Published online Aug 26, 2010. doi: 10.4331/wjbc.v1.i8.248
Revised: June 25, 2010
Accepted: July 2, 2010
Published online: August 26, 2010
Alterations in calcium signaling and/or the expression of calcium pumps and channels are an increasingly recognized property of some cancer cells. Alterations in the expression of plasma membrane calcium ATPase (PMCA) isoforms have been reported in a variety of cancer types, including those of breast and colon, with some studies of cancer cell line differentiation identifying specific PMCA isoforms, which may be altered in some cancers. Some studies have also begun to assess levels of PMCA isoforms in clinical tumor samples and to address mechanisms of altered PMCA expression in cancers. Both increases and decreases in PMCA expression have been reported in different cancer types and in many cases these alterations are isoform specific. In this review, we provide an overview of studies investigating the expression of PMCA in cancer and discuss how both the overexpression and reduced expression of a PMCA isoform in a cancer cell could bestow a growth advantage, through augmenting responses to proliferative stimuli or reducing sensitivity to apoptosis.
- Citation: Roberts-Thomson SJ, Curry MC, Monteith GR. Plasma membrane calcium pumps and their emerging roles in cancer. World J Biol Chem 2010; 1(8): 248-253
- URL: https://www.wjgnet.com/1949-8454/full/v1/i8/248.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v1.i8.248
The versatility of calcium to regulate a variety of key physiological events has made alterations in calcium signaling a target for many researchers seeking to identify potential mechanisms of diseases. The plasma membrane calcium ATPases (PMCAs), an active transport mechanism for Ca2+ efflux across the plasma membrane, determine basal cytosolic free Ca2+ levels ([Ca2+]CYT), the peak [Ca2+]CYT achieved after some cellular stimuli and the recovery phase of [Ca2+]CYT transients[1,2]. The ability of PMCAs to shape the nature of calcium-mediated cell activation suggests that deregulation of this calcium transport protein could result in the altered calcium homeostasis, which underlies different disease states[1-3]. Due to the intrinsic link between calcium and processes such as neurotransmission and muscle contraction, much of the initial research on PMCAs has focused on conditions associated with the central nervous and cardiovascular systems[4-10]. This work has identified potentially very important roles for PMCAs in a variety of disease states[1,3].
In contrast to cardiovascular diseases, an appreciation of the link between calcium signaling and cancer is only more recent. As a consequence, the study of PMCAs in the context of tumorigenesis is still in its infancy. However, there have been a few studies investigating the role of PMCAs in tumor-relevant pathways, or the expression of PMCA isoforms in cancer, or the potential of PMCAs as potential therapeutic targets in specific cancers. In this review, we will briefly outline the key properties of PMCAs potentially important in the context of cancer progression and highlight studies of PMCAs in cancer; we will also discuss how the level of PMCA expression may be critical in determining how altered PMCA expression may bestow a growth advantage to cancer cells.
The PMCA is a calcium efflux pump that resides on the plasma membrane of the cell. Using ATP as the energy source, PMCAs remove calcium from the cell against the concentration gradient i.e. from a low intracellular (cytoplasmic) ionized free calcium concentration of approximately 100 nmol/L to the extracellular space where the concentration is approximately 1-2 mmol/L. PMCAs have been reviewed elsewhere[1-3,11], in this section we have highlighted some of the aspects of PMCAs that have, or may have, significance to future studies of PMCA in cancer.
