Published online Sep 27, 2021. doi: 10.4331/wjbc.v12.i5.70
Peer-review started: March 31, 2021
First decision: June 7, 2021
Revised: June 21, 2021
Accepted: August 3, 2021
Article in press: August 3, 2021
Published online: September 27, 2021
Processing time: 174 Days and 9.5 Hours
The prevalence of type 2 diabetes (T2D) continues to rise despite the amount of research dedicated to finding the culprits of this debilitating disease. Skeletal muscle is arguably the most important contributor to glucose disposal making it a clear target in insulin resistance and T2D research. Within skeletal muscle there is a clear link to metabolic dysregulation during the progression of T2D but the determination of culprits vs consequences of the disease has been elusive. Emerging evidence in skeletal muscle implicates influential cross talk between a key anabolic regulatory protein, the mammalian target of rapamycin (mTOR) and its associated complexes (mTORC1 and mTORC2), and the well-described cano
Core Tip: The prevalence of type 2 diabetes (T2D) continues to rise despite the amount of research dedicated to finding the culprits of this debilitating disease. Within skeletal muscle there is a clear link to metabolic dysregulation during the progression of T2D but the determination of culprits vs consequences of the disease has been elusive. Emerging evidence in skeletal muscle implicates influential cross talk between the mammalian target of rapamycin (mTOR) complexes (mTORC1 and mTORC2) during insulin stimulated glucose uptake. This review highlights interactions between protein and glucose regulatory pathways and the implications this may have for the control of glucoregulatory function in skeletal muscle.
- Citation: O'Reilly CL, Uranga S, Fluckey JD. Culprits or consequences: Understanding the metabolic dysregulation of muscle in diabetes. World J Biol Chem 2021; 12(5): 70-86
- URL: https://www.wjgnet.com/1949-8454/full/v12/i5/70.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v12.i5.70
Globally, 462 million individuals are affected by type 2 diabetes (T2D) and it is ranked as the 9th leading cause of mortality[1]. The prevalence of diabetes over the past few decades has continued to rise with no sign of this changing[1]. T2D is characterized by insulin resistance and hyperglycemia and can lead to various other outcomes and comorbidities reducing quality of life in those effected. While the pathogenesis and progression of T2D is still widely debated, it is clear that a complex interplay between the pancreas and peripheral tissues is dependent for maintenance of glucose homeo
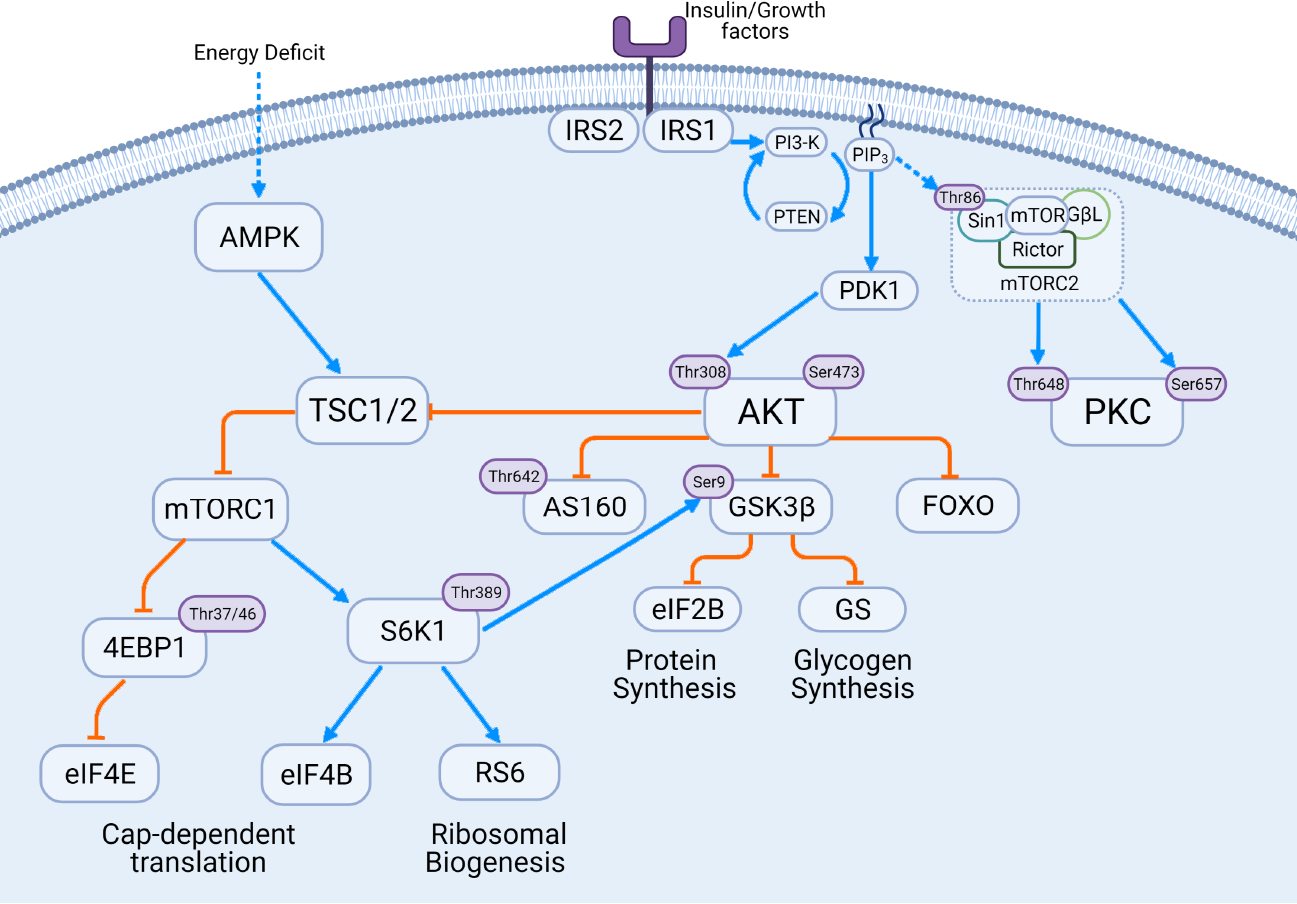
The insulin signaling cascade involves both glucoregulatory and anabolic processes which is outlined in Figure 1. Insulin responsive tissues have insulin receptors (IR) on the cell surface plasma membrane. These IR contain subunits where insulin can bind as well as residues that provide docking sites for downstream signaling molecules including the IR substrates (IRS). The two predominant insulin receptor substrates are IRS1 and IRS2 with similar sequences but specific signaling roles[8,9]. IRS1 appears to be the insulin receptor substrate protein whose primary responsibility is glucose re
This serine/threonine kinase is part of the AGC protein family and is known for its diverse function in growth, survival, proliferation and most importantly substrate metabolism[11-13] AKT is often referred to as one molecule but actually comprises of three distinct isoforms (AKT1, AKT2, AKT3) , while all isoforms are present in skeletal muscle, AKT2 is the most prevalent[12], but varies from low to immeasurable amounts in skeletal muscle[14,15]. While defining the variation and overlap between the AKT isoforms is important and needed, it is beyond the scope of this review but what is known currently can be found in these reviews[12,16] It is important to note that AKT2 is expressed primarily in insulin responsive tissues like fat and skeletal muscle and is the most abundant isoform in skeletal muscle[14,15,17,18]. AKT is as a critical regula
The upstream regulation of AKT, in its most simple iteration, appears to be very similar across isoforms. The two common phosphorylation sites of AKT are Ser473 (Ser474 in AKT2) and Thr308. The insulin receptors IRS1 and IRS2 will activate the PI3K-dependent conversion of PIP2 to PIP3, and PIP3 will recruit Pyruvate Dehydrogenase Kinase 1 (PDK1) and AKT to the membrane where colocalization will allow for phosphorylation at the Thr308 by PDK1[12,13]. Further, some evidence suggests that mitogen-activated protein kinase-associated protein 1 (mSin1) of the mTORC2 comp
The regulation of mTORC2 activity by mSin1 phosphorylation is controversial. It has been proposed that PIP3) promotes mTORC2 activity directly[21,22]. Recent work has indicated a positive feedback loop between AKT and mTORC2 via phosphory
There is also considerable debate over what the phosphorylation of specific AKT sites implicates for AKT activity and substrate specificity. Much of the early work in AKT reported a requirement of phosphorylation at Ser473 for full activation[25-28]. However, more recent work in platelets[29], HEK cells[27,30], and skeletal muscle[31,32] demonstrated that not all downstream substrates are impacted by Ser473 phos
The implications of Ser473 phosphorylation via mTORC2 has been studied in various tissues. In mSin1 knockout mouse embryonic fibroblasts, a regulator of mTORC2 complex formation and stability, Forkhead box 01/03 (FOX01/3a) phospho
As previously mentioned AKT has various downstream substrates that make the action of this kinase quite diverse in cell function. These substrates include members of the mTOR complexes Pras40 and Sin1, Glucose uptake proteins AS160 and GSK-3, Protein synthesis related Tuberous sclerosis 2, and apoptotic signaling through the FOX0 family. This section will focus on signal transduction related to glucose uptake.
