSDs to disrupt RNA binding and LIN28A-mediated oligouridylation of let-7[48]. Studies demonstrated that TPEN targeted LIN28-expressing mouse ESCs but non-LIN28-expressing HeLa cells as well while L171 was effective in LIN28-dependent human leukaemia cells and ESCs. However, TPEN’s zinc chelation is not specific to LIN28A so it could cause apoptosis in cells that do not express LIN28A and L171 has a low potency for its inhibitory effects so both compounds require improvements. Moreover, there have been other potential LIN28A inhibitors such as 5-(methylamino) nicotinic acid that could block LIN28-mediated oligouridylation and gossypol that is hypothesised to hinder growth of LIN28-expressing tumours by suppressing oncoproteins such as Bcl-1 and MSI1, but further research would need to be conducted into their mechanisms and efficacy[48].
LIN28A inhibitors such as C1632 can restore let-7–mediated downregulation of programmed death ligand-1 (PD-L1). PD-L1 is frequently overexpressed in cancer cells and is a mechanism through which these cells evade T-cell recognition of tumour-specific and enhance tumour progression. Furthermore, high expression levels of PD-L1 are associated with more malignant tumour subtypes and poor prognosis in patients. By restoring let-7 levels, C1632 can hamper tumour growth by hindering proliferation of cancer cells and improve the immune surveillance[49]. Currently, C1632 treatment can suppress PD-L1 in antigen-presenting cells such as THP-1 macrophages, and elevate secretion of interferon gamma and tumour necrosis factor alpha to enhance their T-cell mediated anti-tumour activity[49].
In vitro studies involving treatment with C1632 did not seem to exhibit cytotoxic effects as significant increase in apoptosis was not observed. However, this does not necessarily translate to a therapeutically effective dose against cancer in humans. Interestingly, metformin and C1632 produce synergistic anti-tumour effects in oral squamous cell carcinoma (OSCC). Studies suggest that metformin activates Dicer via the AMP-activated protein kinase pathway to enable maturation of let-7. Consequently, a decline in proliferative and migratory capacity of human OSCC cells in vitro (reduced closure of wound) and decline in tumour growth in vivo (reduced weight), without obvious signs of toxicity were observed[50]. While this combined treatment is promising for a non-invasive treatment of OSCC, the side effects and immunoreactivity in humans are unclear so further testing is needed.
In addition, LIN28 expression has been positively correlated with aldehyde dehydrogenase (ALDH) levels, a marker of CSCs and particularly with a subpopulation of tumour cells that are ALDH 1 positive (ALDH1+)[51]. ALDHs could affect mechanisms in DNA repair and radioresistance and thus, contribute to carcinogenesis. Since LIN28 regulates and maintains the ALDH1+ cell population through the let-7 pathway or a let-7 independent pathway through reprogramming factors such as OCT4, inhibiting LIN28 would diminish the tumour cell population. This could be carried out by either manipulating TUTase to increase let-7 or using nanoparticle-delivered LIN28 small interfering RNA or let-7.
In fact, research has explored nanobodies that can directly interact with a functional region on the TUT4 known as 106-reside LIN28: Let-7 interaction (LLI) fragment. These nanobodies can bind to the LLI fragments to interfere with the recruitment of TUTase and impeding LIN28-dependent (i.e. involving TUT4) oligouridylation of pre-let-7 microRNAs and LIN28-independent monouridylation of group II pre-let-7 microRNAs[51]. Hence, inhibition of TUT4 activity leads to elevated levels of mature let-7; countering the potential oncogenic implications of the LIN28/let-7 pathway.
Lastly, ubiquitin-specific protease 28 (USP28) is a deubiquitinase that removes polyubiquitin from proteins such as LIN28A to stop the proteasomal degradation, thereby stabilising and extending its half-life. Studies indicate that USP28 augments LIN28A’s inhibitory and oncogenic function. The former function involves enhanced inhibition of let-7 while the latter function involves enhanced colony formation, cell migration and invasion, and cell anchorage-independent proliferation[52]. Moreover, USP28 is overexpressed in cancers such as colorectal cancer and non-small cell lung cancer. This can be attributed to USP28 stabilising proto-oncogenic factors such as MYC. Considering MYC can facilitate cancer cell proliferation, it is hypothesised that USP28 can influence the LIN28-mediated cancer cell proliferation as well[52]. Therefore, disrupting USP28 itself or targeting other proteins that regulate it could become a viable option for cancer therapy.