PMCAs are large proteins with 10 predicted transmembrane domains that have the majority of the non-transmembrane portions located within the cytosol[12-14]. The cytosolic portions of PMCAs contain the catalytic site for ATP cleavage and harbor the sites essential for the interaction with key regulators of PMCA activity, including protein kinases and calmodulin, as well as a PDZ binding motif that modulates the trafficking and activity of some PMCA variants[14-20]. PMCAs are encoded by four genes (PMCA1-4) and alternative splicing of PMCA mRNA can generate over 30 isoforms[20], which have pronounced differences in their tissue distribution and regulation[20]. Most cell types express PMCA1 and PMCA4 isoforms, whereas PMCA2 and PMCA3 expression is more restricted[14]. For example, PMCA2 is expressed in the central nervous system and is dramatically upregulated in the mammary gland during lactation, where it appears to play a pivotal role in the enrichment of milk with calcium[21-23]. Each PMCA isoform can uniquely shape the parameters of the calcium signal. When overexpressed, the PMCA2 and PMCA3 isoforms are more efficient at extruding calcium from the cell than overexpression of either the PMCA1 or PMCA4 isoforms[24]. For this reason, along with their more restricted tissue distribution, PMCA2 and PMCA3 are believed to play important roles in cells with large calcium fluxes, such as neurons[13,24]. Studies of knockout animals have helped elucidate some of the important roles of specific PMCA isoforms and this has been comprehensively reviewed elsewhere[25]. Briefly, PMCA1 knockout animals are not viable, whereas knockout of PMCA4 results in a more subtle phenotype with the most obvious effect being male infertility[25,26]. PMCA2 knockout mice, consistently have pronounced defects in balance and hearing and appear to have compromised ability to transport calcium into milk during lactation[9,23,25,26], there has not yet been any reports of the phenotype observed in PMCA3 knockout animals. The diverse phenotypes of PMCA isoform knockout animals emphasise the specific roles of these isoforms, and, as discussed later in this review, these roles may be particularly important in the context of cancer subtypes.
The characteristics that define a cancer cell have been described by Hanahan et al[27] and include self-sufficiency in growth signals, insensitivity to signals that limit growth, and the ability to evade apoptosis, to endlessly replicate, to sustain angiogenesis and to invade and metastasise. Many of these processes are regulated by calcium signaling. The role of calcium in tumorigenic pathways has been recently reviewed, and includes mechanisms as diverse as gene transcription, RAS activity and mitochondrial-mediated apoptosis[28]. One of the recent reasons for the increased investigation into calcium signaling in cancer cells has been the identification of the altered expression of specific calcium channels and pumps in cancer cells. The best characterized example of a calcium transporter with altered expression in cancer is the increased expression of the calcium permeable transient receptor potential cation channel, subfamily M, member 8 (TRPM8) in prostate cancer[29,30]. Increased expression of TRPM8, along with reports that inhibition of TRPM8 attenuates prostate cancer cell proliferation[30] and other studies, such as those assessing TRPV6[31,32], has led to the identification of calcium channels and pumps as potential targets for cancer therapy[33]. Some calcium channels and pumps may also be prognostic disease markers, or markers of cancer subtype, such as is the case for TRPV6, where levels correlate with tumor histological grade[34]. Although many calcium channels and pumps have been more extensively characterized in cancer than PMCAs, these calcium efflux pumps have also been linked to specific cancers and tumorigenic pathways. In the next section, we will review these studies and describe how calcium efflux mediated via PMCAs could be a key regulator of cancer relevant processes. We will also describe how a remodelling of PMCA-mediated calcium efflux, through either increased or decreased expression, could contribute to tumorigenesis.
One of the first clues that PMCA expression may be a characterizing feature of some cancers came from the work in the laboratory of Thomas Vanaman, who reported in 1997 that there were lower levels of PMCA protein in SV40 transformed skin and lung fibroblasts compared to controls[35]. The mRNA for both PMCA1b and PMCA4a appeared to be downregulated in the SV-40 transformed cells[35]. Other evidence started to emerge in 2001 from studies involving the assessment of PMCA levels and calcium efflux associated with the differentiation of a human neuroblastoma cell line (IMR-32). Differentiation of the IMR-32 neuroblastoma cell line led to an increase in the recovery rate of [Ca2+]CYT after K+ stimulation in the presence of an inhibitor of sarcoplasmic reticulum Ca2+-ATPases (to inhibit sequestration of Ca2+ from the cytoplasm into intracellular stores)[36]. This increase in recovery rate, consistent with an increase in Ca2+ efflux, was associated with a pronounced upregulation of total PMCA protein. The PMCA4 isoform appeared to be the isoform with the most pronounced increase in expression after differentiation, with concurrent increases in PMCA2 and PMCA3 protein in differentiated cells (PMCA1 protein was not detected)[36]. As will be discussed below, the approach of assessing PMCA expression during the differentiation of cancer cell lines has been used effectively to identify PMCA isoforms for further study in clinical cancer samples. PMCA isoforms upregulated during the differentiation of a cancer cell line may be expected to have lower levels in clinical cancer samples. As yet, there appears to have been no assessment of PMCA isoform expression in clinical neuroblastoma samples, and this despite reports that siRNA mediated inhibition of PMCA2 reduces the viability of SH-SY5Y neuroblastoma cells after stresses such as high extracellular Ca2+, N-methyl-D-aspartate exposure and glucose deprivation[37].