GLUT-4 is the predominant isoform of the GLUT family found in skeletal muscle, and one of insulin’s primary metabolic roles is to promote the translocation of GLUT-4 to the surface membrane. AKT has been linked to downstream substrates that impact insulin-dependent GLUT-4 translocation including GSK-3[41] as well as AKT Subs
AKT phosphorylates TSC2 at Thr1462 which regulates the tuberin-hamartin complex and it’s activity[51-53]. Phosphorylation at this site releases the tuberin-hamartin complex inhibition of the mTORC1 complex and allows for downstream targets to be phosphorylated[51]. mTORC1 is a prolific kinase with multiple downstream sub
It is generally agreed that glucose transport is the rate limiting step of glucose uptake, and the step most impacted by the progression of T2D. The consensus in diabetes research at large is that the translocation or trafficking of glucose transport molecules in skeletal muscle is impaired in T2D[43,62] but the culprit behind this impairment is still widely debated. In skeletal muscle GLUT-4 is the predominantly expressed isoform[63,64] and the localization of GLUT-4 has been confirmed with insulin[65], exercise[65,66] and hypoxia[67]. The first important finding with diabetes is that the limitation in glucose transport cannot be explained by production or maintenance of the GLUT itself, because total GLUT-4 protein is largely unchanged with diabetes[68-70]. This implies that the issue is not related to GLUT-4 expression, per se, but within the signaling cascades that assist in the translocation of GLUT-4 to the surface mem
As the initial step in the insulin signaling cascade, the insulin receptor was a primary target of research related to the breakdown of the glucoregulatory signals. While current data are conflicting on IR activity with some reporting impairment[62,71,72] and others reporting normal activity[73-77], it appears that the important signaling ‘defects’ of T2D are further down the signal cascade. Signaling defects in IRS1 phosphorylation[73,77-79] and PI3K[73,77,78,80,81], activity are consistently found in the diabetic model. More controversial is the activity of AKT with studies reporting significant reductions of insulin stimulated AKT phosphorylation on Ser473 or Thr308[69,75,82,83]. While others report not impact of T2D on insulin dependent phosphorylation[80,81]. Downstream substrates of AKT have also been presented in the diabetic model with reduced glycogen synthase activity with protein levels of GSK-3 reported as being elevated which would inhibit GS activity[84]. Additionally, insulin dependent phosphorylation of AS160 has also been reported to be higher in T2D[42], despite the fact that AKT phosphorylation was not different in the same study.
Despite the continued exploration and detailed understanding of what the signaling cascades are doing during diabetes, there is still no consensus on where these dys
It is well documented that muscle mass and strength decline with T2D[85,86] and contribute to a decline in quality life over time. Interestingly despite a loss in muscle mass, there appears to be an upregulation of protein synthesis and the anabolic signal cascade in diabetic muscle[87,88]. Previously, studies assessing anabolic responses [fractional synthesis rate (FSR)] in diabetic skeletal muscle have been inconsistent, ranging from decreased[89,90], to normal[91,92] but more recently increased FSR has been confirmed by our lab[87,88,93,94] and others[95,96]. In Fatty Zucker rats, a well-documented model for T2D, upregulated protein synthesis in specific muscle fractions and increased phosphorylation of S6K1 were observed despite an overall decrease in muscle mass. This upregulation of S6K1 appears to be linked to a loss of control of upstream mTOR activation. While the hyperactive mTOR activity may be a result of the maintained state of hyperinsulinemia with glucose intolerance, we suspect some
Our recent studies have demonstrated that the constitutive activation of mTOR may be a result of suppressed DEPTOR expression in the diabetic state. DEPTOR is one of the mTOR associated binding partners that can be a part of either mTORC1 or mTORC2 and is a negative regulator of mTOR activity. Similar to several lines of cancer[97]. DEPTOR is substantially lower in obese subjects[87,88]. Since DEPTOR is still a fairly new discovery in the mTOR signaling cascade, the implications of low DEPTOR and the regulation of mTORC1 are still speculative but the low DEPTOR appears to allow the downstream anabolic signals to go unchecked[98] which has implications for mRNA translation[99], as well as glucoregulatory signaling cascades. This is unbridled mTORC1 activity without concomitant muscle mass accretion is indicative of high protein turnover[88], where it may not be warranted or wanted. It is also an important bridge between mTORC1 and mTORC2 which will be discussed in a later section.