In optical neuropathies, retinal ischaemia-reperfusion (RIR) injury is characterised by expedited neuronal cell death due to lack of nutrients and oxygen as well as reactive oxygen species (ROS). Treatment with rasagiline and idebenone can utilise LIN28A’s inhibitory effects on caspase-3 to mitigate oxidative damage by ROS and apoptosis[53]. Next, these drugs can upregulate Dicer expression through the LIN28A/let-7 pathway, which enables cleavage of pre-miRNA into mature miRNA for optic neuroprotection and retinal development. Therefore, this combined treatment can alleviate RIR injury[53].
In auditory neuropathies, sensorineural hearing loss can be due to loss of auditory neurons. In vitro studies demonstrated that LIN28 overexpression activates basic helix-loop-helix (bHLH) transcription factors via let-7 inhibition and upregulates Sox2 and HMGA2, which leads to increased proliferation and reprogramming of inner ear glial cells into neurons[54]. Therefore, neuronal dedifferentiation and proliferation could possibly restore auditory function. These findings indicate the potential of LIN28A in cell replacement therapy.
Since its discovery, LIN28A has been found to regulate several physiological processes such as stem cell renewal and differentiation, tissue repair, and glucose metabolism through let-7 dependent and independent pathways. While downstream signalling pathways such as the insulin-Akt-mTor pathway and certain mRNA targets such as HMGA2 have been identified, the exact mechanisms have not been completely understood. Next, it remains unclear how these pathways eventuate in different and sometimes, contrasting effects in cell types. Pathologically, overexpression of LIN28A is generally correlated with poor prognosis in certain cancers, and negative outcomes in some biliary diseases and neuropathies. Consequently, therapeutic approaches have been developed, which either target LIN28A or other proteins which interact with LIN28A. By inhibiting LIN28A expression or function and manipulating its pathways, cellular proliferation and differentiation, and tissue repair can be regulated, which would especially be imperative in cancer therapy and tissue regeneration. However, further research is required before the efficacy of these approaches can be verified.
I would like to thank Dr. Rajaraman Eri for his guidance in writing this review. I would also like to thank him and Tauseef Ahmad for providing constructive feedback.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Biochemistry and molecular biology
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yang YZ, China S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Mayr F, Heinemann U. Mechanisms of Lin28-mediated miRNA and mRNA regulation--a structural and functional perspective. Int J Mol Sci. 2013;14:16532-16553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 2. | Tsialikas J, Romer-Seibert J. LIN28: roles and regulation in development and beyond. Development. 2015;142:2397-2404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (1)] |
| 3. | Shyh-Chang N, Daley GQ. Lin28: primal regulator of growth and metabolism in stem cells. Cell Stem Cell. 2013;12:395-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 389] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 4. | Amen AM, Ruiz-Garzon CR, Shi J, Subramanian M, Pham DL, Meffert MK. A Rapid Induction Mechanism for Lin28a in Trophic Responses. Mol Cell. 2017;65:490-503.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Ustianenko D, Chiu HS, Treiber T, Weyn-Vanhentenryck SM, Treiber N, Meister G, Sumazin P, Zhang C. LIN28 Selectively Modulates a Subclass of Let-7 MicroRNAs. Mol Cell. 2018;71:271-283.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 6. | Lee H, Han S, Kwon CS, Lee D. Biogenesis and regulation of the let-7 miRNAs and their functional implications. Protein Cell. 2016;7:100-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 250] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 7. | Kim SK, Lee H, Han K, Kim SC, Choi Y, Park SW, Bak G, Lee Y, Choi JK, Kim TK, Han YM, Lee D. SET7/9 methylation of the pluripotency factor LIN28A is a nucleolar localization mechanism that blocks let-7 biogenesis in human ESCs. Cell Stem Cell. 2014;15:735-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Hafner M, Max KE, Bandaru P, Morozov P, Gerstberger S, Brown M, Molina H, Tuschl