INTRODUCTION
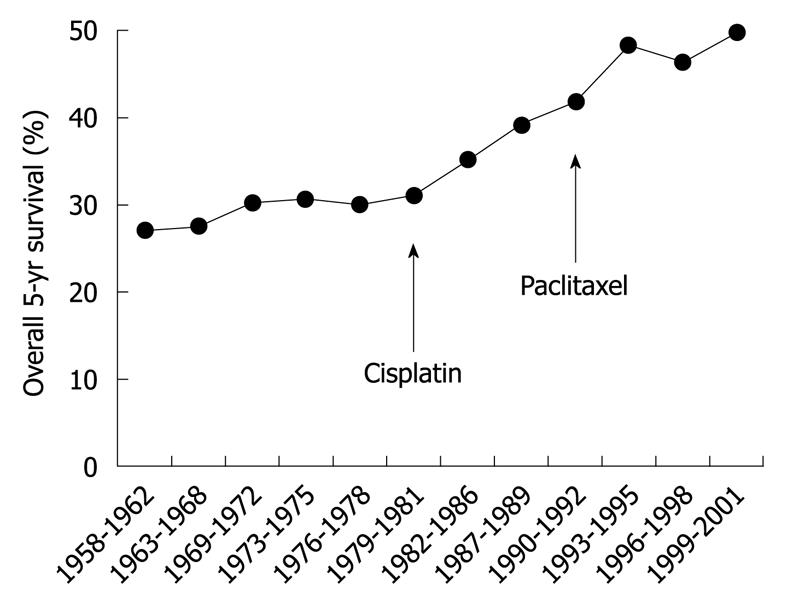
Ovarian cancer is the leading cause of death in women with gynecological cancer. The incidence of ovarian cancer was projected in 2007 to be 230 555 new cases and 141 452 deaths worldwide, which represents 4.3% of all cancer deaths in women[1]. The 5-year survival rate of ovarian cancer ranges from 19% to 90%, depending on the spread of disease at diagnosis. More than 70% of patients with ovarian cancer are diagnosed at the advanced stage, which is associated with high morbidity and mortality[2]. However, new therapeutic agents introduced recently have improved overall survival from ovarian cancer (Figure 1)[2].
Figure 1 Overall 5-year survival by year.
Therapy by alkylating agents (e.g. melphalan) was the standard of care for epithelial ovarian cancer until the 1970s. The US Food and Drug Administration approved the therapeutic agent cisplatin in 1978 and paclitaxel at the end of 1992, as indicated in the figure. Overall 5-year survival of epithelial ovarian cancer was increased after introduction of these agents.
Until 1996, standard treatment for advanced ovarian cancer included surgical tumor debulking, followed by adjuvant chemotherapy that consisted of a platinum compound and an alkylating agent[3]. Two pivotal trials, the Gynecologic Oncology Group (GOG) 111 and a European-Canadian study, known as OV-10, have shown that incorporating paclitaxel into first-line therapy improves the survival rate of patients with International Federation of Gynecology and Obstetrics (FIGO) stage III or IV ovarian cancer[4,5]. A subsequent international study, the GOG 182 International Collaborative Ovarian Neoplasm 5 (ICON5) study, sought to improve the efficacy of standard platinum-taxane therapy by incorporating newer cytotoxic agents in sequential doublet and triplet combinations[6]. Unfortunately, no combination of several agents used in standard therapy has improved overall survival; thus, carboplatin with paclitaxel remains the standard regimen as first-line treatment for ovarian cancer and the response rate exceeds 80%. However, approximately 70% of patients with FIGO stage III or IV ovarian cancer recur within 5 years and drug resistance emerges[2,7]. These patients with platinum- and taxane-resistant disease are usually treated with other agents, such as liposomal doxorubicin, gemcitabine, topotecan, or etoposide. The overall response rates with these other drugs, however, are only 10%-25% with relatively short durations of response[8]. Therefore, novel treatment strategies are required to improve outcomes for women with advanced ovarian cancer.
Recently, various molecular targeted agents have been developed and used in the management of a variety of malignancies, including ovarian cancer[9]. Unlike most traditional cytotoxic anticancer drugs, these drugs target tumor cells, tumor stroma, tumor vasculature, and cellular signaling mechanisms that are aberrant in tumor tissue. Tumor cells are effectively selected and growth retardation and apoptosis are induced with minimizing toxicity to normal cells. Thus, targeted therapy agents, the majority of which are monoclonal antibodies and small-molecule protein-kinase inhibitors, are expected to be attractive treatment options for malignancies. This article reviews the molecular mechanisms of various targeted therapies and drugs that are under early-phase clinical evaluation in ovarian cancer.