Studies in breast cell lines have shown a modest upregulation of PMCA1 mRNA in a panel of cancer cell lines compared to control (non-cancer breast derived) cell lines, and pronounced up-regulation of PMCA2 mRNA in specific breast cancer cell lines, in particular ZR-75-1 cells[38,39]. Breast cancer cell lines also tended to have lower levels of PMCA4 mRNA than breast cell lines derived from noncancerous tissue[39]. The upregulation of PMCA2 and downregulation of PMCA4 in some breast cancer cell lines may relate to events seen in the normal mammary gland during lactation. PMCA2 levels are dramatically increased during lactation, which is associated with a downregulation of PMCA4[21], hence some breast cancer cells may adopt, in the context of PMCA isoform expression, a lactation-like phenotype with PMCA2 overexpression and a downregulation of PMCA4. Also of note is the observation that, in MCF-7 breast cancer cells, calcineurin interacts with PMCA2 and PMCA4, but not PMCA1, and the PMCA2-calcineurin interaction is associated with an inhibition of the calcium-dependent transcription factor NFAT[40]. Further study is required to assess PMCA expression in clinical breast cancer samples, however, as discussed below, a reduction in total levels of PMCA expression is associated with reduced proliferation of the MCF-7 breast cancer cell line[41].
PMCA1 expression has also been assessed in oral cancers, where primary oral squamous cell carcinoma-derived cell lines were shown to have a PMCA1 gene that was epigenetically inactivated[42]. PMCA1 protein expression levels were also reduced in over 40% of oral squamous cell carcinomas and oral premalignant lesions[42]. These studies pointed at a possible role of PMCA1 downregulation in some of the initial processes involved in some oral cancers, and, for the first time, started to explore the mechanisms for the altered expression of PMCAs and their levels in clinical tumor samples[42].
Altered expression of PMCAs also appears to be a feature of some colon cancers. Two research groups independently identified isoform-specific changes in PMCA expression associated with the differentiation of colon cancer cell lines. Aung et al[43], showed that, in the HT29 colon cancer cell line, both chemical (sodium butyrate, 3 mmol/L) and culturing post-confluency (7-28 d) induced differentiation resulted in a significant upregulation of PMCA4 mRNA and protein, but that these changes were associated with changes in the other PMCA isoform expressed in this cell line, PMCA1. Similarly, Ribiczey et al[44] showed selective PMCA4 upregulation together with differentiation in multiple colon and gastric cancer cell lines treated with a variety of differentiation inducers. They also demonstrated upregulation of PMCA4 in Caco-2 colon cancer cells differentiated as a result of post-confluency culturing[44]. Moreover, PMCA-mediated calcium transport was shown to be increased in valerate and butyrate differentiated KATO-III and DLD-1 colon cancer cell lines[44]. The increased expression of PMCA4 in differentiated colon cancer cells suggests that reduced levels of PMCA4 may be a characterizing feature of some colon cancers. Subsequent work by Aung et al[45], assessing microarray data, showed that PMCA4 mRNA levels were significantly reduced in adenocarcinoma and benign colon tumors compared to normal samples. These studies also demonstrated that PMCA4 protein was localized to the plasma membrane of differentiated HT29 colon cancer cells[45]. The possible functional consequences of altered PMCA expression were assessed through siRNA-mediated inhibition of PMCA4 expression in HT29 colon cancer cells, which augmented [Ca2+]CYT responses elicited by ATP and neurotensin, suggesting that reduced PMCA4 levels may increase the sensitivity to some growth stimuli[45]. Indeed, reversing the remodelling of PMCA4 expression, which may occur in colon cancer, by stable overexpression of PMCA4 in HT29 colon cancer cells, reduced cellular proliferation[45].