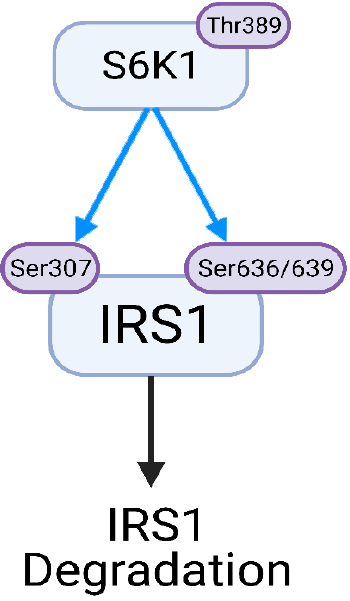
A relatively recent but important discovery in the connection of anabolic and glucoregulatory signaling paths is an inhibitory pathway that directly links S6K1 to IRS1. IRS1 can be serine phosphorylated through many pathways including c-Jun NH2-terminal kinase, IkB kinase, PKC, and S6K1[100,101]. It is now known that the insulin receptor contains multiple phosphorylation sites[102] and even in a basal state it is highly phosphorylated[103]. Ser/Thr phosphorylation of IRS-1 has been linked to the de
While we are gaining perspective in the current literature about the interaction between mTORC1 signaling for protein synthesis and the disruption of insulin sig
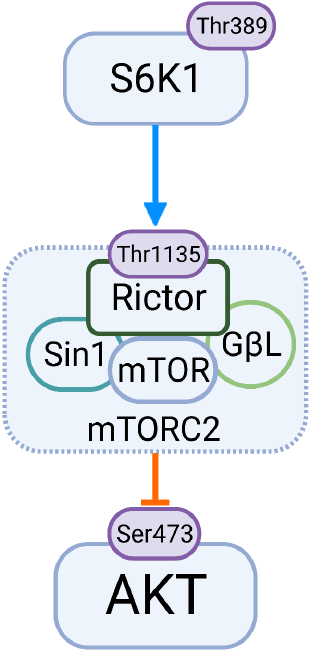
The mTORC2 complex is best known for its involvement in cell survival but is known to phosphorylate AKT through Ser473[25,114-117] as well as the PKC family[40,116-119]. This complex is composed of binding partners mSin1, DEPTOR, Protor1, mLST8 and RICTOR. While all of these binding partners play roles in mTORC2 ac
Work by others indicated that the muscle-specific deletion of RICTOR led to de
Exercise and physical activity are effective, low cost interventions for insulin resistance and T2D[126,127]. The benefits of aerobic exercise on glucose tolerance are well es
It is often speculated that insulin-resistant skeletal muscle is desensitized or ‘re
Aside from insulin sensitivity, there are benefits to regular exercise, whether it is of an aerobic or anaerobic nature. It is important to note here that there are insulin independent pathways that trigger glucose uptake that are directly related to skeletal muscle contraction. This pathway is triggered by muscle contraction and involves a distinct subset of GLUT-4[66,151-153]. These pathways can involve nitric oxide[154] and activation of AMPK[155,156] as well as cytosolic calcium[130] but these effects are distinct and additive to those of insulin mediated glucose uptake[2,157-159]. Probably most important for T2D research is that these contraction mediated glucose pathways are not only present in T2D but are fully functional[160,161].
Interestingly, in insulin resistant muscle there seems to be a difference in the control of muscle protein synthesis. It appears that in tissue where the upstream activators of the mTORC1 pathway are impaired there are alterations to the use in protein syn
One key player that may have an impact on muscle protein synthesis in response to insulin is a serine/threonine kinase called PKC. PKC has long been considered as a regulatory contributor during mRNA translation in a number of tissues[162,163] but more recently specific isoforms of PKC have been implicated in the regulation of glucose uptake. Specifically, the conventional family of PKCs (α, β, γ) lead to atte
The regulation of PKC, like many of the enzymes related to insulin signal trans
Dysregulation of mTOR signaling is a key player in the development of many disease states including diabetes. While decades of research have been dedicated to under
Manuscript source: Invited manuscript
Specialty type: Biochemistry and molecular biology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhang LL S-Editor: Fan JR L-Editor: A P-Editor: Yuan YY
| 1. | Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of Type 2 Diabetes - Global Burden of Disease and Forecasted Trends. J Epidemiol Glob Health. 2020;10:107-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 523] [Cited by in RCA: 1652] [Article Influence: 413.0] [Reference Citation Analysis (2)] |
| 2. | DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30:1000-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1170] [Cited by in RCA: 1274] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 3. | DeFronzo RA, Gunnarsson R, Björkman O, Olsson M, Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest. 1985;76:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 832] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 4. | Issa-Chergui B, Guttmann RD, Seemayer TA, Kelley VE, Colle E. The effect of diet on the spontaneous insulin dependent diabetic syndrome in the rat. Diabetes Res. 1988;9:81-86. [PubMed] |
| 5. | Moore MC, Cherrington AD, Wasserman DH. Regulation of hepatic and peripheral glucose disposal. Best Pract Res Clin Endocrinol Metab. 2003;17:343-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Sato M, Dehvari N, Oberg AI, Dallner OS, Sandström AL, Olsen JM, Csikasz RI, Summers RJ, Hutchinson DS, Bengtsson T. Improving type 2 diabetes through a distinct adrenergic signaling pathway involving mTORC2 that mediates glucose uptake in skeletal muscle. Diabetes. 2014;63:4115-4129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 7. | Ren JM, Marshall BA, Gulve EA, Gao J, Johnson DW, Holloszy JO, Mueckler M. Evidence from transgenic mice that glucose transport is rate-limiting for glycogen deposition and glycolysis in skeletal muscle. J Biol Chem. 1993;268:16113-16115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 190] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Huang C, Thirone AC, Huang X, Klip A. Differential contribution of insulin receptor substrates 1 versus 2 to insulin signaling and glucose uptake in l6 myotubes. J Biol Chem. 2005;280:19426-19435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 131] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 9. | Bouzakri K, Zachrisson A, Al-Khalili L, Zhang BB, Koistinen HA, Krook A, Zierath JR. siRNA-based gene silencing reveals specialized roles of IRS-1/Akt2 and IRS-2/Akt1 in glucose and lipid metabolism in human skeletal muscle. Cell Metab. 2006;4:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 167] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 10. | Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1195] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 11. | Zhang Z, Liu H, Liu J. Akt activation: A potential strategy to ameliorate insulin resistance. Diabetes Res Clin Pract. 2019;156:107092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 12. | Gonzalez E, McGraw TE. The Akt kinases: isoform specificity in metabolism and cancer. Cell Cycle. 2009;8:2502-2508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 383] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 13. | Manning BD, Toker A. AKT/PKB Signaling: Navigating the Network. Cell. 2017;169:381-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1732] [Cited by in RCA: 2648] [Article Influence: 331.0] [Reference Citation Analysis (0)] |
| 14. | Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276:38349-38352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 771] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 15. | Garofalo RS, Orena SJ, Rafidi K, Torchia AJ, Stock JL, Hildebrandt AL, Coskran T, Black SC, Brees DJ, Wicks JR, McNeish JD, Coleman KG. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J Clin Invest. 2003;112:197-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 566] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 16. | Schultze SM, Jensen J, Hemmings BA, Tschopp O, Niessen M. Promiscuous affairs of PKB/AKT isoforms in metabolism. Arch Physiol Biochem. 2011;117:70-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Cleasby ME, Reinten TA, Cooney GJ, James DE, Kraegen EW. Functional studies of Akt isoform specificity in skeletal muscle in vivo; maintained insulin sensitivity despite reduced insulin receptor substrate-1 expression. Mol Endocrinol. 2007;21:215-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Jaiswal N, Gavin MG, Quinn WJ 3rd, Luongo TS, Gelfer RG, Baur JA, Titchenell PM. The role of skeletal muscle Akt in the regulation of muscle mass and glucose homeostasis. Mol Metab. 2019;28:1-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 118] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 19. | Yuan HX, Guan KL. The SIN1-PH Domain Connects mTORC2 to PI3K. Cancer Discov. 2015;5:1127-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Yang Q, Inoki K, Ikenoue T, Guan KL. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev. 2006;20:2820-2832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 398] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 21. | Liu P, Gan W, Chin YR, Ogura K, Guo J, Zhang J, Wang B, Blenis J, Cantley LC, Toker A, Su B, Wei W. PtdIns(3,4,5)P3-Dependent Activation of the mTORC2 Kinase Complex. Cancer Discov. 