TARGETING ANGIOGENESIS
Angiogenesis, the formation of new blood vessels (neovascularization) from existing vasculature, is one of the crucial processes for tumor growth, invasion, and metastasis[10]. This process is regulated by a number of growth factor receptor pathways[11]. One of the major pathways involved in tumor angiogenesis is the vascular endothelium growth factor (VEGF) family and its receptors (VEGFR). The mammalian VEGF family consists of seven structurally related glycoproteins including VEGF-A (usually referred to as VEGF), VEGF-B, VEGF-C, VEGF-D, VEGF-E, and placental growth factors 1 and 2[12]. The major mediator of tumor angiogenesis in the VEGF family is VEGF-A, which is expressed as various mature isoforms of 121, 145, 165, 183, 189, and 206 amino acids through alternative splicing of the VEGF-A gene. VEGF-A165 is the predominant isoform and is commonly overexpressed in a variety of human tumors. The expression of VEGF is often upregulated in tumors by numerous environmental factors, such as hypoxia-inducible transcription factors 1α and 2α, low pH, inflammatory cytokines (e.g. interleukin-6), growth factors [e.g. basic fibroblast growth factor and platelet-derived growth factors (PDGFs)], sex hormones (androgens and estrogens), and chemokines (e.g. stromal-cell-derived factor 1)[13]. Activation of oncogenes [e.g. ras, src, epidermal growth factor receptor (EGFR), and human epidermal growth factor receptor 2 (HER2)] and loss or mutational inactivation of tumor-suppressor genes (e.g. p53, VHL, and PTEN) have also been reported as genetic factors for VEGF induction (Figure 2).
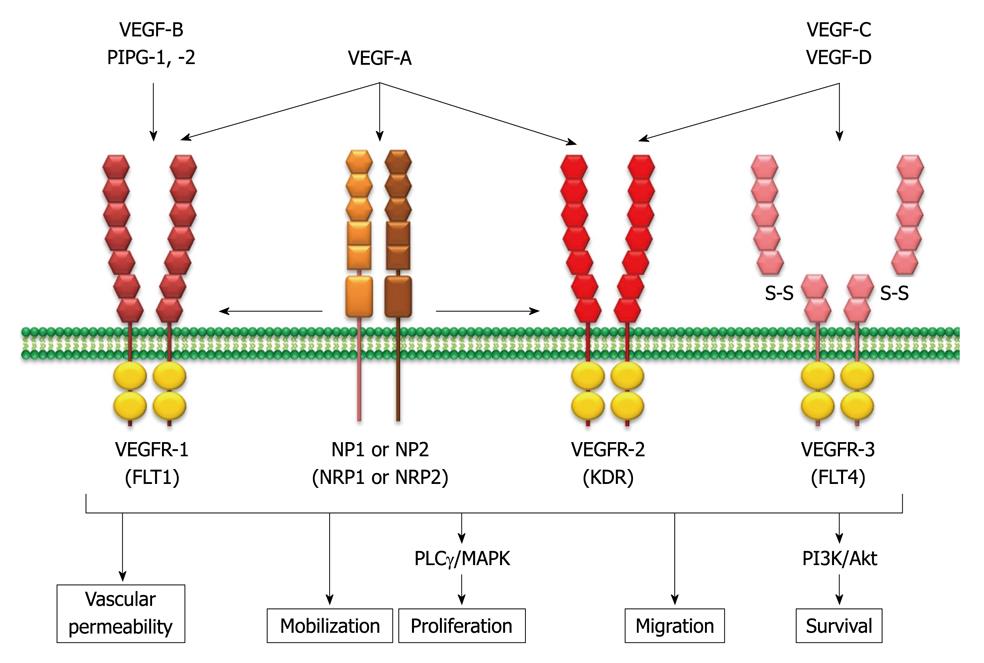
Figure 2 Vascular endothelium growth factor family members and their specific binding ligands.
The mammalian vascular endothelium growth factor (VEGF) family consists of seven structurally related glycoproteins with VEGF-A as the major mediator of tumor angiogenesis among them. The VEGF ligands bind to three structurally similar receptors, and each tyrosine kinase activates the intracellular signaling cascade, including mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3,4,5-kinase (PI3K)/Akt pathways. Subsequently, the pro-angiogenic signaling pathways are activated. PLCγ: Phospholipase C γ; NP: Neuropilin; VEGFR: VEGF receptors; FLT: Fms-related tyrosine kinase; KDR: Kinase insert domain receptor.
