Published online May 26, 2010. doi: 10.4331/wjbc.v1.i5.81
Revised: May 14, 2010
Accepted: May 21, 2010
Published online: May 26, 2010
Tau is an intracellular protein, found mainly in neurons, but it can also be found in the extracellular space in pathological situations. Here we discuss whether intracellular tau, in aggregated form or modified by phosphorylation, could be toxic inside a neuron. On the other hand, it has been proposed that extracellular tau could be toxic. In this review, we address the question if the elimination of tau would be a possible therapeutic method to avoid tauopathy disorder and we suggest ways to eliminate intracellular and extracellular tau as treatment.
- Citation: Barreda EG, Avila J. Is tau a suitable therapeutical target in tauopathies? World J Biol Chem 2010; 1(5): 81-84
- URL: https://www.wjgnet.com/1949-8454/full/v1/i5/81.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v1.i5.81
Neuron morphology is determined by its cytoskeleton. Microtubules, one of the cytoskeleton components, are particularly abundant in neurons[1]. Neuron microtubules are less dynamic than other cell microtubules due to the presence of some microtubule associated proteins (MAPs)[2] that copolymerize with tubulin and that stabilize microtubule polymers[3]. One of these MAPs is tau protein, which has a function as a microtubule stabilizer[4,5], a role that can be complemented, in its absence, by the presence of other neuronal MAPs[2]. However, in some neurodegenerative disorders known as tauopathies[6], tau can be modified by post translational modifications. This modified tau can be toxic.
In the tauopathies, tau can be found in an aggregated form. A clear example is the most predominant tauopathy, Alzheimer disease (AD), in which tau protein polymerizes into filaments (paired helical filaments)[7,8] that are the components of neurofibrillary tangles, one of the hallmarks of AD[9]. In paired helical filaments, tau is present in an abnormally hyperphosphorylated form[10].
Tau phosphorylation can be performed by different protein kinases, with glycogen synthase kinase-3 (GSK3) phosphorylating more phosphorytable sites in the tau molecule[11]. It is not clear if GSK3 dependent phosphorylation of tau could be toxic for a neuron. A GSK3β transgenic mouse model showed tau hyperphosphorylation and increased neuronal death in the hippocampus[12]. Moreover, in double transgenic mice which overexpressed GSK3β and mutated tau (human tau with three mutations associated with frontotemporal dementia FTDP-17), neurodegeneration appeared to be accelerated[13]. Recently, a new transgenic mouse model has been described that overexpresses GSK3β in a tau knockout background[14]. These mice show a slower progression of the degenerative process induced by GSK3β overexpression and attenuated learning deficits. This evidence supports the suggested toxicity of phosphorylated tau. Due to GSK3 induced neurodegeneration, this enzyme has been proposed as a therapeutical target to avoid neurodegeneration in tauopathies[15,16].
Also, it has been suggested that tau aggregation could induce neuron death in tauopathies, like AD, although this point is under discussion[17]. In addition, it has been shown that tau overexpression could be toxic for neurons[18]. Thus, an increase in tau, or in phosphotau or aggregated tau, might have pathological consequences.
On the other hand, two different tau-deficient mice models, isolated by gene-targeting, were viable and only some slight differences (muscle weakness and behavioral deficits) were observed in the preliminary analysis[19,20].Taking into account all the previous observations, it can be suggested that tau depletion might be a way to prevent the development of tauopathies.