2015;5:1194-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 295] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 22. | Gan X, Wang J, Su B, Wu D. Evidence for direct activation of mTORC2 kinase activity by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 2011;286:10998-11002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 166] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 23. | Yang G, Murashige DS, Humphrey SJ, James DE. A Positive Feedback Loop between Akt and mTORC2 via SIN1 Phosphorylation. Cell Rep. 2015;12:937-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 231] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 24. | Humphrey SJ, Yang G, Yang P, Fazakerley DJ, Stöckli J, Yang JY, James DE. Dynamic adipocyte phosphoproteome reveals that Akt directly regulates mTORC2. Cell Metab. 2013;17:1009-1020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 302] [Cited by in RCA: 331] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 25. | Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4837] [Cited by in RCA: 5257] [Article Influence: 262.9] [Reference Citation Analysis (0)] |
| 26. | Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541-6551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2225] [Cited by in RCA: 2230] [Article Influence: 76.9] [Reference Citation Analysis (0)] |
| 27. | Scheid MP, Marignani PA, Woodgett JR. Multiple phosphoinositide 3-kinase-dependent steps in activation of protein kinase B. Mol Cell Biol. 2002;22:6247-6260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 265] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 28. | Hemmings BA. Akt signaling: linking membrane events to life and death decisions. Science. 1997;275:628-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 394] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 29. | Moore SF, Hunter RW, Hers I. mTORC2 Protein-mediated Protein Kinase B (Akt) Serine 473 Phosphorylation Is Not Required for Akt1 Activity in Human Platelets*. J Biol Chem. 2011;286:24553-24560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | Ananthanarayanan B, Fosbrink M, Rahdar M, Zhang J. Live-cell molecular analysis of Akt activation reveals roles for activation loop phosphorylation. J Biol Chem. 2007;282:36634-36641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Kramer HF, Witczak CA, Fujii N, Jessen N, Taylor EB, Arnolds DE, Sakamoto K, Hirshman MF, Goodyear LJ. Distinct signals regulate AS160 phosphorylation in response to insulin, AICAR, and contraction in mouse skeletal muscle. Diabetes. 2006;55:2067-2076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 253] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 32. | Kumar N, Afeyan R, Sheppard S, Harms B, Lauffenburger DA. Quantitative analysis of Akt phosphorylation and activity in response to EGF and insulin treatment. Biochem Biophys Res Commun. 2007;354:14-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Cozzone D, Fröjdö S, Disse E, Debard C, Laville M, Pirola L, Vidal H. Isoform-specific defects of insulin stimulation of Akt/protein kinase B (PKB) in skeletal muscle cells from type 2 diabetic patients. Diabetologia. 2008;51:512-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Brozinick JT Jr, Roberts BR, Dohm GL. Defective signaling through Akt-2 and -3 but not Akt-1 in insulin-resistant human skeletal muscle: potential role in insulin resistance. Diabetes. 2003;52:935-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 138] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 35. | Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1045] [Cited by in RCA: 1108] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 36. | Tang Y, Wallace M, Sanchez-Gurmaches J, Hsiao WY, Li H, Lee PL, Vernia S, Metallo CM, Guertin DA. Adipose tissue mTORC2 regulates ChREBP-driven de novo lipogenesis and hepatic glucose metabolism. Nat Commun. 2016;7:11365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 141] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 37. | Hagiwara A, Cornu M, Cybulski N, Polak P, Betz C, Trapani F, Terracciano L, Heim MH, Rüegg MA, Hall MN. Hepatic mTORC2 activates glycolysis and lipogenesis through Akt, glucokinase, and SREBP1c. Cell Metab. 2012;15:725-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 437] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 38. | Zhang HH, Lipovsky AI, Dibble CC, Sahin M, Manning BD. S6K1 regulates GSK3 under conditions of mTOR-dependent feedback inhibition of Akt. Mol Cell. 2006;24:185-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 244] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 39. | Wang Q, Zhou Y, Evers BM. Neurotensin phosphorylates GSK-3alpha/beta through the activation of PKC in human colon cancer cells. Neoplasia. 2006;8:781-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Facchinetti V, Ouyang W, Wei H, Soto N, Lazorchak A, Gould C, Lowry C, Newton AC, Mao Y, Miao RQ, Sessa WC, Qin J, Zhang P, Su B, Jacinto E. The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C. EMBO J. 2008;27:1932-1943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 422] [Cited by in RCA: 418] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 41. | Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3826] [Cited by in RCA: 4055] [Article Influence: 135.2] [Reference Citation Analysis (0)] |
| 42. | Karlsson HK, Zierath JR, Kane S, Krook A, Lienhard GE, Wallberg-Henriksson H. Insulin-stimulated phosphorylation of the Akt substrate AS160 is impaired in skeletal muscle of type 2 diabetic subjects. Diabetes. 2005;54:1692-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 219] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 43. | Sano H, Kane S, Sano E, Mîinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem. 2003;278:14599-14602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 715] [Cited by in RCA: 723] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 44. | Chen CH, Shaikenov T, Peterson TR, Aimbetov R, Bissenbaev AK, Lee SW, Wu J, Lin HK, Sarbassov dos D. ER stress inhibits mTORC2 and Akt signaling through GSK-3β-mediated phosphorylation of rictor. Sci Signal. 2011;4:ra10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 45. | Koo J, Wu X, Mao Z, Khuri FR, Sun SY. Rictor Undergoes Glycogen Synthase Kinase 3 (GSK3)-dependent, FBXW7-mediated Ubiquitination and Proteasomal Degradation. J Biol Chem. 2015;290:14120-14129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 46. | Eguez L, Lee A, Chavez JA, Miinea CP, Kane S, Lienhard GE, McGraw TE. Full intracellular retention of GLUT4 requires AS160 Rab GTPase activating protein. Cell Metab. 2005;2:263-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 255] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 47. | Larance M, Ramm G, Stöckli J, van Dam EM, Winata S, Wasinger V, Simpson F, Graham M, Junutula JR, Guilhaus M, James DE. Characterization of the role of the Rab GTPase-activating protein AS160 in insulin-regulated GLUT4 trafficking. J Biol Chem. 2005;280:37803-37813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 313] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 48. | Zeigerer A, McBrayer MK, McGraw TE. Insulin stimulation of GLUT4 exocytosis, but not its inhibition of endocytosis, is dependent on RabGAP AS160. Mol Biol Cell. 2004;15:4406-4415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 181] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 49. | Bruss MD, Arias EB, Lienhard GE, Cartee GD. Increased phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. Diabetes. 