These VEGF ligands bind to three structurally similar receptors: VEGFR1 [also known as fms-related tyrosine kinase 1 (FLT1)]; VEGRF2 (also known as kinase insert domain receptor); and VEGFR3 (also known as FLT4). VEGF-A binds both VEGFR1 and VEGFR2, which are found mainly on vascular endothelial cells, although VEFGR2 has a predominant role[14]. VEGF3 has been reported to be important for lymphangiogenesis. Neuropilin (NP)1 and NP2 (also known as NRP1 and NRP2) act as co-receptors for the VEGFRs, increasing the binding affinity of VEGFs to their receptors. After ligand binding to VEGFRs, each tyrosine kinase activates the intracellular signaling cascade, including mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3,4,5-kinase (PI3K)/Akt pathways[13]. Subsequently, pro-angiogenic effects, such as stimulation of endothelial progenitor cell mobilization from the bone marrow, promotion of endothelial cell proliferation, migration, survival, and differentiation are activated. VEGF also increases vascular permeability and vasodilation[13].
Overexpression of VEGF is often observed in many solid tumors and has been associated with increased risk of metastatic disease and poor prognosis in a variety of malignancies including ovarian cancer[15-17]. Furthermore, coexpression of VEGF and VEGFR2 has recently been discovered in both ovarian cancer cells and ovarian tumor tissues, which might indicate excision of the autocrine VEGFA/VEFGR2 loop in ovarian cancer[18-20]. Therefore, the VEGF signaling pathways are thought to be promising targets to treat ovarian cancer patients.
VEGF inhibitors
Bevacizumab (Avastin): Bevacizumab is a recombinant humanized monoclonal IgG1 antibody that targets VEGF-A, and shows clinical benefit in patients with metastatic colorectal cancer, non-small cell lung cancer, and breast cancer[21]. This antibody binds and neutralizes all biologically active forms of VEGF-A (e.g. VEGF-A165) and then suppresses tumor growth and inhibits metastatic disease progression[22,23]. In addition, anti-VEGF drugs are thought to enhance the effects of chemotherapy[24]. Tumor vasculature is structurally and functionally abnormal. It has been proposed that bevacizumab improves the structure and function of tumor vessels (normalization). These morphological changes lead to functional change (e.g. decreased interstitial fluid pressure, increased tumor oxygenation, and improved penetration of drugs in these tumors), thus making tumors more sensitive to chemotherapy.
Two phase II studies of bevacizumab monotherapy (15 mg/kg intravenously every 21 d) for patients with ovarian cancer or peritoneal cancer have recently been conducted[25,26]. In the GOG 170D study, 62 patients, including 26 platinum-resistant patients, were eligible and assessable, and 13 of these (21.0%) experienced clinical response with two complete responses[25]. The major grade 3/4 adverse events were hypertension (9.7%), gastrointestinal (GI) events (6.5%) without GI perforation, venous thrombosis (1.6%), and proteinuria (1.6%). In the other phase II trial on 44 platinum-resistant patients, partial response (PR) was observed in seven patients (15.9%). Serious adverse events occurred in 18 patients (40.9%) and included GI perforation (11.4%), arterial thromboembolic events (6.8%), and death (6.8%). Grade 3/4 adverse events included hypertension (9.1%), proteinuria (15.9%), bleeding (2.3%) and wound-healing complications (2.3%).
Several phase II trials have been conducted to assess the efficacy and safety of bevacizumab in combination with other targeted therapy or chemotherapy[27-29]. In a phase II study, 13 patients with recurrent ovarian, primary peritoneal and fallopian tube cancer were given bevacizumab (15 mg/kg every 21 d), together with erlotinib (150 mg/d), an EGFR tyrosine kinase inhibitor[29]. There were two major objective responses for a response rate of 15.4%. However, this study was stopped because two patients had fatal GI perforations. Two phase II trials of bevacizumab (10 mg/kg every 2 wk) in combination with oral cyclophosphamide (50 mg/d) for patients with recurrent ovarian cancer have been reported[27,28]. These two studies demonstrated objective response rates of 24% (17 patients) and 53% (eight patients), respectively. Four episodes (5.7%) of GI perforation of fistulae and three treatment-related deaths were observed in one study[28]. Two phase III studies, GOG 218 and ICON7, to examine standard chemotherapy (paclitaxel + carboplatin) and combined effects with bevacizumab are on-going, and the results are expected soon. Another on-going phase III study, the Ovarian Cancer Education Awareness Network trial, is evaluating the efficacy of bevacizumab in combination with carboplatin and gemcitabine in patients with ovarian, primary peritoneal, or fallopian tube cancer.