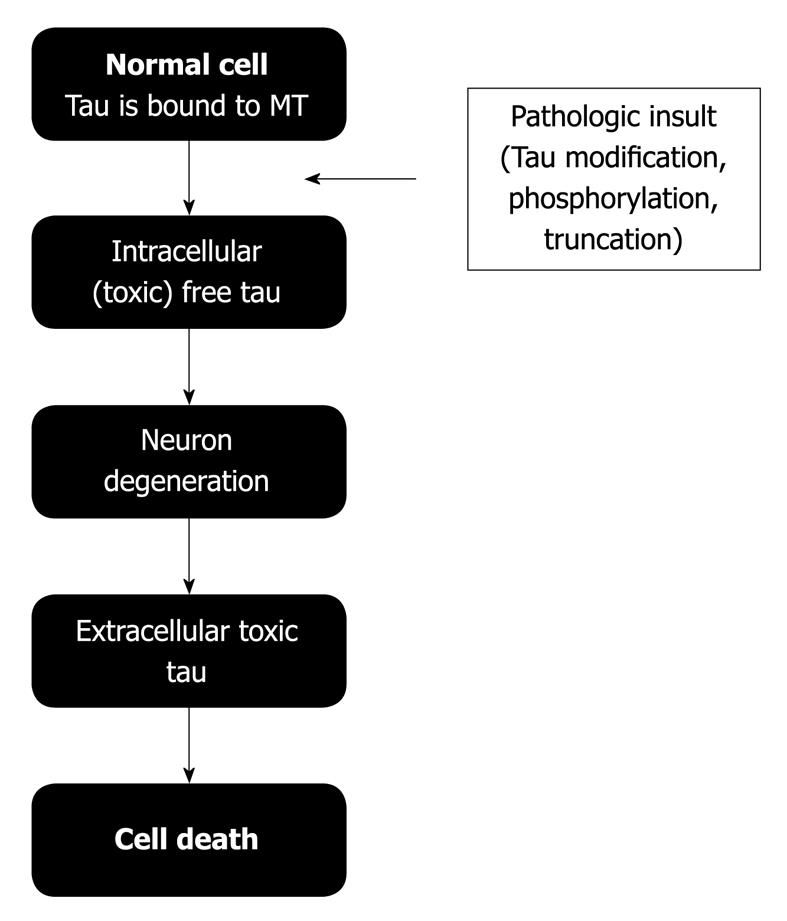
Previously, it was indicated that tau protein is associated with microtubules mainly in the cytoplasm. This association could be decreased when tau protein was phosphorylated[6]. Intracellular phosphotau could be toxic for a neuron and it could result in neuronal death. After neuronal death, cytoplasmic proteins are in the extracellular space. Some of these proteins could be toxic agents. Recently, it was shown that tau could be one of these toxic extracellular proteins[21-25] (Figure 1). Extracellular tau can bind to neuron receptors[25], promoting neuron degeneration and the formation of new extracellular (and toxic) tau. If this process is repeated, it could explain how tau pathology could spread through the brain, promoting the development of tauopathies such as AD.
If the origin of tauopathies is related to an excess of intracellular tau, phosphotau or aggregated tau, it would be of interest to know the main localizations of tau protein in the brain. It has been proposed that tau RNA and tau protein are mainly present in the temporal and frontal lobes[26,27], which are the lobes that are close to the nose in a mammal. A possible way to deplete intracellular tau in vivo could be the delivery of interference RNA (against tau) intranasally. Previous reports have described intranasal delivery of molecules to the central nervous system in rodents, primates and humans[28-31]. As an AD treatment, it has been proposed that the intranasal administration of insulin might improve memory in AD patients[32,33]. Preliminary data with intranasal siRNA (small interfering RNA) tau treatment in mice suggests that it can reach the brain, mainly the temporal and frontal lobes (Gomez de Barreda et al, unpublished data). In the case of extracellular tau, a possible way to deplete it would be the use of a tau vaccine[34].
All previous observations support the notion that tau depletion could be beneficial to avoid the development of tauopathies. However, it would be important to know the consequences of the lack of tau. Depletion of tau protein would obviously affect the different functions of tau. Some of those functions could be complemented by other proteins, but it is not clear if it would occur with all tau functions.
In fact, tau protein is a sticky protein that not only binds to tubulin but also to actin[35-37], presenilin-1[38], α-synuclein[39,40], calmodulin[41], phospholipase C-γ[42-44], ferritin[45], hGas7b[46] or even itself[47-49]. Moreover, in its phosphorylated state, it can also bind other proteins such as the chaperone protein Pin-1[50-52], 14-3-3 protein[53,54], c-Jun N-terminal kinase-interacting protein 1 (JIP1)[55], and many protein phosphatases (i.e. PP1, PP2A, PP2B and PP5)[56-60].