2005;54:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 210] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 50. | Kramer HF, Witczak CA, Taylor EB, Fujii N, Hirshman MF, Goodyear LJ. AS160 regulates insulin- and contraction-stimulated glucose uptake in mouse skeletal muscle. J Biol Chem. 2006;281:31478-31485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 200] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 51. | Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002;10:151-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1178] [Cited by in RCA: 1221] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 52. | Aicher LD, Campbell JS, Yeung RS. Tuberin phosphorylation regulates its interaction with hamartin. Two proteins involved in tuberous sclerosis. J Biol Chem. 2001;276:21017-21021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 53. | Dan HC, Sun M, Yang L, Feldman RI, Sui XM, Ou CC, Nellist M, Yeung RS, Halley DJ, Nicosia SV, Pledger WJ, Cheng JQ. Phosphatidylinositol 3-kinase/Akt pathway regulates tuberous sclerosis tumor suppressor complex by phosphorylation of tuberin. J Biol Chem. 2002;277:35364-35370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 283] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 54. | Pause A, Belsham GJ, Gingras AC, Donzé O, Lin TA, Lawrence JC Jr, Sonenberg N. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5'-cap function. Nature. 1994;371:762-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 955] [Cited by in RCA: 977] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 55. | Pullen N, Thomas G. The modular phosphorylation and activation of p70s6k. FEBS Lett. 1997;410:78-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 436] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 56. | Weng QP, Kozlowski M, Belham C, Zhang A, Comb MJ, Avruch J. Regulation of the p70 S6 kinase by phosphorylation in vivo. Analysis using site-specific anti-phosphopeptide antibodies. J Biol Chem. 1998;273:16621-16629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 307] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 57. | Peterson RT, Schreiber SL. Translation control: connecting mitogens and the ribosome. Curr Biol. 1998;8:R248-R250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 130] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 58. | Jefferies HB, Fumagalli S, Dennis PB, Reinhard C, Pearson RB, Thomas G. Rapamycin suppresses 5'TOP mRNA translation through inhibition of p70s6k. EMBO J. 1997;16:3693-3704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 720] [Cited by in RCA: 745] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 59. | Tzatsos A, Kandror KV. Nutrients suppress phosphatidylinositol 3-kinase/Akt signaling via raptor-dependent mTOR-mediated insulin receptor substrate 1 phosphorylation. Mol Cell Biol. 2006;26:63-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 329] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 60. | Ueno M, Carvalheira JB, Tambascia RC, Bezerra RM, Amaral ME, Carneiro EM, Folli F, Franchini KG, Saad MJ. Regulation of insulin signalling by hyperinsulinaemia: role of IRS-1/2 serine phosphorylation and the mTOR/p70 S6K pathway. Diabetologia. 2005;48:506-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 132] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 61. | Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1256] [Cited by in RCA: 1327] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 62. | Maegawa H, Shigeta Y, Egawa K, Kobayashi M. Impaired autophosphorylation of insulin receptors from abdominal skeletal muscles in nonobese subjects with NIDDM. Diabetes. 1991;40:815-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 63. | Birnbaum MJ. Identification of a novel gene encoding an insulin-responsive glucose transporter protein. Cell. 1989;57:305-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 452] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 64. | Fukumoto H, Kayano T, Buse JB, Edwards Y, Pilch PF, Bell GI, Seino S. Cloning and characterization of the major insulin-responsive glucose transporter expressed in human skeletal muscle and other insulin-responsive tissues. J Biol Chem. 1989;264:7776-7779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 282] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 65. | Thorell A, Hirshman MF, Nygren J, Jorfeldt L, Wojtaszewski JF, Dufresne SD, Horton ES, Ljungqvist O, Goodyear LJ. Exercise and insulin cause GLUT-4 translocation in human skeletal muscle. Am J Physiol. 1999;277:E733-E741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 81] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 66. | Douen AG, Ramlal T, Rastogi S, Bilan PJ, Cartee GD, Vranic M, Holloszy JO, Klip A. Exercise induces recruitment of the "insulin-responsive glucose transporter". Evidence for distinct intracellular insulin- and exercise-recruitable transporter pools in skeletal muscle. J Biol Chem. 1990;265:13427-13430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 310] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 67. | Ryder JW, Yang J, Galuska D, Rincón J, Björnholm M, Krook A, Lund S, Pedersen O, Wallberg-Henriksson H, Zierath JR, Holman GD. Use of a novel impermeable biotinylated photolabeling reagent to assess insulin- and hypoxia-stimulated cell surface GLUT4 content in skeletal muscle from type 2 diabetic patients. Diabetes. 2000;49:647-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 157] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 68. | Garvey WT, Maianu L, Hancock JA, Golichowski AM, Baron A. Gene expression of GLUT4 in skeletal muscle from insulin-resistant patients with obesity, IGT, GDM, and NIDDM. Diabetes. 1992;41:465-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 152] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 69. | Pedersen O, Bak JF, Andersen PH, Lund S, Moller DE, Flier JS, Kahn BB. Evidence against altered expression of GLUT1 or GLUT4 in skeletal muscle of patients with obesity or NIDDM. Diabetes. 1990;39:865-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 188] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 70. | Ciaraldi TP, Kong AP, Chu NV, Kim DD, Baxi S, Loviscach M, Plodkowski R, Reitz R, Caulfield M, Mudaliar S, Henry RR. Regulation of glucose transport and insulin signaling by troglitazone or metformin in adipose tissue of type 2 diabetic subjects. Diabetes. 2002;51:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 83] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 71. | Arner P, Pollare T, Lithell H, Livingston JN. Defective insulin receptor tyrosine kinase in human skeletal muscle in obesity and type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1987;30:437-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 116] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 72. | Nolan JJ, Freidenberg G, Henry R, Reichart D, Olefsky JM. Role of human skeletal muscle insulin receptor kinase in the in vivo insulin resistance of noninsulin-dependent diabetes mellitus and obesity. J Clin Endocrinol Metab. 1994;78:471-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 73. | Krook A, Björnholm M, Galuska D, Jiang XJ, Fahlman R, Myers MG Jr, Wallberg-Henriksson H, Zierath JR. Characterization of signal transduction and glucose transport in skeletal muscle from type 2 diabetic patients. Diabetes. 2000;49:284-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 259] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 74. | Klein HH, Vestergaard H, Kotzke G, Pedersen O. Elevation of serum insulin concentration during euglycemic hyperinsulinemic clamp studies leads to similar activation of insulin receptor kinase in skeletal muscle of subjects with and without NIDDM. Diabetes. 1995;44:1310-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 75. | Meyer MM, Levin K, Grimmsmann T, Beck-Nielsen H, Klein HH. Insulin signalling in skeletal muscle of subjects with or without Type II-diabetes and first degree relatives of patients with the disease. Diabetologia. 2002;45:813-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 76. | Caro JF, Sinha MK, Raju SM, Ittoop O, Pories WJ, Flickinger EG, Meelheim D, Dohm GL. Insulin receptor kinase in human skeletal muscle from obese subjects with and without noninsulin dependent diabetes. J Clin Invest. 1987;79:1330-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 247] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 77. | Kim YB, Kotani K, Ciaraldi TP, Henry RR, Kahn BB. Insulin-stimulated protein kinase C lambda/zeta activity is reduced in skeletal muscle of humans with obesity and type 2 diabetes: reversal with weight reduction. Diabetes. 