Cediranib (AZD2171; Recentin): Cediranib is a highly potent, small-molecule, oral tyrosine kinase inhibitor of VEGFR1, 2 and 3, and c-Kit, which competes for the ATP-binding site within the receptor kinase domain[30,31]. Cediranib, therefore, is thought to be effective in prevention of tumor progression, not only by inhibiting VEGFR-2 activity and angiogenesis, but also by concomitantly inhibiting VEGFR-3 activity and lymphangiogenesis.
In a phase II study on patients with recurrent ovarian, fallopian tube, and peritoneal cancer, cediranib was administered orally[32]. The original dose was 45 mg/d, but the dose was lowered to 30 mg because of toxicity observed in the first 11 patients. Forty-six patients were treated and eight showed a PR, which gave an objective response rate of 17.4%. Major grade 3 toxicities included hypertension (46%), fatigue (24%), and diarrhea (13%). Grade 4 toxicities included central nervous system hemorrhage (n = 1), hypertriglyceridemia/hypercholesterolemia/elevated lipase (n = 1), and dehydration/elevated creatinine (n = 1). No GI perforations or fistulas occurred. Thus, cediranib has been shown to be an active drug in recurrent ovarian cancer, with the predictable toxicities observed with other tyrosine kinase inhibitors. A phase III randomized study (ICON6) on patients with ovarian, fallopian tube, and primary peritoneal carcinoma is comparing three treatment arms: (1) chemotherapy alone (carboplatin and paclitaxel); (2) concurrent cediranib; and (3) concurrent and maintenance cediranib.
VEGF Trap (AVE-0005; Aflibercept): VEGF Trap is a fusion protein that combined the Fc region of IgG1 with domain two of VEGFR1 and domain three of VEGFR2 (VEGFRδ1R2) that acts as a decoy receptor, binding with high affinity to the VEGF-A ligand and thus preventing VEGFR1 and VEGFR2 binding and subsequent stimulation[33]. It also has strong binding affinity for PIGF.
Preliminary results from a randomized phase II trial of VEGF Trap in patients with recurrent ovarian cancer have demonstrated a PR in 8% of patients and ascites resolution in 29%[34]. The most frequent grade 3/4 adverse events included hypertension (18%), proteinuria (7%), and headache (4%). GI perorations were observed in two patients (1%). A phase I/II trial of VEGF Trap in combination with docetaxel in patients with recurrent ovarian cancer, primary peritoneal cancer, and fallopian tube cancer is ongoing.
PDGF inhibitors
The families of PDGFs and its receptors (PDGFRs) modulate angiogenesis by regulating endothelial cell survival and pericyte/vascular smooth muscle cell recruitment[35-37]. The PDGF family includes five dimeric isoforms (PDGF-AA, -AB, -BB, -CC, and -DD) that have distinct abilities to bind to and activate the PDGFRs (PDGFRα/β heterodimers, PDGFRα and β homodimers).
Furthermore, PDGF enhances the proliferation of human ovarian surface epithelial cells and ovarian cancer cells[38,39]. Expression of PDGF and PDGFα was found in 73.3% and 35.6% of malignant ovarian tumors, respectively, but not in any benign tumors or normal ovaries[40]. In addition, the expression of PDGFRα was an independent poor prognostic factor in patients with ovarian cancer. Thus, PDGF signaling pathways could be novel targets for ovarian cancer therapy.
Imatinib mesylate (STI571; Gleevec or Glivec): Imatinib, a derivative of 2-phenylaminopyrimidine, has been created using the structure of the ATP-binding site of the Abl protein kinase[41]. Imatinib also inhibits PDGFR and the stem-cell factor receptor c-Kit (CD117) tyrosine kinases and is used to treat chronic myelogenous leukemia, Philadelphia-chromosome-positive acute lymphoid leukemia, and c-Kit-positive GI stromal tumors[42].