T. Identification of mRNAs bound and regulated by human LIN28 proteins and molecular requirements for RNA recognition. RNA. 2013;19:613-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 9. | Cho J, Chang H, Kwon SC, Kim B, Kim Y, Choe J, Ha M, Kim YK, Kim VN. LIN28A is a suppressor of ER-associated translation in embryonic stem cells. Cell. 2012;151:765-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 183] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 10. | Wilbert ML, Huelga SC, Kapeli K, Stark TJ, Liang TY, Chen SX, Yan BY, Nathanson JL, Hutt KR, Lovci MT, Kazan H, Vu AQ, Massirer KB, Morris Q, Hoon S, Yeo GW. LIN28 binds messenger RNAs at GGAGA motifs and regulates splicing factor abundance. Mol Cell. 2012;48:195-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 224] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 11. | Qiu C, Ma Y, Wang J, Peng S, Huang Y. Lin28-mediated post-transcriptional regulation of Oct4 expression in human embryonic stem cells. Nucleic Acids Res. 2010;38:1240-1248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 180] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 12. | Li C, Sako Y, Imai A, Nishiyama T, Thompson K, Kubo M, Hiwatashi Y, Kabeya Y, Karlson D, Wu SH, Ishikawa M, Murata T, Benfey PN, Sato Y, Tamada Y, Hasebe M. A Lin28 homologue reprograms differentiated cells to stem cells in the moss Physcomitrella patens. Nat Commun. 2017;8:14242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Parisi S, Passaro F, Russo L, Musto A, Navarra A, Romano S, Petrosino G, Russo T. Lin28 is induced in primed embryonic stem cells and regulates let-7-independent events. FASEB J. 2017;31:1046-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Seabrook JL, Cantlon JD, Cooney AJ, McWhorter EE, Fromme BA, Bouma GJ, Anthony RV, Winger QA. Role of LIN28A in mouse and human trophoblast cell differentiation. Biol Reprod. 2013;89:95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Yang M, Yang SL, Herrlinger S, Liang C, Dzieciatkowska M, Hansen KC, Desai R, Nagy A, Niswander L, Moss EG, Chen JF. Lin28 promotes the proliferative capacity of neural progenitor cells in brain development. Development. 2015;142:1616-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 16. | Li N, Zhong X, Lin X, Guo J, Zou L, Tanyi JL, Shao Z, Liang S, Wang LP, Hwang WT, Katsaros D, Montone K, Zhao X, Zhang L. Lin-28 homologue A (LIN28A) promotes cell cycle progression via regulation of cyclin-dependent kinase 2 (CDK2), cyclin D1 (CCND1), and cell division cycle 25 homolog A (CDC25A) expression in cancer. J Biol Chem. 2012;287:17386-17397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Shyh-Chang N, Zhu H, Yvanka de Soysa T, Shinoda G, Seligson MT, Tsanov KM, Nguyen L, Asara JM, Cantley LC, Daley GQ. Lin28 enhances tissue repair by reprogramming cellular metabolism. Cell. 2013;155:778-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 304] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 18. | Wang XW, Li Q, Liu CM, Hall PA, Jiang JJ, Katchis CD, Kang S, Dong BC, Li S, Zhou FQ. Lin28 Signaling Supports Mammalian PNS and CNS Axon Regeneration. Cell Rep. 2018;24:2540-2552.e6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 19. | Shinoda G, Shyh-Chang N, Soysa TY, Zhu H, Seligson MT, Shah SP, Abo-Sido N, Yabuuchi A, Hagan JP, Gregory RI, Asara JM, Cantley LC, Moss EG, Daley GQ. Fetal deficiency of lin28 programs life-long aberrations in growth and glucose metabolism. Stem Cells. 2013;31:1563-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 20. | Zacharewicz E, Lamon S, Russell AP. MicroRNAs in skeletal muscle and their regulation with exercise, ageing, and disease. Front Physiol. 2013;4:266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 21. | Corre C, Shinoda G, Zhu H, Cousminer DL, Crossman C, Bellissimo C, Goldenberg A, Daley GQ, Palmert MR. Sex-specific regulation of weight and puberty by the Lin28/let-7 axis. J Endocrinol. 2016;228:179-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Docherty CK, Salt IP, Mercer JR. Lin28A induces energetic switching to glycolytic metabolism in human embryonic kidney cells. Stem Cell Res Ther. 2016;7:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Kim JD, Toda C, Ramírez CM, Fernández-Hernando C, Diano S. Hypothalamic Ventromedial Lin28a Enhances Glucose Metabolism in Diet-Induced Obesity. Diabetes. 