Hence, many studies now suggest that alterations in the expression of specific PMCA isoforms may be a characterizing feature of some cancers. These changes are summarized in Table 1. PMCA expression is now being increasingly assessed in clinical patient samples, but further work is required to determine how changes in PMCA expression identified in cancer cell lines relate to in situ cancers in patients. Likewise, whether PMCA expression levels relate to prognosis requires more thorough investigation. The mechanisms whereby PMCA expression may be altered in some cancers is also still in its infancy, with some epigenetic changes being reported. In some cases, alterations in PMCA expression may be due to compensatory changes triggered by altered calcium signaling associated with the acquisition of the tumor phenotype. Recent years have also seen the first attempts at addressing the consequences of inhibiting and/or overexpressing PMCA isoforms in cancer cell lines. These studies have suggested PMCA as a potential regulator of cancer cell proliferation. However, further studies are required to identify the roles of PMCA in other tumorigenic processes. Among the interesting observations, there are reports that some cancers may be associated with downregulation of PMCA. Since other cancers may be characterized by elevated PMCA levels, as discussed below, this raises the issue regarding the mechanisms by which PMCAs may play a role in tumorigenesis.
| Cancer subtype | PMCA isoform | Relative change in pump expression compared to non-tumorigenic control | Ref. | |
| mRNA | Protein | |||
| Skin: SV40 transformed fibroblasts | PMCA1 | ↓ | ↓ | [35] |
| PMCA4 | ↓ | ND | [35] | |
| Lung: SV40 transformed fibroblasts | PMCA1 | ↓ | ↓ | [35] |
| PMCA4 | ↓ | ND | [35] | |
| Breast cancer: cell lines | PMCA1 | ↑ | ND | [38,39] |
| PMCA2 | ↑ | ND | [38,39] | |
| PMCA4 | ↓ | ND | [39] | |
| Oral cancer: squamous cell carcinoma, patient tissue samples and cell lines | PMCA1 | ↓ | ↓ | [42] |
| Colon: adenocarcinoma and benign colon tumors | PMCA4 | ↓ | ND | [45] |
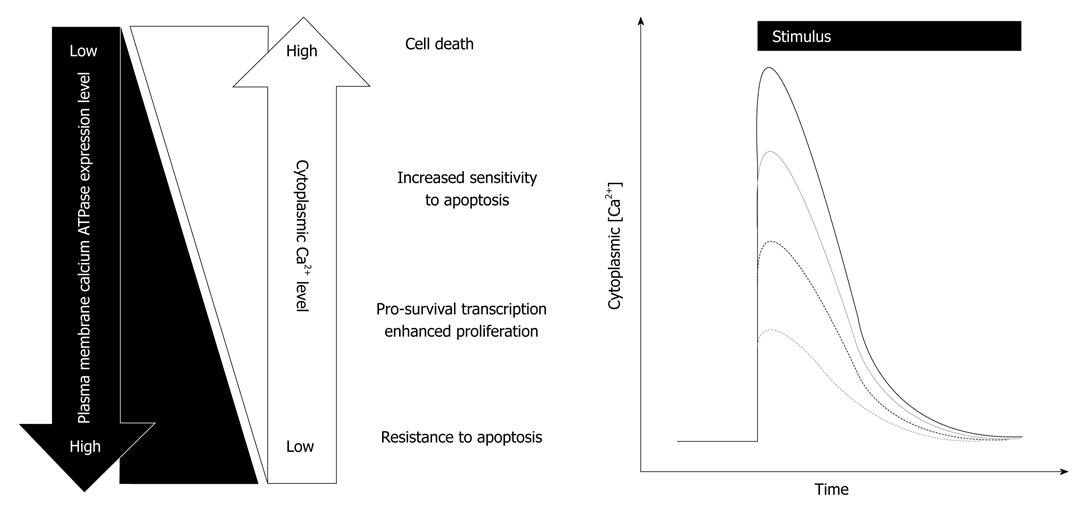
The observation that some cancers may have reduced levels of PMCA while others have increased levels suggests that some cancers are more efficient at removing Ca2+ from the cytoplasm while others may be less effective at extruding Ca2+ after cellular stimuli. This raises the question as to how a reduction or an increase in PMCA expression could contribute to tumorigenic pathways. This may be explained by the diverse array of processes whereby calcium signaling regulates cell function and by the way the calcium signal, such as the amplitude of the response, dictates the fate of the cell[46]. The potential consequences of altered PMCA isoform expression are summarized in Figure 1.