Two phase II studies have evaluated imatinib in patients with recurrent ovarian cancer or primary peritoneal carcinoma[43,44]. In the University of Texas M.D. Anderson Cancer Center trial, imatinib was given orally at 600 mg/d[43]. However, no complete or partial responses were documented in the 12 evaluable patients. In the GOG 170E trial, 56 patients were treated with imatinib at 400 mg twice daily, but only one patient responded. Thus, imatinib monotherapy has limited activity in patients with recurrent ovarian cancer.
The combination effect of imatinib and docetaxel was evaluated in patients with platinum-resistant ovarian cancer[45]. However, a response rate was reported in 21.7% (5/23) and there was no clear benefit of this combination over docetaxel alone.
Other antiangiogenic drugs
In a phase II trial, vandetanib (ZD6474; Zactima), a small-molecule, oral tyrosine kinase inhibitor of VEFGR and EGFR, was given as monotherapy in patients with recurrent ovarian cancer[46]. Twelve patients entered the study; however, no significant clinical benefit in this disease setting has been reported.
Other multi-targeted tyrosine kinase inhibitors, with the targets including VEGFRs, such as sunitinib (SU11248; Sutent), sorafenib (BAY43-9006; Nexavar), pazopanib (GW786034; Votrient), motesanib, and BIBF 1200, are also being evaluated in phase II or III settings.
AGENTS TARGETING THE HUMAN EGFR FAMILY
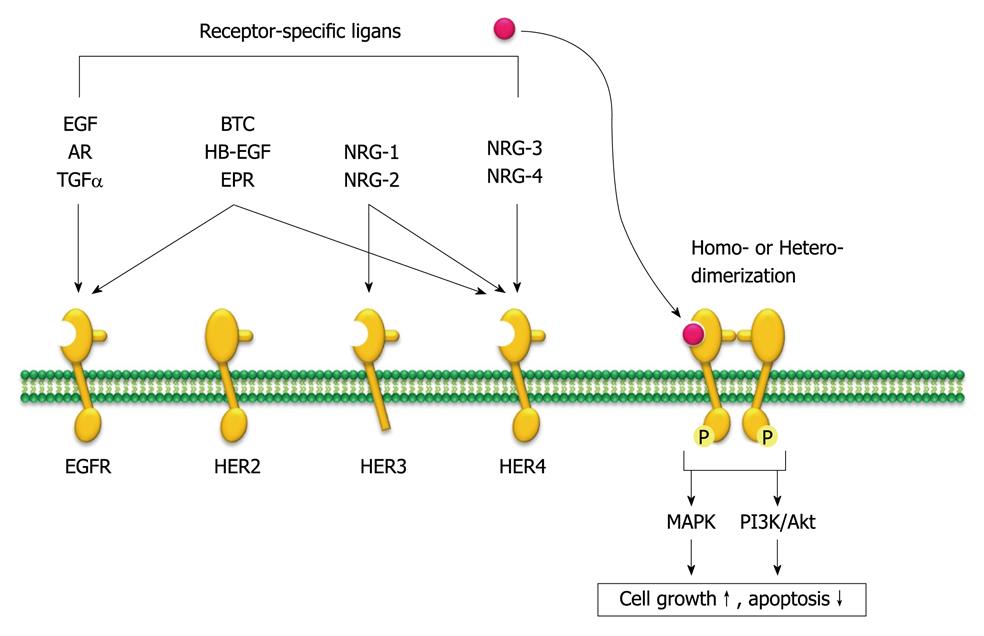
The HER family consists of four distinct transmembrane tyrosine kinase receptors: HER-1 (EGFR/erbB1), HER-2/neu (erbB2), HER-3 (erbB3) and HER-4 (erbB4). These receptors are closely related structurally and share a structure that consists of a ligand-binding extracellular domain (except for HER3), a functional intracellular kinase domain, and a C-terminal signaling tail. Although HER2 has no known ligand, 10 different ligands can selectively bind to each receptor. The receptors form multiple combinations by homodimerization or heterodimerization that leads to receptor autophosphorylation on several tyrosine residues in the intracellular kinase domain through tyrosine kinase activity. Autophosphorylation leads to a series of downstream signaling pathways, such as MAPK and PI3K/Akt pathways, which are involved in cancer-cell proliferation, blocking apoptosis, activating invasion and metastasis, and stimulating tumor-induced neovascularization, thus making it an attractive target for anticancer therapies (Figure 3)[47].
Figure 3 Human epidermal growth factor receptor family members and their specific ligands.