2017;66:2102-2111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Balzeau J, Menezes MR, Cao S, Hagan JP. The LIN28/let-7 Pathway in Cancer. Front Genet. 2017;8:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 352] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 25. | Thornton JE, Gregory RI. How does Lin28 let-7 control development and disease? Trends Cell Biol. 2012;22:474-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 304] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 26. | Roncarati R, Lupini L, Shankaraiah RC, Negrini M. The Importance of microRNAs in RAS Oncogenic Activation in Human Cancer. Front Oncol. 2019;9:988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Ma X, Li C, Sun L, Huang D, Li T, He X, Wu G, Yang Z, Zhong X, Song L, Gao P, Zhang H. Lin28/let-7 axis regulates aerobic glycolysis and cancer progression via PDK1. Nat Commun. 2014;5:5212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 142] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 28. | Zhang Y, Li C, Hu C, Wu Q, Cai Y, Xing S, Lu H, Wang L, Huang, Sun L, Li T, He X, Zhong X, Wang J, Gao P, Smith ZJ, Jia W, Zhang H. Lin28 enhances de novo fatty acid synthesis to promote cancer progression via SREBP-1. EMBO Rep. 2019;20:e48115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Wang T, He Y, Zhu Y, Chen M, Weng M, Yang C, Zhang Y, Ning N, Zhao R, Yang W, Jin Y, Li J, Redpath RJ, Zhang L, Jin X, Zhong Z, Zhang F, Wei Y, Shen G, Wang D, Liu Y, Wang G, Li X. Comparison of the expression and function of Lin28A and Lin28B in colon cancer. Oncotarget. 2016;7:79605-79616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Mizuno R, Kawada K, Sakai Y. The Molecular Basis and Therapeutic Potential of Let-7 MicroRNAs against Colorectal Cancer. Can J Gastroenterol Hepatol. 2018;2018:5769591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 31. | Xiong H, Zhao W, Wang J, Seifer BJ, Ye C, Chen Y, Jia Y, Chen C, Shen J, Wang L, Sui X, Zhou J. Oncogenic mechanisms of Lin28 in breast cancer: new functions and therapeutic opportunities. Oncotarget. 2017;8:25721-25735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Shen B, Yuan Y, Zhang Y, Yu S, Peng W, Huang X, Feng J. Long non-coding RNA FBXL19-AS1 plays oncogenic role in colorectal cancer by sponging miR-203. Biochem Biophys Res Commun. 2017;488:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Zhang Y, Xiao X, Zhou W, Hu J, Zhou D. LIN28A-stabilized FBXL19-AS1 promotes breast cancer migration, invasion and EMT by regulating WDR66. In Vitro Cell Dev Biol Anim. 2019;55:426-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | King CE, Wang L, Winograd R, Madison BB, Mongroo PS, Johnstone CN, Rustgi AK. LIN28B fosters colon cancer migration, invasion and transformation through let-7-dependent and -independent mechanisms. Oncogene. 2011;30:4185-4193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 35. | Liu Y, Li H, Feng J, Cui X, Huang W, Li Y, Su F, Liu Q, Zhu J, Lv X, Chen J, Huang D, Yu F. Lin28 induces epithelial-to-mesenchymal transition and stemness via downregulation of let-7a in breast cancer cells. PLoS One. 2013;8:e83083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 36. | Zhong Y, Yang S, Wang W, Wei P, He S, Ma H, Yang J, Wang Q, Cao L, Xiong W, Zhou M, Li G, Shuai C, Peng S. The interaction of Lin28A/Rho associated coiled-coil containing protein kinase2 accelerates the malignancy of ovarian cancer. Oncogene. 2019;38:1381-1397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Enriquez VA, Cleys ER, Da Silveira JC, Spillman MA, Winger QA, Bouma GJ. High LIN28A Expressing Ovarian Cancer Cells Secrete Exosomes That Induce Invasion and Migration in HEK293 Cells. Biomed Res Int. 2015;2015:701390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 38. | Fang LL, Wang XH, Sun BF, Zhang XD, Zhu XH, Yu ZJ, Luo H. Expression, regulation and mechanism of action of the miR-17-92 cluster in tumor cells (Review). Int J Mol Med. 2017;40:1624-1630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 39. | Song H, Xu W, Song J, Liang Y, Fu W, Zhu XC, Li C, Peng JS, Zheng JN. Overexpression of Lin28 inhibits the proliferation, migration and cell cycle progression and induces apoptosis of BGC-823 gastric cancer cells. Oncol Rep. 2015;33:997-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | McDaniel K, Hall C, Sato K, Lairmore T, Marzioni M, Glaser S, Meng F, Alpini G. Lin28 and let-7: roles and regulation in liver diseases. Am J Physiol Gastrointest Liver Physiol. 