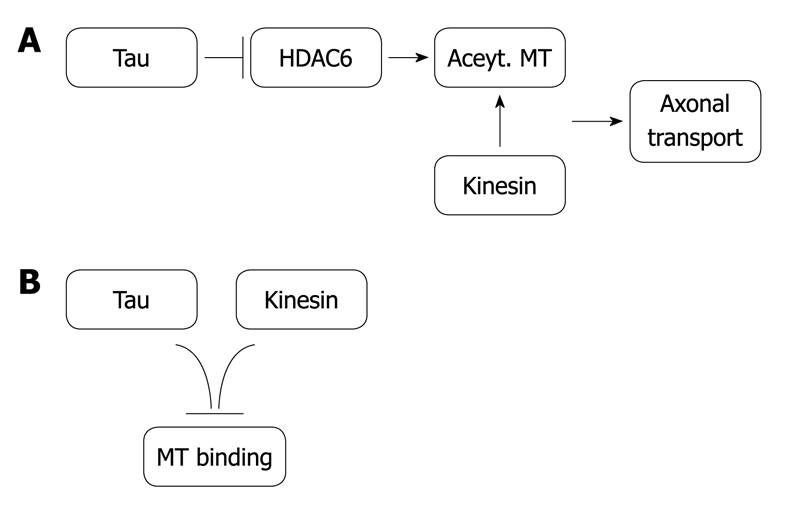
Recently, it has been found that tau protein binds to histone deacetylase 6 (HDAC6), and the consequence of that binding is an inhibition of HDAC6[61]. This protein deacetylases tubulin assembled in microtubules, which favors axonal vesicle transport[62]. Thus, the presence of tau protein would induce microtubule acetylation and, consequently, axonal transport. On the other hand, since tau competes with microtubule motors (involved in axonal transport) for the same tubulin binding site, an excess of tau protein may impair axonal transport. Thus, it would be an optimal amount of tau, neither too much nor too little, that would favor axonal transport (Figure 2). This optimal amount of tau could be right for other tau functions.
Modification of tau protein by phosphorylation or aggregation could result in a gain of toxic function in different tauopathies. It suggests that tau depletion could be beneficial in avoiding the development of those tauopathies. However, the absence of tau could promote other dysfunctions in a neuron.
Peer reviewer: Hiroshi Takahashi, MD, PhD, Director, Department of Neurology, National Hospital Organization, Tottori Medical Center, 876 Mitsu, Tottori, 689-0203, Japan
S- Editor Cheng JX L- Editor Lutze M E- Editor Zheng XM
| 2. | Matus A. Microtubule-associated proteins: their potential role in determining neuronal morphology. Annu Rev Neurosci. 1988;11:29-44. |
| 3. | Panda D, Goode BL, Feinstein SC, Wilson L. Kinetic stabilization of microtubule dynamics at steady state by tau and microtubule-binding domains of tau. Biochemistry. 1995;34:11117-11127. |
| 4. | Cleveland DW, Hwo SY, Kirschner MW. Purification of tau, a microtubule-associated protein that induces assembly of microtubules from purified tubulin. J Mol Biol. 1977;116:207-225. |
| 5. | Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proc Natl Acad Sci USA. 1975;72:1858-1862. |
| 6. | Avila J, Lucas JJ, Perez M, Hernandez F. Role of tau protein in both physiological and pathological conditions. Physiol Rev. 2004;84:361-384. |
| 7. | Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986;261:6084-6089. |
| 8. | Kidd M. Paired helical filaments in electron microscopy of Alzheimer's disease. Nature. 1963;197:192-193. |
| 9. | Alzheimer A. Über eine eigenartige erkrankung der hirnrinde. Allg Z Psychiat. 1907;64:146-148. |
| 10. | Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA. 1986;83:4913-4917. |
| 11. | Hanger DP, Anderton BH, Noble W. Tau phosphorylation: the therapeutic challenge for neurodegenerative disease. Trends Mol Med. 2009;15:112-119. |
| 12. | Lucas JJ, Hernández F, Gómez-Ramos P, Morán MA, Hen R, Avila J. Decreased nuclear beta-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3beta conditional transgenic mice. EMBO J. 2001;20:27-39. |
| 13. | Engel T, Lucas JJ, Gómez-Ramos P, Moran MA, Avila J, Hernández F. Cooexpression of FTDP-17 tau and GSK-3beta in transgenic mice induce tau polymerization and neurodegeneration. Neurobiol Aging. 2006;27:1258-1268. |