The human epidermal growth factor receptor (HER) family consists of four distinct transmembrane tyrosine kinase receptors, and 10 different ligands can selectively bind to each of them. The receptor undergoes homo- or hetero-dimerization that leads to receptor autophosphorylation that activates a series of downstream signaling pathways, such as mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3,4,5-kinase (PI3K)/Akt pathways that control cell growth and apoptotic signaling. AR: Amphiregulin; TGFα: Transforming growth factor α; BTC: β-cellulin; HB-EGF: Heparin-binding epidermal growth factor; EPR: Epiregulin; NRG: Neuregulins; P: Phosphate; EGFR: Epidermal growth factor receptor.
The HER family is commonly expressed in many human malignancies. In ovarian cancer, a wide variety of HER family expression has been reported [EFGR, 4%-100% (average 48%); HER-2, 0%-100% (average 40%); HER-3, 3%-90% (average 48%); and HER-4, 45%-92% (average 71%)][48]. HER overexpression, especially EGFR and HER-2, is thought to be correlated with poor prognosis and decreased therapeutic responsiveness in ovarian cancer patients, although the clinical data are contradictory. Therefore, several EGFR and HER-2 inhibitors, including tyrosine kinases inhibitors (gefitinib and erlotinib) and monoclonal antibodies (cetuximab, trastuzumab, and pertuzumab), are being tested in ovarian cancer patients.
EGFR inhibitors
Gefitinib (ZD1839; Iressa): Gefitinib is an orally active, low-molecular-weight synthetic anilinoquinazoline that selectively inhibits the activation of EGFR tyrosine kinase through competitive binding of the ATP-binding domain of the receptor[49]. Treatment of cancer cells with gefitinib increases expression of the cyclin-dependent kinase inhibitor p27KIP1, which leads to cell cycle arrest at the G0-G1 boundary in a dose- and time-dependent manner[50]. Clinical response to gefitinib is thought to correlate with activated mutations of the EGFR gene in patients with non-small cell lung cancer[51,52]. In addition, gefitinib enhances cytotoxic effects of anticancer agents (cisplatin, carboplatin, oxaliplatin, paclitaxel, docetaxel, doxorubicin, etoposide, topotecan, and raltitrexed) in various human cancer cell lines, including ovarian cancer[53].
In a phase II study (GOG170C), gefitinib was given orally at 500 mg/d to 27 patients with relapsed/persistent ovarian or primary peritoneal carcinoma[54]. PR was observed in only one patient (3.7%). The response rate, however, for patients with EGFR-positive tumors was 9% (1/11). Thus, prescreening patients for activated mutations in EGFR might improve the response rate to gefitinib. The most commonly observed grade 3 toxicities were dermatological (15%, 4/27) and diarrhea (30%, 8/27).
A phase II study on gefitinib in combination with paclitaxel and carboplatin for second-line treatment of patients with ovarian, tubal or peritoneal adenocarcinoma has been conducted[55]. This combination therapy provided a good clinical response with high overall response rates [19.2% (5/26) for resistant/refractory and 61.9% (26/42) for sensitive disease], but was associated with an increased risk of hematological disorders.
Erlotinib (OSI-774; Tarceva): Erlotinib, like gefitinib, is a potent, selective, and reversible inhibitor of EGFR tyrosine kinase and reduces EGFR autophosphorylation[56]. This inhibitor blocks cell cycle progression at the G1 phase by accumulation of retinoblastoma protein in its underphosphorylated form and accumulation of p27KIP1, and also triggers apoptosis.
Thirty-four patients with refractory, recurrent, EGFR-positive ovarian carcinoma received 150 mg of erlotinib orally once daily in a phase II study[57]. Two patients had PRs, which gave an objective response rate of 5.9%. The most frequent adverse events were rash (68%) and diarrhea (38%).
A phase Ib trial has shown the feasibility of a combination of erlotinib with docetaxel and carboplatin in patients with ovarian, fallopian tube, and primary peritoneal cancers[58]. However, further investigations are awaited.
Cetuximab (C225; Erbitux): Cetuximab is a chimeric (mouse/human) monoclonal antibody to EGFR used for treatment of advanced colorectal, head and neck cancers that binds to the extracellular domain of EGFR and blocks the binding of EGF ligand to EGFR and its subsequent activation by inhibiting ligand induced autophosphorylation of the EGFR[59]. Cetuximab downregulates the receptor from the cell surface through internalization of the EGFR and also induces antibody-dependent cellular cytotoxicity (ADCC)[60]. In addition, in vitro and in vivo studies have shown that cetuximab enhances cytotoxic effects of anticancer agents (doxorubicin, cisplatin, docetaxel, gemcitabine, and topotecan) in various human cancer cell lines[61].