2003;52:1935-1942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 123] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 78. | Cusi K, Maezono K, Osman A, Pendergrass M, Patti ME, Pratipanawatr T, DeFronzo RA, Kahn CR, Mandarino LJ. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J Clin Invest. 2000;105:311-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 829] [Cited by in RCA: 779] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 79. | Bouzakri K, Roques M, Gual P, Espinosa S, Guebre-Egziabher F, Riou JP, Laville M, Le Marchand-Brustel Y, Tanti JF, Vidal H. Reduced activation of phosphatidylinositol-3 kinase and increased serine 636 phosphorylation of insulin receptor substrate-1 in primary culture of skeletal muscle cells from patients with type 2 diabetes. Diabetes. 2003;52:1319-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 224] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 80. | Beeson M, Sajan MP, Dizon M, Grebenev D, Gomez-Daspet J, Miura A, Kanoh Y, Powe J, Bandyopadhyay G, Standaert ML, Farese RV. Activation of protein kinase C-zeta by insulin and phosphatidylinositol-3,4,5-(PO4)3 is defective in muscle in type 2 diabetes and impaired glucose tolerance: amelioration by rosiglitazone and exercise. Diabetes. 2003;52:1926-1934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 125] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 81. | Kim YB, Nikoulina SE, Ciaraldi TP, Henry RR, Kahn BB. Normal insulin-dependent activation of Akt/protein kinase B, with diminished activation of phosphoinositide 3-kinase, in muscle in type 2 diabetes. J Clin Invest. 1999;104:733-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 337] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 82. | Krook A, Roth RA, Jiang XJ, Zierath JR, Wallberg-Henriksson H. Insulin-stimulated Akt kinase activity is reduced in skeletal muscle from NIDDM subjects. Diabetes. 1998;47:1281-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 197] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 83. | Prada PO, Coelho MS, Zecchin HG, Dolnikoff MS, Gasparetti AL, Furukawa LN, Saad MJ, Heimann JC. Low salt intake modulates insulin signaling, JNK activity and IRS-1ser307 phosphorylation in rat tissues. J Endocrinol. 2005;185:429-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 84. | Nikoulina SE, Ciaraldi TP, Mudaliar S, Mohideen P, Carter L, Henry RR. Potential role of glycogen synthase kinase-3 in skeletal muscle insulin resistance of type 2 diabetes. Diabetes. 2000;49:263-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 271] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 85. | Park SW, Goodpaster BH, Strotmeyer ES, de Rekeneire N, Harris TB, Schwartz AV, Tylavsky FA, Newman AB. Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes. 2006;55:1813-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 539] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 86. | Kim KS, Park KS, Kim MJ, Kim SK, Cho YW, Park SW. Type 2 diabetes is associated with low muscle mass in older adults. Geriatr Gerontol Int. 2014;14 Suppl 1:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 87. | Nilsson MI, Greene NP, Dobson JP, Wiggs MP, Gasier HG, Macias BR, Shimkus KL, Fluckey JD. Insulin resistance syndrome blunts the mitochondrial anabolic response following resistance exercise. Am J Physiol Endocrinol Metab. 2010;299:E466-E474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 88. | Nilsson MI, Dobson JP, Greene NP, Wiggs MP, Shimkus KL, Wudeck EV, Davis AR, Laureano ML, Fluckey JD. Abnormal protein turnover and anabolic resistance to exercise in sarcopenic obesity. FASEB J. 2013;27:3905-3916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 89. | Durschlag RP, Layman DK. Skeletal muscle growth in lean and obese Zucker rats. Growth. 1983;47:282-291. [PubMed] |
| 90. | Guillet C, Delcourt I, Rance M, Giraudet C, Walrand S, Bedu M, Duche P, Boirie Y. Changes in basal and insulin and amino acid response of whole body and skeletal muscle proteins in obese men. J Clin Endocrinol Metab. 2009;94:3044-3050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 129] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 91. | Lobley GE, Webster AJ, Reeds PJ. Protein synthesis in lean and obese Zucker rats. Proc Nutr Soc. 1978;37:20A. [PubMed] |
| 92. | Halvatsiotis P, Short KR, Bigelow M, Nair KS. Synthesis rate of muscle proteins, muscle functions, and amino acid kinetics in type 2 diabetes. Diabetes. 2002;51:2395-2404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 111] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 93. | Fluckey JD, Cortright RN, Tapscott E, Koves T, Smith L, Pohnert S, Dohm GL. Active involvement of PKC for insulin-mediated rates of muscle protein synthesis in Zucker rats. Am J Physiol Endocrinol Metab. 2004;286:E753-E758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 94. | Fluckey JD, Pohnert SC, Boyd SG, Cortright RN, Trappe TA, Dohm GL. Insulin stimulation of muscle protein synthesis in obese Zucker rats is not via a rapamycin-sensitive pathway. Am J Physiol Endocrinol Metab. 2000;279:E182-E187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 95. | Bell JA, Volpi E, Fujita S, Cadenas JG, Sheffield-Moore M, Rasmussen BB. Skeletal muscle protein anabolic response to increased energy and insulin is preserved in poorly controlled type 2 diabetes. J Nutr. 2006;136:1249-1255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 96. | She P, Olson KC, Kadota Y, Inukai A, Shimomura Y, Hoppel CL, Adams SH, Kawamata Y, Matsumoto H, Sakai R, Lang CH, Lynch CJ. Leucine and protein metabolism in obese Zucker rats. PLoS One. 2013;8:e59443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 97. | Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, Gray NS, Sabatini DM. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137:873-886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 966] [Cited by in RCA: 953] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 98. | Shimkus KL, Wudeck EV, Shirazi-Fard Y, Nilsson MI, Greene NP, Hogan HA, Fluckey JD. DEPTOR Expression Correlates with Muscle Protein Synthesis. Int J Exerc Sci. 2013;7. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 99. | Deaver JW, López SM, Ryan PJ, Nghiem PP, Riechman SE, Fluckey JD. Regulation of cellular anabolism by mTOR: Or how I learned to stop worrying and love translation. Sports Health. 2020;. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 100. | Tremblay F, Brûlé S, Hee Um S, Li Y, Masuda K, Roden M, Sun XJ, Krebs M, Polakiewicz RD, Thomas G, Marette A. Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. Proc Natl Acad Sci USA. 2007;104:14056-14061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 351] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 101. | Gual P, Le Marchand-Brustel Y, Tanti JF. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie. 2005;87:99-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 605] [Cited by in RCA: 653] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 102. | Sun XJ, Rothenberg P, Kahn CR, Backer JM, Araki E, Wilden PA, Cahill DA, Goldstein BJ, White MF. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 1991;352:73-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1094] [Cited by in RCA: 1158] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 103. | Haruta T, Uno T, Kawahara J, Takano A, Egawa K, Sharma PM, Olefsky JM, Kobayashi M. A rapamycin-sensitive pathway down-regulates insulin signaling via phosphorylation and proteasomal degradation of insulin receptor substrate-1. Mol Endocrinol. 2000;14:783-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 308] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 104. | Rice KM, Turnbow MA, Garner CW. Insulin stimulates the degradation of IRS-1 in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 1993;190:961-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 105. | Smith LK, Vlahos CJ, Reddy KK, Falck JR, Garner CW. Wortmannin and LY294002 inhibit the insulin-induced down-regulation of IRS-1 in 3T3-L1 adipocytes. Mol Cell Endocrinol. 1995;113:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 106. | Smith LK, Rice KM, Garner CW. The insulin-induced down-regulation of IRS-1 in 3T3-L1 adipocytes is mediated by a calcium-dependent thiol protease. Mol Cell Endocrinol. 