| 14. | Gómez de Barreda E, Pérez M, Gómez Ramos P, de Cristobal J, Martín-Maestro P, Morán A, Dawson HN, Vitek MP, Lucas JJ, Hernández F. Tau-knockout mice show reduced GSK3-induced hippocampal degeneration and learning deficits. Neurobiol Dis. 2010;37:622-629. |
| 15. | Noble W, Planel E, Zehr C, Olm V, Meyerson J, Suleman F, Gaynor K, Wang L, LaFrancois J, Feinstein B. Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc Natl Acad Sci USA. 2005;102:6990-6995. |
| 16. | Pérez M, Hernández F, Lim F, Díaz-Nido J, Avila J. Chronic lithium treatment decreases mutant tau protein aggregation in a transgenic mouse model. J Alzheimers Dis. 2003;5:301-308. |
| 17. | Hernández F, Avila J. Tauopathies. Cell Mol Life Sci. 2007;64:2219-2233. |
| 18. | Andorfer C, Acker CM, Kress Y, Hof PR, Duff K, Davies P. Cell-cycle reentry and cell death in transgenic mice expressing nonmutant human tau isoforms. J Neurosci. 2005;25:5446-5454. |
| 19. | Dawson HN, Ferreira A, Eyster MV, Ghoshal N, Binder LI, Vitek MP. Inhibition of neuronal maturation in primary hippocampal neurons from tau deficient mice. J Cell Sci. 2001;114:1179-1187. |
| 20. | Harada A, Oguchi K, Okabe S, Kuno J, Terada S, Ohshima T, Sato-Yoshitake R, Takei Y, Noda T, Hirokawa N. Altered microtubule organization in small-calibre axons of mice lacking tau protein. Nature. 1994;369:488-491. |
| 21. | Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, Fraser G, Stalder AK, Beibel M, Staufenbiel M. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11:909-913. |
| 22. | Frost B, Jacks RL, Diamond MI. Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem. 2009;284:12845-12852. |
| 23. | Gómez-Ramos A, Díaz-Hernández M, Cuadros R, Hernández F, Avila J. Extracellular tau is toxic to neuronal cells. FEBS Lett. 2006;580:4842-4850. |
| 24. | Gómez-Ramos A, Díaz-Hernández M, Rubio A, Díaz-Hernández JI, Miras-Portugal MT, Avila J. Characteristics and consequences of muscarinic receptor activation by tau protein. Eur Neuropsychopharmacol. 2009;19:708-717. |
| 25. | Gómez-Ramos A, Díaz-Hernández M, Rubio A, Miras-Portugal MT, Avila J. Extracellular tau promotes intracellular calcium increase through M1 and M3 muscarinic receptors in neuronal cells. Mol Cell Neurosci. 2008;37:673-681. |
| 26. | Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168-176. |
| 27. | Santa-María I, Hernández F, Smith MA, Perry G, Avila J, Moreno FJ. Neurotoxic dopamine quinone facilitates the assembly of tau into fibrillar polymers. Mol Cell Biochem. 2005;278:203-212. |
| 28. | Alcalá-Barraza SR, Lee MS, Hanson LR, McDonald AA, Frey WH 2nd, McLoon LK. Intranasal delivery of neurotrophic factors BDNF, CNTF, EPO, and NT-4 to the CNS. J Drug Target. 2010;18:179-190. |
| 29. | Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514-516. |
| 30. | Hanson LR, Frey WH 2nd. Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008;9 Suppl 3:S5. |
| 31. | Thorne RG, Frey WH 2nd. Delivery of neurotrophic factors to the central nervous system: pharmacokinetic considerations. Clin Pharmacokinet. 2001;40:907-946. |