A remodelling of PMCA expression, with consequently reduced calcium efflux, could augment responses to growth or migratory stimuli, giving cancer cells a growth advantage. However, a remodelling of PMCA expression whereby PMCA was at even lower levels, leading to a pronounced reduction in PMCA-mediated efflux, would sensitise the cells to apoptotic stimuli, which would not give cancer cells a growth advantage. Selection pressure would likely result in clonal expansion of cancer cells with reduced levels of PMCA expression that give them a growth advantage but without increasing their sensitivity to apoptotic stimuli. Indeed, siRNA inhibition of PMCA4 expression in HT29 colon cancer cells, at levels that augment [Ca2+]CYT increases after stimulation with ATP and neurotensin, does not sensitize cells to tumor necrosis factor-regulated apoptosis-inducing ligand-mediated cell death[45]. For cancers where PMCA expression is up-regulated, the cells may have an increased capacity to extrude Ca2+ from the cytoplasm, giving these cancer cells a survival advantage, by reducing their sensitivity to apoptotic stimuli, which acts in part through increasing [Ca2+]CYT. Indeed, HeLa cells over-expressing PMCA are less sensitive to ceramide-mediated cell death[47].
Work over recent years has seen an increased interest in understanding how cancer cells may remodel calcium signaling to obtain a growth or a survival advantage. Although not as extensively studied as some other channels and pumps in cancer cells, alterations in PMCA isoform expression appears to be a characterising feature of some cancers. Although mostly assessed in cancer cell lines, increasing evidence is emerging from patient tumour samples. Studies are also beginning to explore the mechanism underlying the altered expression of PMCAs in some cancers and the consequences of their altered expression. The coming years should see a shift towards relating PMCA isoform expression to patient prognosis and the possible role of altered expression on tumorigenic pathways other than proliferation.
Peer reviewers: Rosario Donato, MD, Professor, Director and Chairman, Department Exp. Med. and Biochem. Sci, University of Perugia, Via del Giochetto, C.P. 81 Succ. 3, 06122 Perugia, Italy; Sekhar P Reddy, PhD, Professor, Department of Environmental Health Sciences and Oncology, Johns Hopkins Bloomberg School of Public Health, 615 N. Wolfe Street, Room E7547, Baltimore, MD 21205, United States
S- Editor Cheng JX L- Editor Negro F E- Editor Yang C
| 1. | Brini M, Carafoli E. Calcium pumps in health and disease. Physiol Rev. 2009;89:1341-1378. |
| 2. | Monteith GR, Roufogalis BD. The plasma membrane calcium pump--a physiological perspective on its regulation. Cell Calcium. 1995;18:459-470. |
| 3. | Strehler EE, Treiman M. Calcium pumps of plasma membrane and cell interior. Curr Mol Med. 2004;4:323-335. |
| 4. | Monteith GR, Kable EP, Chen S, Roufogalis BD. Plasma membrane calcium pump-mediated calcium efflux and bulk cytosolic free calcium in cultured aortic smooth muscle cells from spontaneously hypertensive and Wistar-Kyoto normotensives rats. J Hypertens. 1996;14:435-442. |
| 5. | Martin V, Bredoux R, Corvazier E, Papp B, Enouf J. Platelet Ca(2+)ATPases : a plural, species-specific, and multiple hypertension-regulated expression system. Hypertension. 2000;35:91-102. |
| 6. | Blankenship KA, Dawson CB, Aronoff GR, Dean WL. Tyrosine phosphorylation of human platelet plasma membrane Ca(2+)-ATPase in hypertension. Hypertension. 2000;35:103-107. |