In a phase II study, patients with resistant/recurrent ovarian or primary peritoneal carcinoma received cetuximab intravenously at 400 mg/m2 initially and then 250 mg/m2 weekly for two 3-wk cycles[62]. Rash (96%) was the most common drug-related adverse event. One of 25 patients achieved partial remission, with an overall response rate of 4%. Thus cetuximab monotherapy showed minimal activity in patients with recurrent ovarian cancer.
The GOG146P phase II trial assessed the activity of cetuximab in combination with carboplatin in relapsed platinum-sensitive ovarian or primary peritoneal carcinoma and reported that this combination therapy had only modest activity in patients with EGFR-positive carcinoma[62]. Similarly, a phase II study was conducted to determine the efficacy of cetuximab plus paclitaxel and carboplatin as initial treatment of stage III/IV ovarian cancer[63]. However, this combination did not demonstrate prolongation of progression-free survival when compared with historical data.
Trastuzumab (Herceptin): Trastuzumab, a recombinant humanized monoclonal antibody, binds to the juxta-membrane portion of the extracellular domain of the HER2 receptor and blocks activation of its intracellular signal-transduction pathways, such as PI3K/Akt and MAPK[64]. Other proposed mechanisms of action include inhibition of the formation of a truncated membrane-bound fragment (p95), activation of ADCC, prevention of HER2-receptor dimerization, and increased endocytotic destruction of the receptor.
Results from a phase II study (GOG160) of trastuzumab showed that 2+ or 3+ HER2 overexpression was observed in only 95 (11.4%) of 837 patients with recurrent or refractory ovarian or primary peritoneal carcinoma. Forty-one eligible patients with HER2 overexpression were given intravenous trastuzumab at 4 mg/kg initially and then at 2 mg/kg weekly. Although there was only mild toxicity (e.g. anemia, GI events, neuropathy, or fatigue), the overall response rate was low at 7.3%. The clinical value of trastuzumab monotherapy in recurrent ovarian cancer is limited by the low frequency of HER2 overexpression and low rate of objective response among patients with HER2 overexpression.
Pertuzumab (2C4; Omnitarg): Pertuzumab, a recombinant, humanized monoclonal antibody, binds to the HER2 dimerization domain (near the center of domain II), sterically blocking a binding pocket that is necessary for receptor dimerization with its partner receptors, and inhibits the signaling cascades[65]. Pertuzumab binding to HER2 induces activation of ADCC effects but does not block the truncation of HER2 in the same way as trastuzumab binding does[66].
In a phase II study of 123 recurrent ovarian cancer patients, 55 patients in cohort 1 and 62 in cohort 2 were assessable for efficacy[67]. The patients in cohort 1 received a loading dose of 840 mg of pertuzumab intravenously followed by 420 mg every 3 wk; the patients in cohort 2 received 1050 mg every 3 wk, and showed an overall response rate of 4.3%. The main adverse events observed were diarrhea (11%, grade 3) and asymptomatic left ventricular ejection fraction decreases of < 50% (one patient in cohort 1 and four patients in cohort 2).
Combination therapy of pertuzumab with gemcitabine was tested in a randomized phase II trial in 130 patients with platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer[68]. The patients were randomly assigned to gemcitabine (800 mg/m2 on days 1 and 8 of a 21-d cycle) plus either placebo or pertuzumab (840 mg loading dose followed by 420 mg every 3 wk), and showed objective response rates of 13.8% and 4.6%, respectively. In patients whose tumors had low HER3 mRNA expression, an increased treatment benefit was observed in the gemcitabine + pertuzumab arm. Therefore, pertuzumab may be effective in platinum-resistant ovarian cancer and low HER3 mRNA expression may predict pertuzumab clinical benefit.
AGENTS TARGETING EPIGENETIC MODULATORS
Epigenetic modulation of gene expression is important in orchestrating key biological processes that include DNA methylation, histone covalent modifications, and nucleosomal remodeling[73]. Several enzymes, such as DNA methyltransferases (DNMTs), histone deacetylases (HDACs), histone methyltransferases, and complex nucleosomal remodeling factors contribute to these gene regulations. Aberrant epigenetic regulation of gene expression has been reported to contribute to carcinogenesis through silencing of tumor suppressor genes, activation of oncogenes, loss of imprinting, genomic instability, X-chromosome inactivation, and harmful expression of inserted viral sequences. In addition, the degree of epigenetic abnormalities increases during malignant transformation[74]. DNMT and HDAC inhibitors are two of the most studied enzymes in the anticancer therapy.