1996;122:81-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 107. | Li J, DeFea K, Roth RA. Modulation of insulin receptor substrate-1 tyrosine phosphorylation by an Akt/phosphatidylinositol 3-kinase pathway. J Biol Chem. 1999;274:9351-9356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 166] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 108. | Shah OJ, Wang Z, Hunter T. Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr Biol. 2004;14:1650-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 632] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 109. | Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, Barnett J, Leslie NR, Cheng S, Shepherd PR, Gout I, Downes CP, Lamb RF. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166:213-223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 861] [Cited by in RCA: 889] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 110. | Takano A, Usui I, Haruta T, Kawahara J, Uno T, Iwata M, Kobayashi M. Mammalian target of rapamycin pathway regulates insulin signaling via subcellular redistribution of insulin receptor substrate 1 and integrates nutritional signals and metabolic signals of insulin. Mol Cell Biol. 2001;21:5050-5062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 203] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 111. | Barbour LA, McCurdy CE, Hernandez TL, Friedman JE. Chronically increased S6K1 is associated with impaired IRS1 signaling in skeletal muscle of GDM women with impaired glucose tolerance postpartum. J Clin Endocrinol Metab. 2011;96:1431-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 112. | Khamzina L, Veilleux A, Bergeron S, Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology. 2005;146:1473-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 441] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 113. | Fu W, Hall MN. Regulation of mTORC2 Signaling. Genes (Basel). 2020;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 140] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 114. | Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1972] [Cited by in RCA: 2094] [Article Influence: 99.7] [Reference Citation Analysis (0)] |
| 115. | Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11:859-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1079] [Cited by in RCA: 1157] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 116. | García-Martínez JM, Alessi DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1). Biochem J. 2008;416:375-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 711] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 117. | Ikenoue T, Inoki K, Yang Q, Zhou X, Guan KL. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. EMBO J. 2008;27:1919-1931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 497] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 118. | Hresko RC, Mueckler M. mTOR.RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J Biol Chem. 2005;280:40406-40416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 503] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 119. | Dibble CC, Asara JM, Manning BD. Characterization of Rictor phosphorylation sites reveals direct regulation of mTOR complex 2 by S6K1. Mol Cell Biol. 2009;29:5657-5670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 357] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 120. | Jacinto E, Loewith R, Schmidt A, Lin S, Rüegg MA, Hall A, Hall MN. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1552] [Cited by in RCA: 1623] [Article Influence: 77.3] [Reference Citation Analysis (0)] |
| 121. | Frias MA, Thoreen CC, Jaffe JD, Schroder W, Sculley T, Carr SA, Sabatini DM. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr Biol. 2006;16:1865-1870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 528] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 122. | Julien LA, Carriere A, Moreau J, Roux PP. mTORC1-activated S6K1 phosphorylates Rictor on threonine 1135 and regulates mTORC2 signaling. Mol Cell Biol. 2010;30:908-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 340] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 123. | Kumar A, Harris TE, Keller SR, Choi KM, Magnuson MA, Lawrence JC Jr. Muscle-specific deletion of rictor impairs insulin-stimulated glucose transport and enhances Basal glycogen synthase activity. Mol Cell Biol. 2008;28:61-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 179] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 124. | Treins C, Warne PH, Magnuson MA, Pende M, Downward J. Rictor is a novel target of p70 S6 kinase-1. Oncogene. 2010;29:1003-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 125. | Boulbes D, Chen CH, Shaikenov T, Agarwal NK, Peterson TR, Addona TA, Keshishian H, Carr SA, Magnuson MA, Sabatini DM, Sarbassov dos D. Rictor phosphorylation on the Thr-1135 site does not require mammalian target of rapamycin complex 2. Mol Cancer Res. 2010;8:896-906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 126. | Laaksonen DE, Lakka HM, Niskanen LK, Kaplan GA, Salonen JT, Lakka TA. Metabolic syndrome and development of diabetes mellitus: application and validation of recently suggested definitions of the metabolic syndrome in a prospective cohort study. Am J Epidemiol. 2002;156:1070-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 611] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 127. | Lakka TA, Laaksonen DE. Physical activity in prevention and treatment of the metabolic syndrome. Appl Physiol Nutr Metab. 2007;32:76-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 295] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 128. | Schwaab B, Kafsack F, Markmann E, Schütt M. Effects of aerobic and anaerobic exercise on glucose tolerance in patients with coronary heart disease and type 2 diabetes mellitus. Cardiovasc Endocrinol Metab. 2020;9:3-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 129. | de Mello MA, de Souza CT, Braga LR, dos Santos JW, Ribeiro IA, Gobatto CA. Glucose tolerance and insulin action in monosodium glutamate (MSG) obese exercise-trained rats. Physiol Chem Phys Med NMR. 2001;33:63-71. [PubMed] |
| 130. | Holloszy JO, Schultz J, Kusnierkiewicz J, Hagberg JM, Ehsani AA. Effects of exercise on glucose tolerance and insulin resistance. Brief review and some preliminary results. Acta Med Scand Suppl. 1986;711:55-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 131. | Mikines KJ, Sonne B, Farrell PA, Tronier B, Galbo H. Effect of physical exercise on sensitivity and responsiveness to insulin in humans. Am J Physiol. 1988;254:E248-E259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 209] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 132. | Mikines KJ, Sonne B, Farrell PA, Tronier B, Galbo H. Effect of training on the dose-response relationship for insulin action in men. J Appl Physiol (1985). 1989;66:695-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 133. | Linke SE, Gallo LC, Norman GJ. Attrition and adherence rates of sustained vs. intermittent exercise interventions. Ann Behav Med. 2011;42:197-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 134. | Yang Z, Scott CA, Mao C, Tang J, Farmer AJ. Resistance exercise versus aerobic exercise for type 2 diabetes: a systematic review and meta-analysis. Sports Med. 2014;44:487-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 198] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 135. | Irvine L, Barton GR, Gasper AV, Murray N, Clark A, Scarpello T, Sampson M. Cost-effectiveness of a lifestyle intervention in preventing Type 2 diabetes. Int J Technol Assess Health Care. 2011;27:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 136. | Kelley GA, Kelley KS, Hootman JM, Jones DL. Exercise and Health-Related Quality of Life in Older Community-Dwelling Adults: A Meta-Analysis of Randomized Controlled Trials. J Appl Gerontol. 2009;28:369-394. [RCA] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 137. | Miller WJ, Sherman WM, Ivy JL. Effect of strength training on glucose tolerance and post-glucose insulin response. Med Sci Sports Exerc. 