| 32. | Reger MA, Watson GS, Frey WH 2nd, Baker LD, Cholerton B, Keeling ML, Belongia DA, Fishel MA, Plymate SR, Schellenberg GD. Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiol Aging. 2006;27:451-458. |
| 33. | Reger MA, Watson GS, Green PS, Wilkinson CW, Baker LD, Cholerton B, Fishel MA, Plymate SR, Breitner JC, DeGroodt W. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2008;70:440-448. |
| 34. | Kayed R, Jackson GR. Prefilament tau species as potential targets for immunotherapy for Alzheimer disease and related disorders. Curr Opin Immunol. 2009;21:359-363. |
| 35. | Correas I, Padilla R, Avila J. The tubulin-binding sequence of brain microtubule-associated proteins, tau and MAP-2, is also involved in actin binding. Biochem J. 1990;269:61-64. |
| 36. | Farias GA, Muñoz JP, Garrido J, Maccioni RB. Tubulin, actin, and tau protein interactions and the study of their macromolecular assemblies. J Cell Biochem. 2002;85:315-324. |
| 37. | Griffith LM, Pollard TD. The interaction of actin filaments with microtubules and microtubule-associated proteins. J Biol Chem. 1982;257:9143-9151. |
| 38. | Takashima A, Murayama M, Murayama O, Kohno T, Honda T, Yasutake K, Nihonmatsu N, Mercken M, Yamaguchi H, Sugihara S. Presenilin 1 associates with glycogen synthase kinase-3beta and its substrate tau. Proc Natl Acad Sci USA. 1998;95:9637-9641. |
| 39. | Benussi L, Ghidoni R, Paterlini A, Nicosia F, Alberici AC, Signorini S, Barbiero L, Binetti G. Interaction between tau and alpha-synuclein proteins is impaired in the presence of P301L tau mutation. Exp Cell Res. 2005;308:78-84. |
| 40. | Jensen PH, Hager H, Nielsen MS, Hojrup P, Gliemann J, Jakes R. alpha-synuclein binds to Tau and stimulates the protein kinase A-catalyzed tau phosphorylation of serine residues 262 and 356. J Biol Chem. 1999;274:25481-25489. |
| 41. | Padilla R, Maccioni RB, Avila J. Calmodulin binds to a tubulin binding site of the microtubule-associated protein tau. Mol Cell Biochem. 1990;97:35-41. |
| 42. | Hwang SC, Jhon DY, Bae YS, Kim JH, Rhee SG. Activation of phospholipase C-gamma by the concerted action of tau proteins and arachidonic acid. J Biol Chem. 1996;271:18342-18349. |
| 43. | Jenkins SM, Johnson GV. Tau complexes with phospholipase C-gamma in situ. Neuroreport. 1998;9:67-71. |
| 44. | Reynolds CH, Garwood CJ, Wray S, Price C, Kellie S, Perera T, Zvelebil M, Yang A, Sheppard PW, Varndell IM. Phosphorylation regulates tau interactions with Src homology 3 domains of phosphatidylinositol 3-kinase, phospholipase Cgamma1, Grb2, and Src family kinases. J Biol Chem. 2008;283:18177-18186. |
| 45. | Pérez M, Valpuesta JM, de Garcini EM, Quintana C, Arrasate M, López Carrascosa JL, Rábano A, García de Yébenes J, Avila J. Ferritin is associated with the aberrant tau filaments present in progressive supranuclear palsy. Am J Pathol. 1998;152:1531-1539. |
| 46. | Akiyama H, Gotoh A, Shin RW, Koga T, Ohashi T, Sakamoto W, Harada A, Arai H, Sawa A, Uchida C. A novel role for hGas7b in microtubular maintenance: possible implication in tau-associated pathology in Alzheimer disease. J Biol Chem. 2009;284:32695-32699. |