| 7. | Monteith GR, Kable EP, Kuo TH, Roufogalis BD. Elevated plasma membrane and sarcoplasmic reticulum Ca2+ pump mRNA levels in cultured aortic smooth muscle cells from spontaneously hypertensive rats. Biochem Biophys Res Commun. 1997;230:344-346. |
| 8. | Nicot A, Ratnakar PV, Ron Y, Chen CC, Elkabes S. Regulation of gene expression in experimental autoimmune encephalomyelitis indicates early neuronal dysfunction. Brain. 2003;126:398-412. |
| 9. | Street VA, McKee-Johnson JW, Fonseca RC, Tempel BL, Noben-Trauth K. Mutations in a plasma membrane Ca2+-ATPase gene cause deafness in deafwaddler mice. Nat Genet. 1998;19:390-394. |
| 10. | Mamic TM, Holman NA, Roberts-Thomson SJ, Monteith GR. PMCA1 mRNA expression in rat aortic myocytes: a real-time RT-PCR study. Biochem Biophys Res Commun. 2000;276:1024-1027. |
| 11. | Carafoli E. Biogenesis: plasma membrane calcium ATPase: 15 years of work on the purified enzyme. FASEB J. 1994;8:993-1002. |
| 12. | Guerini D, Coletto L, Carafoli E. Exporting calcium from cells. Cell Calcium. 2005;38:281-289. |
| 13. | Monteith GR, Wanigasekara Y, Roufogalis BD. The plasma membrane calcium pump, its role and regulation: new complexities and possibilities. J Pharmacol Toxicol Methods. 1998;40:183-190. |
| 14. | Carafoli E, Stauffer T. The plasma membrane calcium pump: functional domains, regulation of the activity, and tissue specificity of isoform expression. J Neurobiol. 1994;25:312-324. |
| 15. | Di Leva F, Domi T, Fedrizzi L, Lim D, Carafoli E. The plasma membrane Ca2+ ATPase of animal cells: structure, function and regulation. Arch Biochem Biophys. 2008;476:65-74. |
| 16. | Kruger WA, Yun CC, Monteith GR, Poronnik P. Muscarinic-induced recruitment of plasma membrane Ca2+-ATPase involves PSD-95/Dlg/Zo-1-mediated interactions. J Biol Chem. 2009;284:1820-1830. |
| 17. | Kruger WA, Monteith GR, Poronnik P. The plasma membrane Ca(2+)-ATPase: regulation by PSD-95/Dlg/Zo-1 scaffolds. Int J Biochem Cell Biol. 2010;42:805-808. |
| 18. | Garside ML, Turner PR, Austen B, Strehler EE, Beesley PW, Empson RM. Molecular interactions of the plasma membrane calcium ATPase 2 at pre- and post-synaptic sites in rat cerebellum. Neuroscience. 2009;162:383-395. |
| 19. | Padányi R, Pászty K, Strehler EE, Enyedi A. PSD-95 mediates membrane clustering of the human plasma membrane Ca2+ pump isoform 4b. Biochim Biophys Acta. 2009;1793:1023-1032. |
| 20. | Strehler EE, Zacharias DA. Role of alternative splicing in generating isoform diversity among plasma membrane calcium pumps. Physiol Rev. 2001;81:21-50. |
| 21. | Reinhardt TA, Filoteo AG, Penniston JT, Horst RL. Ca(2+)-ATPase protein expression in mammary tissue. Am J Physiol Cell Physiol. 2000;279:C1595-C1602. |
| 22. | Reinhardt TA, Horst RL. Ca2+-ATPases and their expression in the mammary gland of pregnant and lactating rats. Am J Physiol. 1999;276:C796-C802. |
| 23. | Reinhardt TA, Lippolis JD, Shull GE, Horst RL. Null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2 impairs calcium transport into milk. J Biol Chem. 2004;279:42369-42373. |
| 24. | Brini M, Coletto L, Pierobon N, Kraev N, Guerini D, Carafoli E. A comparative functional analysis of plasma membrane Ca2+ pump isoforms in intact cells. J Biol Chem. 2003;278:24500-24508. |
| 25. | Prasad V, Okunade GW, Miller ML, Shull GE. Phenotypes of SERCA and PMCA knockout mice. Biochem Biophys Res Commun. 2004;322:1192-1203. |
| 26. | Okunade GW, Miller ML, Pyne GJ, Sutliff RL, O'Connor KT, Neumann JC, Andringa A, Miller DA, Prasad V, Doetschman T. Targeted ablation of plasma membrane Ca2+-ATPase (PMCA) 1 and 4 indicates a major housekeeping function for PMCA1 and a critical role in hyperactivated sperm motility and male fertility for PMCA4. J Biol Chem. 2004;279:33742-33750. |