1984;16:539-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 87] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 138. | Ibañez J, Izquierdo M, Argüelles I, Forga L, Larrión JL, García-Unciti M, Idoate F, Gorostiaga EM. Twice-weekly progressive resistance training decreases abdominal fat and improves insulin sensitivity in older men with type 2 diabetes. Diabetes Care. 2005;28:662-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 257] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 139. | Fluckey JD, Hickey MS, Brambrink JK, Hart KK, Alexander K, Craig BW. Effects of resistance exercise on glucose tolerance in normal and glucose-intolerant subjects. J Appl Physiol (1985). 1994;77:1087-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 69] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 140. | De Filippis E, Alvarez G, Berria R, Cusi K, Everman S, Meyer C, Mandarino LJ. Insulin-resistant muscle is exercise resistant: evidence for reduced response of nuclear-encoded mitochondrial genes to exercise. Am J Physiol Endocrinol Metab. 2008;294:E607-E614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 141. | Katta A, Kakarla S, Wu M, Paturi S, Gadde MK, Arvapalli R, Kolli M, Rice KM, Blough ER. Altered regulation of contraction-induced Akt/mTOR/p70S6k pathway signaling in skeletal muscle of the obese Zucker rat. Exp Diabetes Res. 2009;2009:384683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 142. | Wong TS, Booth FW. Skeletal muscle enlargement with weight-lifting exercise by rats. J Appl Physiol (1985). 1988;65:950-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 143. | Fluckey JD, Vary TC, Jefferson LS, Farrell PA. Augmented insulin action on rates of protein synthesis after resistance exercise in rats. Am J Physiol. 1996;270:E313-E319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 144. | Fluckey JD, Vary TC, Jefferson LS, Evans WJ, Farrell PA. Insulin stimulation of protein synthesis in rat skeletal muscle following resistance exercise is maintained with advancing age. J Gerontol A Biol Sci Med Sci. 1996;51:B323-B330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 145. | Dardevet D, Sornet C, Vary T, Grizard J. Phosphatidylinositol 3-kinase and p70 s6 kinase participate in the regulation of protein turnover in skeletal muscle by insulin and insulin-like growth factor I. Endocrinology. 1996;137:4087-4094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 146. | Graves LM, Bornfeldt KE, Argast GM, Krebs EG, Kong X, Lin TA, Lawrence JC Jr. cAMP- and rapamycin-sensitive regulation of the association of eukaryotic initiation factor 4E and the translational regulator PHAS-I in aortic smooth muscle cells. Proc Natl Acad Sci USA. 1995;92:7222-7226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 180] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 147. | Lin TA, Kong X, Saltiel AR, Blackshear PJ, Lawrence JC Jr. Control of PHAS-I by insulin in 3T3-L1 adipocytes. Synthesis, degradation, and phosphorylation by a rapamycin-sensitive and mitogen-activated protein kinase-independent pathway. J Biol Chem. 1995;270:18531-18538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 204] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 148. | von Manteuffel SR, Gingras AC, Ming XF, Sonenberg N, Thomas G. 4E-BP1 phosphorylation is mediated by the FRAP-p70s6k pathway and is independent of mitogen-activated protein kinase. Proc Natl Acad Sci USA. 1996;93:4076-4080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 209] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 149. | Kirwan JP, Hickner RC, Yarasheski KE, Kohrt WM, Wiethop BV, Holloszy JO. Eccentric exercise induces transient insulin resistance in healthy individuals. J Appl Physiol (1985). 1992;72:2197-2202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 100] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 150. | Fluckey JD, Ploug T, Galbo H. Attenuated insulin action on glucose uptake and transport in muscle following resistance exercise in rats. Acta Physiol Scand. 1999;167:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 151. | Nesher R, Karl IE, Kipnis DM. Dissociation of effects of insulin and contraction on glucose transport in rat epitrochlearis muscle. Am J Physiol. 1985;249:C226-C232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 174] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 152. | Ploug T, Galbo H, Richter EA. Increased muscle glucose uptake during contractions: no need for insulin. Am J Physiol. 1984;247:E726-E731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 77] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 153. | Richter EA, Ploug T, Galbo H. Increased muscle glucose uptake after exercise. No need for insulin during exercise. Diabetes. 1985;34:1041-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 154. | Higaki Y, Hirshman MF, Fujii N, Goodyear LJ. Nitric oxide increases glucose uptake through a mechanism that is distinct from the insulin and contraction pathways in rat skeletal muscle. Diabetes. 2001;50:241-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 172] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 155. | Lefort N, St-Amand E, Morasse S, Côté CH, Marette A. The alpha-subunit of AMPK is essential for submaximal contraction-mediated glucose transport in skeletal muscle in vitro. Am J Physiol Endocrinol Metab. 2008;295:E1447-E1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 156. | Winder WW, Hardie DG. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol. 1996;270:E299-E304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 379] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 157. | Ploug T, Galbo H, Vinten J, Jørgensen M, Richter EA. Kinetics of glucose transport in rat muscle: effects of insulin and contractions. Am J Physiol. 1987;253:E12-E20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 45] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 158. | Dela F, Mikines KJ, Larsen JJ, Galbo H. Glucose clearance in aged trained skeletal muscle during maximal insulin with superimposed exercise. J Appl Physiol (1985). 1999;87:2059-2067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 159. | Christ-Roberts CY, Pratipanawatr T, Pratipanawatr W, Berria R, Belfort R, Mandarino LJ. Increased insulin receptor signaling and glycogen synthase activity contribute to the synergistic effect of exercise on insulin action. J Appl Physiol (1985). 2003;95:2519-2529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 160. | King PA, Betts JJ, Horton ED, Horton ES. Exercise, unlike insulin, promotes glucose transporter translocation in obese Zucker rat muscle. Am J Physiol. 1993;265:R447-R452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 161. | Brozinick JT Jr, Etgen GJ Jr, Yaspelkis BB 3rd, Ivy JL. Contraction-activated glucose uptake is normal in insulin-resistant muscle of the obese Zucker rat. J Appl Physiol (1985). 1992;73:382-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 162. | Angenstein F, Evans AM, Settlage RE, Moran ST, Ling SC, Klintsova AY, Shabanowitz J, Hunt DF, Greenough WT. A receptor for activated C kinase is part of messenger ribonucleoprotein complexes associated with polyA-mRNAs in neurons. J Neurosci. 2002;22:8827-8837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 163. | Shih SC, Mullen A, Abrams K, Mukhopadhyay D, Claffey KP. Role of protein kinase C isoforms in phorbol ester-induced vascular endothelial growth factor expression in human glioblastoma cells. J Biol Chem. 1999;274:15407-15414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 164. | Itani SI, Pories WJ, Macdonald KG, Dohm GL. Increased protein kinase C theta in skeletal muscle of diabetic patients. Metabolism. 2001;50:553-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 165. | Kanoh Y, Sajan MP, Bandyopadhyay G, Miura A, Standaert ML, Farese RV. Defective activation of atypical protein kinase C zeta and lambda by insulin and phosphatidylinositol-3,4,5-(PO4)(3) in skeletal muscle of rats following high-fat feeding and streptozotocin-induced diabetes. Endocrinology. 2003;144:947-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 166. | Cortright RN, Azevedo JL Jr, Zhou Q, Sinha M, Pories WJ, Itani SI, Dohm GL. Protein kinase C modulates insulin action in human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;278:E553-E562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 2.7] [Reference Citation Analysis (0)] |