Copyright
©2010 Baishideng Publishing Group Co.
World J Biol Chem. Mar 26, 2010; 1(3): 31-40
Published online Mar 26, 2010. doi: 10.4331/wjbc.v1.i3.31
Published online Mar 26, 2010. doi: 10.4331/wjbc.v1.i3.31
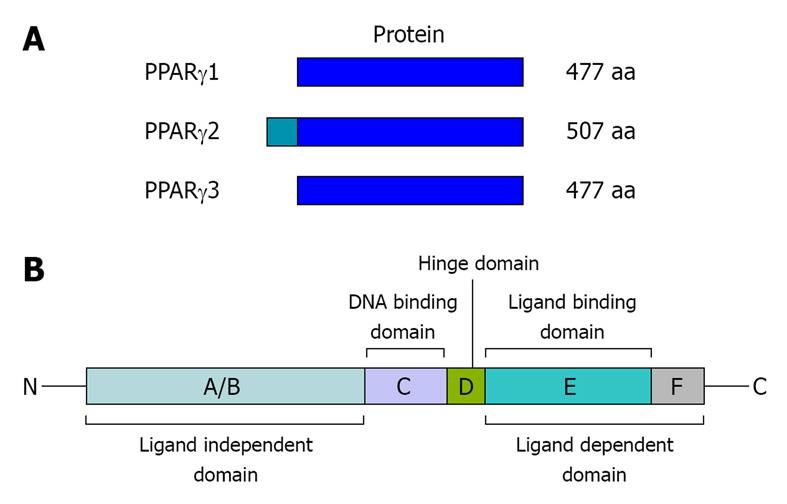
Figure 1 Structure of human peroxisome proliferator-activated receptor (PPAR)γ protein isoforms and domains.
A: AR proteins. The three subtypes of mRNAs give rise to two different PPARγ proteins. Transcription of the PPARγ1 and 3 promoters result in the same protein of 477 amino acids (aa). The PPARγ2 protein of 507 amino acids is produced by transcription from the promoter γ2 area; B: main structure of PPARγ. PPARs contain the following functional regions: an N-terminal A/B domain (ligand-independent domain), a C-domain (DNA-binding domain), a D-domain (hinge domain), and a C-terminal domain (E-F ligand-dependent domain).
- Citation: Han SW, Roman J. Anticancer actions of PPARγ ligands: Current state and future perspectives in human lung cancer. World J Biol Chem 2010; 1(3): 31-40
- URL: https://www.wjgnet.com/1949-8454/full/v1/i3/31.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v1.i3.31