| 28. | Roderick HL, Cook SJ. Ca2+ signalling checkpoints in cancer: remodelling Ca2+ for cancer cell proliferation and survival. Nat Rev Cancer. 2008;8:361-375. |
| 29. | Prevarskaya N, Zhang L, Barritt G. TRP channels in cancer. Biochim Biophys Acta. 2007;1772:937-946. |
| 30. | Zhang L, Barritt GJ. Evidence that TRPM8 is an androgen-dependent Ca2+ channel required for the survival of prostate cancer cells. Cancer Res. 2004;64:8365-8373. |
| 31. | Bolanz KA, Kovacs GG, Landowski CP, Hediger MA. Tamoxifen inhibits TRPV6 activity via estrogen receptor-independent pathways in TRPV6-expressing MCF-7 breast cancer cells. Mol Cancer Res. 2009;7:2000-2010. |
| 32. | Lehen'kyi V, Flourakis M, Skryma R, Prevarskaya N. TRPV6 channel controls prostate cancer cell proliferation via Ca(2+)/NFAT-dependent pathways. Oncogene. 2007;26:7380-7385. |
| 33. | Monteith GR, McAndrew D, Faddy HM, Roberts-Thomson SJ. Calcium and cancer: targeting Ca2+ transport. Nat Rev Cancer. 2007;7:519-530. |
| 34. | Fixemer T, Wissenbach U, Flockerzi V, Bonkhoff H. Expression of the Ca2+-selective cation channel TRPV6 in human prostate cancer: a novel prognostic marker for tumor progression. Oncogene. 2003;22:7858-7861. |
| 35. | Reisner PD, Brandt PC, Vanaman TC. Analysis of plasma membrane Ca(2+)-ATPase expression in control and SV40-transformed human fibroblasts. Cell Calcium. 1997;21:53-62. |
| 36. | Usachev YM, Toutenhoofd SL, Goellner GM, Strehler EE, Thayer SA. Differentiation induces up-regulation of plasma membrane Ca(2+)-ATPase and concomitant increase in Ca(2+) efflux in human neuroblastoma cell line IMR-32. J Neurochem. 2001;76:1756-1765. |
| 37. | Fernandes D, Zaidi A, Bean J, Hui D, Michaelis ML. RNA--induced silencing of the plasma membrane Ca2+-ATPase 2 in neuronal cells: effects on Ca2+ homeostasis and cell viability. J Neurochem. 2007;102:454-465. |
| 38. | Lee WJ, Roberts-Thomson SJ, Holman NA, May FJ, Lehrbach GM, Monteith GR. Expression of plasma membrane calcium pump isoform mRNAs in breast cancer cell lines. Cell Signal. 2002;14:1015-1022. |
| 39. | Lee WJ, Roberts-Thomson SJ, Monteith GR. Plasma membrane calcium-ATPase 2 and 4 in human breast cancer cell lines. Biochem Biophys Res Commun. 2005;337:779-783. |
| 40. | Holton M, Yang D, Wang W, Mohamed TM, Neyses L, Armesilla AL. The interaction between endogenous calcineurin and the plasma membrane calcium-dependent ATPase is isoform specific in breast cancer cells. FEBS Lett. 2007;581:4115-4119. |
| 41. | Lee WJ, Robinson JA, Holman NA, McCall MN, Roberts-Thomson SJ, Monteith GR. Antisense-mediated Inhibition of the plasma membrane calcium-ATPase suppresses proliferation of MCF-7 cells. J Biol Chem. 2005;280:27076-27084. |
| 42. | Saito K, Uzawa K, Endo Y, Kato Y, Nakashima D, Ogawara K, Shiba M, Bukawa H, Yokoe H, Tanzawa H. Plasma membrane Ca2+ ATPase isoform 1 down-regulated in human oral cancer. Oncol Rep. 2006;15:49-55. |
| 43. | Aung CS, Kruger WA, Poronnik P, Roberts-Thomson SJ, Monteith GR. Plasma membrane Ca2+-ATPase expression during colon cancer cell line differentiation. Biochem Biophys Res Commun. 2007;355:932-936. |
| 44. | Ribiczey P, Tordai A, Andrikovics H, Filoteo AG, Penniston JT, Enouf J, Enyedi A, Papp B, Kovács T. Isoform-specific up-regulation of plasma membrane Ca2+ATPase expression during colon and gastric cancer cell differentiation. Cell Calcium. 2007;42:590-605. |
| 45. | Aung CS, Ye W, Plowman G, Peters AA, Monteith GR, Roberts-Thomson SJ. Plasma membrane calcium ATPase 4 and the remodeling of calcium homeostasis in human colon cancer cells. Carcinogenesis. 2009;30:1962-1969. |