DNMTs catalyze the transfer of a methyl group derived from S-adenosyl-methionine to the carbon-5 position of the cytosine ring within the structure of cytosine-guanine (CpG) dinucleotides[75]. Methylation of the CpG islands associated with gene promoter regions leads to transcriptional silencing. Hypermethylation of CpG islands is observed commonly in a variety of cancers, including ovarian cancer, and is associated with tumor initiation, progression, and drug resistance[73,76,77]. Several hypermethylated genes have been reported in ovarian cancers, including classical tumor suppressors [breast and ovarian cancer susceptibility gene (BRCA)1, p16, and MLH1], putative tumor suppressors (OPCML, SPARC, ANGPTL2, CTGF, and RASSF1A, imprinted genes (ARH1, PEG3, DLEC1, ARL11, and TCEAL7), proapoptotic genes (LOT1, DAPK, TMS1/ASC, and PAR-4), cell adhesion (ICAM-1, CDH1), cell signaling (HSulf-1), genome stability (PALB2), taxane resistance (TUBB3), and embryonic development (HOXA10 and HOXA11)[78,79].
Histone acetyltransferases (HATs) and HDACs also regulate transcription of DNA[9]. Histone acetylation by HATs loosen DNA binding around histones and enable transcription factors and RNA polymerases to access the DNA. In contrast, HDACs remove acetyl groups from an ε-N-acetyl lysine amino acid on a histone associated with transcriptional gene silencing through direct interaction with DNMTs[73]. Interestingly, HDAC inhibitors have been shown to induce arrest of cellular growth and apoptosis in cancer cells, including ovarian cancer, by restoring gene expression (e.g. tumor suppressor genes)[80-82].
DNMT inhibitors
Cytosine nucleoside (cytidine) analogs of azacytdine (5-azacitidine; Vidaza) and decitabine (5-aza-2’-deoxycytidine; Dacogen) given at much higher doses were developed initially as chemotherapeutic agents. At lower doses, however, these agents replace cytosine during DNA replication. Subsequent incorporation of these agents into DNA binds to and inhibits DNMTs. Azacytidine is also incorporated into RNA during transcription and inhibits translation of proteins[9].
Azacytdine and decitabine are approved to treat myelodysplastic syndrome. Phase I and II clinical trials are ongoing to examine treatment of ovarian cancer[79]. A phase I study has been completed recently of decitabine combined with carboplatin in patients with recurrent platinum-resistant EOC[83].
HDAC inhibitors
Vorinostat [suberoylanilide hydroxamic acid; Zolinza]: Vorinostat is an oral small-molecule HDAC inhibitor approved for treatment of cutaneous T-cell lymphoma. It binds to the catalytic zinc-pocket on class I, II, and IV HDAC and inhibits these activities[84].
Thirty-seven patients with recurrent or persistent ovarian or primary peritoneal carcinoma received a 400 mg daily oral dose of vorinostat in a phase II trial (GOG170H)[85]. One patient showed response PR with an objective response rate of 3.7%. Two grade 4 toxicities (one leukopenia and one neutropenia) were reported, and the most common grade 3 toxicities were constitutional (11%) and GI (11%) symptoms. The most frequent adverse events were rash (68%) and diarrhea (38%). Therefore, vorinostat has minimal activity as a single-agent in unscreened patients with ovarian cancer.
Belinostat (PXD101): Belinostat is a low-molecular-weight, hydroxamic acid inhibitor of class I and II HDAC. It has been investigated for relapsed or refractory peripheral T-cell lymphoma and for cancer of unknown primary.
In a phase II trial, 18 patients with metastatic or recurrent platinum-resistant EOC and 14 patients with micropapillary/borderline (LMP) ovarian tumors were treated intravenously with 1000 mg/m2 of belinostat on days 1-5 and every 21 d thereafter[86]. One patient with an LMP tumor had a PR. The most frequent adverse events were grade 3 thrombosis (9%), hypersensitivity (3%), and elevated alkaline phosphatase (3%). Thus, belinostat is tolerated well and shows some activity in patients with LMP disease.