| 47. | Montejo de Garcini E, Avila J. In vitro conditions for the self-polymerization of the microtubule-associated protein, tau factor. J Biochem. 1987;102:1415-1421. |
| 48. | Montejo de Garcini E, Carrascosa JL, Correas I, Nieto A, Avila J. Tau factor polymers are similar to paired helical filaments of Alzheimer's disease. FEBS Lett. 1988;236:150-154. |
| 49. | Montejo de Garcini E, Serrano L, Avila J. Self assembly of microtubule associated protein tau into filaments resembling those found in Alzheimer disease. Biochem Biophys Res Commun. 1986;141:790-796. |
| 50. | Lim J, Balastik M, Lee TH, Nakamura K, Liou YC, Sun A, Finn G, Pastorino L, Lee VM, Lu KP. Pin1 has opposite effects on wild-type and P301L tau stability and tauopathy. J Clin Invest. 2008;118:1877-1889. |
| 51. | Lu PJ, Wulf G, Zhou XZ, Davies P, Lu KP. The prolyl isomerase Pin1 restores the function of Alzheimer-associated phosphorylated tau protein. Nature. 1999;399:784-788. |
| 52. | Zhou XZ, Kops O, Werner A, Lu PJ, Shen M, Stoller G, Küllertz G, Stark M, Fischer G, Lu KP. Pin1-dependent prolyl isomerization regulates dephosphorylation of Cdc25C and tau proteins. Mol Cell. 2000;6:873-883. |
| 53. | Hashiguchi M, Sobue K, Paudel HK. 14-3-3zeta is an effector of tau protein phosphorylation. J Biol Chem. 2000;275:25247-25254. |
| 54. | Truong AB, Masters SC, Yang H, Fu H. Role of the 14-3-3 C-terminal loop in ligand interaction. Proteins. 2002;49:321-325. |
| 55. | Ittner LM, Ke YD, Götz J. Phosphorylated Tau interacts with c-Jun N-terminal kinase-interacting protein 1 (JIP1) in Alzheimer disease. J Biol Chem. 2009;284:20909-20916. |
| 56. | Gong CX, Grundke-Iqbal I, Damuni Z, Iqbal K. Dephosphorylation of microtubule-associated protein tau by protein phosphatase-1 and -2C and its implication in Alzheimer disease. FEBS Lett. 1994;341:94-98. |
| 57. | Gong CX, Grundke-Iqbal I, Iqbal K. Dephosphorylation of Alzheimer's disease abnormally phosphorylated tau by protein phosphatase-2A. Neuroscience. 1994;61:765-772. |
| 58. | Gong CX, Lidsky T, Wegiel J, Zuck L, Grundke-Iqbal I, Iqbal K. Phosphorylation of microtubule-associated protein tau is regulated by protein phosphatase 2A in mammalian brain. Implications for neurofibrillary degeneration in Alzheimer's disease. J Biol Chem. 2000;275:5535-5544. |
| 59. | Gong CX, Singh TJ, Grundke-Iqbal I, Iqbal K. Alzheimer's disease abnormally phosphorylated tau is dephosphorylated by protein phosphatase-2B (calcineurin). J Neurochem. 1994;62:803-806. |
| 60. | Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur J Neurosci. 2005;22:1942-1950. |
| 61. | Perez M, Santa-Maria I, Gomez de Barreda E, Zhu X, Cuadros R, Cabrero JR, Sanchez-Madrid F, Dawson HN, Vitek MP, Perry G. Tau--an inhibitor of deacetylase HDAC6 function. J Neurochem. 2009;109:1756-1766. |
| 62. | Kazantsev AG, Thompson LM. Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nat Rev Drug Discov. 2008;7:854-868. |