2016;310:G757-G765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 41. | Nguyen LH, Robinton DA, Seligson MT, Wu L, Li L, Rakheja D, Comerford SA, Ramezani S, Sun X, Parikh MS, Yang EH, Powers JT, Shinoda G, Shah SP, Hammer RE, Daley GQ, Zhu H. Lin28b is sufficient to drive liver cancer and necessary for its maintenance in murine models. Cancer Cell. 2014;26:248-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 160] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 42. | Yang H, Li TW, Peng J, Tang X, Ko KS, Xia M, Aller MA. A mouse model of cholestasis-associated cholangiocarcinoma and transcription factors involved in progression. Gastroenterology. 2011;141:378-388, 388.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 43. | Gori M, Arciello M, Balsano C. MicroRNAs in nonalcoholic fatty liver disease: novel biomarkers and prognostic tools during the transition from steatosis to hepatocarcinoma. Biomed Res Int. 2014;2014:741465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 44. | Chang MY, Oh B, Choi JE, Sulistio YA, Woo HJ, Jo A, Kim J, Kim EH, Kim SW, Hwang J, Park J, Song JJ, Kwon OC, Henry Kim H, Kim YH, Ko JY, Heo JY, Lee MJ, Lee M, Choi M, Chung SJ, Lee HS, Lee SH. LIN28A loss of function is associated with Parkinson's disease pathogenesis. EMBO J. 2019;38:e101196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 45. | Kim JJ, Savas JN, Miller MT, Hu X, Carromeu C, Lavallée-Adam M, Freitas BCG, Muotri AR, Yates JR 3rd, Ghosh A. Proteomic analyses reveal misregulation of LIN28 expression and delayed timing of glial differentiation in human iPS cells with MECP2 loss-of-function. PLoS One. 2019;14:e0212553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Roos M, Pradère U, Ngondo RP, Behera A, Allegrini S, Civenni G, Zagalak JA, Marchand JR, Menzi M, Towbin H, Scheuermann J, Neri D, Caflisch A, Catapano CV, Ciaudo C, Hall J. A Small-Molecule Inhibitor of Lin28. ACS Chem Biol. 2016;11:2773-2781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 114] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 47. | Kim CW, Vo MT, Kim HK, Lee HH, Yoon NA, Lee BJ, Min YJ, Joo WD, Cha HJ, Park JW, Cho WJ. Ectopic over-expression of tristetraprolin in human cancer cells promotes biogenesis of let-7 by down-regulation of Lin28. Nucleic Acids Res. 2012;40:3856-3869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 48. | Wang L, Rowe RG, Jaimes A, Yu C, Nam Y, Pearson DS, Zhang J, Xie X, Marion W, Heffron GJ, Daley GQ, Sliz P. Small-Molecule Inhibitors Disrupt let-7 Oligouridylation and Release the Selective Blockade of let-7 Processing by LIN28. Cell Rep. 2018;23:3091-3101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 49. | Yang X, Lin X, Zhong X, Kaur S, Li N, Liang S, Lassus H, Wang L, Katsaros D, Montone K, Zhao X, Zhang Y, Bützow R, Coukos G, Zhang L. Double-negative feedback loop between reprogramming factor LIN28 and microRNA let-7 regulates aldehyde dehydrogenase 1-positive cancer stem cells. Cancer Res. 2010;70:9463-9472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 50. | Yu C, Wang L, Rowe RG, Han A, Ji W, McMahon C, Baier AS, Huang YC, Marion W, Pearson DS, Kruse AC, Daley GQ, Wu H, Sliz P. A nanobody targeting the LIN28:let-7 interaction fragment of TUT4 blocks uridylation of let-7. Proc Natl Acad Sci U S A. 2020;117:4653-4663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 51. | Chen Y, Xie C, Zheng X, Nie X, Wang Z, Liu H, Zhao Y. LIN28/let-7/PD-L1 Pathway as a Target for Cancer Immunotherapy. Cancer Immunol Res. 2019;7:487-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 52. | Haq S, Das S, Kim DH, Chandrasekaran AP, Hong SH, Kim KS, Ramakrishna S. The stability and oncogenic function of LIN28A are regulated by USP28. Biochim Biophys Acta Mol Basis Dis. 2019;1865:599-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 53. | Lei D, Shao Z, Zhou X, Yuan H. Synergistic neuroprotective effect of rasagiline and idebenone against retinal ischemia-reperfusion injury via the Lin28-let-7-Dicer pathway. Oncotarget. 2018;9:12137-12153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 54. | Kempfle JS, Luu NC, Petrillo M, Al-Asad R, Zhang A, Edge ASB. Lin28 reprograms inner ear glia to a neuronal fate. Stem Cells. 2020;38:890-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |