Copyright
©The Author(s) 2017.
World J Biol Chem. Aug 26, 2017; 8(3): 163-174
Published online Aug 26, 2017. doi: 10.4331/wjbc.v8.i3.163
Published online Aug 26, 2017. doi: 10.4331/wjbc.v8.i3.163
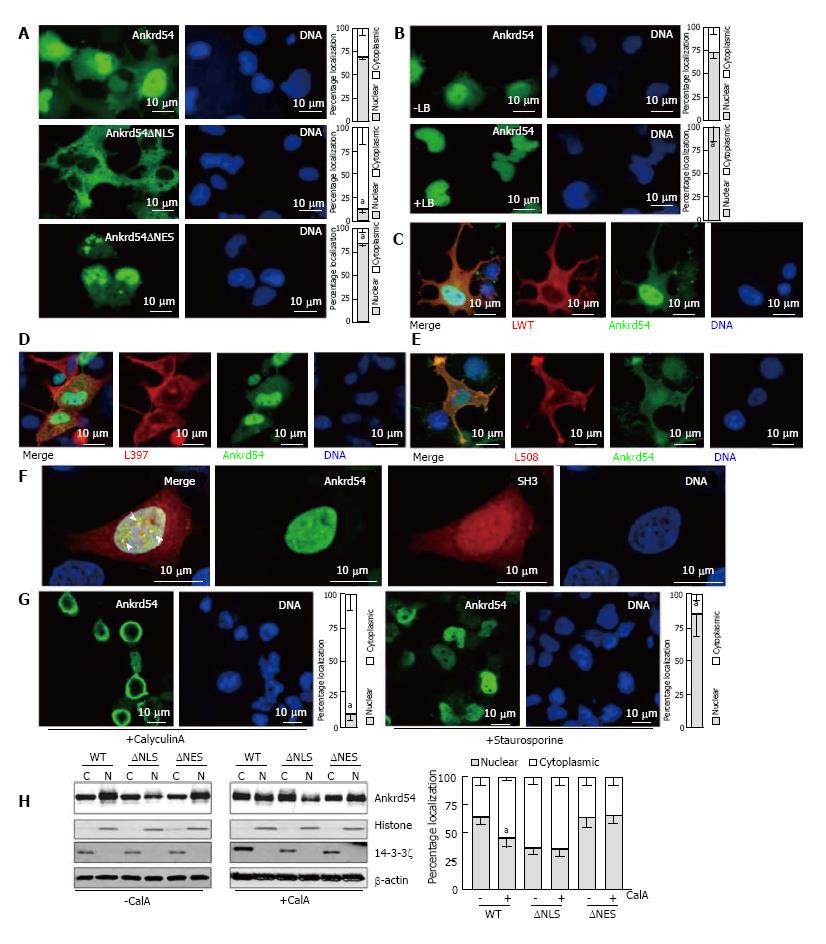
Figure 1 Ankrd54 shuttles between nuclear and cytoplasmic compartments and phosphorylation regulates this subcellular compartmentalization.
A: Localization analysis of eGFP-tagged Ankrd54 (top panel), eGFP-Ankrd54 with the NLS deleted (Ankrd54∆NLS, middle panel), and eGFP-Ankrd54 with the NES deleted (Ankrd54∆NES, bottom panel) in HEK293 cells. Delineation of the nucleus by Hoechst staining of the DNA is on the right, eGFP fluorescence is on the left. Nuclear and cytoplasmic localization of Ankrd54 was enumerated (graph at right), aP < 0.05; B: Wild-type eGFP-Ankrd54 expressing HEK293 cells were analysed after treatment with Leptomycin B (+LB) for 2 h (0.4 ng/mL), eGFP fluorescence (left) DNA counter staining (right), and quantitation of nuclear/cytoplasmic localization (graph at right), aP < 0.05; C: Localization analysis of co-expressed eGFP-Ankrd54 (green) and Lyn wild-type (LWT, red), with DNA counterstained (blue). Merged and individual channels are shown; D: Localization analysis of co-expressed eGFP-Ankrd54 (green) and kinase inactive Lyn (L397, red), with DNA counterstained (blue). Merged and individual channels are shown; E: Localization analysis of co-expressed eGFP-Ankrd54 (green) and dominant active Lyn (L598, red), with DNA counterstained (blue). Merged and individual channels are shown; F: Localization analysis of eGFP-Ankrd54 and an mCherry-fusion of the SH3 domain of Lyn, with DNA counterstained. Merged image (left panel) illustrates co-localizing nuclear puncta (arrow heads); G: Effect of CalyculinA (50 nmol/L, 60 min) or Staurosporine (100 nmol/L, 60 min) on eGFP-Ankrd54 subcellular localization. DNA counterstained (blue), eGFP fluorescence (green). Quantitation of nuclear/cytoplasmic localization (graph at right), aP < 0.05; H: Immunoblot of subcellular fractionation analysis of eGFP-Ankrd54 (WT), eGFP-Ankrd54∆NLS (∆NLS) and eGFP-Ankrd54∆NES (∆NES), without (left) and with (right) the addition of CalyculinA (50 nmol/L, 60min). Nuclear (N) and cytoplasmic (C) fractions of transfected HEK293 cells were immunoblotted using anti-Ankrd54, anti-Histone (H3), anti-14-3-3ζ, and anti-β-actin antibodies. Quantitation of nuclear/cytoplasmic localization of Ankrd54 bands depicted in graph at right, aP < 0.05.
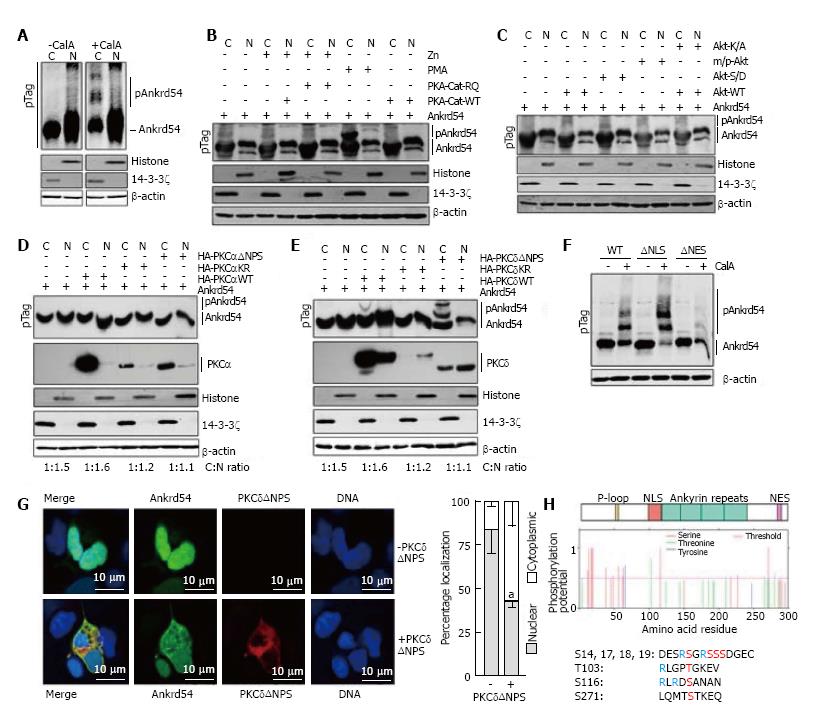
Figure 2 Phosphorylation by PKCδ regulates subcellular compartmentalization of Ankrd54.
A: Immunoblot analysis of nuclear (N) and cytoplasmic (C) fractionation of eGFP-Ankrd54 using Phos-Tag™ SDS-PAGE (pTag), with (+CalA) and without (-CalA) CalyculinA treatment (50 nmol/L, 60 min). Transfected HEK293 cells were immunoblotted using anti-Ankrd54, anti-Histone (H3), anti-14-3-3ζ, and anti-β-actin antibodies. Mobility shifted Ankrd54, due to phosphorylation, is indicated (pAnkrd54); B: Immunoblot analysis of nuclear (N) and cytoplasmic (C) fractionation of eGFP-Ankrd54 using Phos-Tag™ SDS-PAGE (pTag), co-transfected with PKA expression plasmids, or with addition of PMA (200 nmol/L, 60 min). The wild-type (PKA-Cat-WT) catalytic subunit of PKA, or a dominant active mutant (PKA-Cat-RQ) were induced to express by the addition of Zn. Transfected HEK293 cells were immunoblotted using anti-Ankrd54, anti-Histone (H3), anti-14-3-3ζ, and anti-β-actin antibodies. Mobility shifted Ankrd54, due to phosphorylation, is indicated (pAnkrd54); C: Immunoblot analysis of nuclear (N) and cytoplasmic (C) fractionation of eGFP-Ankrd54 using Phos-Tag™ SDS-PAGE (pTag), co-transfected with Akt expression plasmids. Plasmids expressed either wild-type Akt (Akt-WT), a kinase inactive mutant (Akt-K/A), an oncogenic myristoylated/palmitylated Akt (m/p-Akt), or an activation phospho-mimic (Akt-S/D). Transfected HEK293 cells were immunoblotted using anti-Ankrd54, anti-Histone (H3), anti-14-3-3ζ, and anti-β-actin antibodies. Mobility shifted Ankrd54, due to phosphorylation, is indicated (pAnkrd54); D: Immunoblot analysis of nuclear (N) and cytoplasmic (C) fractionation of eGFP-Ankrd54 using Phos-Tag™ SDS-PAGE (pTag), co-transfected with HA-tagged PKCα expression plasmids. Plasmids expressed either wild-type PKCα (HA-PKAαWT), a kinase inactive mutant (HA-PKCαKR), or an activation mutant (HA-PKCαΔNPS). Transfected HEK293 cells were immunoblotted using anti-Ankrd54, anti-HA (PKCα), anti-Histone (H3), anti-14-3-3ζ, and anti-β-actin antibodies. Mobility shifted Ankrd54, due to phosphorylation, is indicated (pAnkrd54). Quantitation of cytoplasmic:nuclear ratios are displayed; E: Immunoblot analysis of nuclear (N) and cytoplasmic (C) fractionation of eGFP-Ankrd54 using Phos-Tag™ SDS-PAGE (pTag), co-transfected with HA-tagged PKCδ expression plasmids. Plasmids expressed either wild-type PKCδ (HA-PKAδWT), a kinase inactive mutant (HA-PKCδKR), or an activation mutant (HA-PKCδ∆NPS). Transfected HEK293 cells were immunoblotted using anti-Ankrd54, anti-HA (PKCδ), anti-Histone (H3), anti-14-3-3ζ, and anti-β-actin antibodies. Mobility shifted Ankrd54, due to phosphorylation, is indicated (pAnkrd54). Quantitation of cytoplasmic:nuclear ratios are displayed; F: Immunoblot analysis of cytoplasmic fractionation of eGFP-Ankrd54 using Phos-Tag™ SDS-PAGE (pTag), with and without the addition of CalyculinA (50 nmol/L, 60 min). Transfected HEK293 cells were immunoblotted using anti-Ankrd54 and anti-β-actin antibodies. Mobility shifted Ankrd54, due to phosphorylation, is indicated (pAnkrd54); G: Localization analysis of eGFP-Ankrd54 with and without co-expression of dominant active PKCδ (PKCδΔNPS) in HEK293 cells. Delineation of the nucleus by Hoechst staining of the DNA is on the right, eGFP fluorescence (green), PKCδ (HA-tag, red), with merged image (left). Nuclear and cytoplasmic localization of eGFP-Ankrd54 was enumerated (graph at right), aP < 0.05; H: Prediction of phosphorylated residues in Ankrd54. Top, schematic of Ankrd54 domain structure showing location of P-loop (yellow), NLS (red), ankyrin repeats (green) and NES (purple). Middle, graph of potential phosphorylated residues, predicted by NetPhos3.1. Below, amino acid sequences surrounding top predicted motifs as indicated.
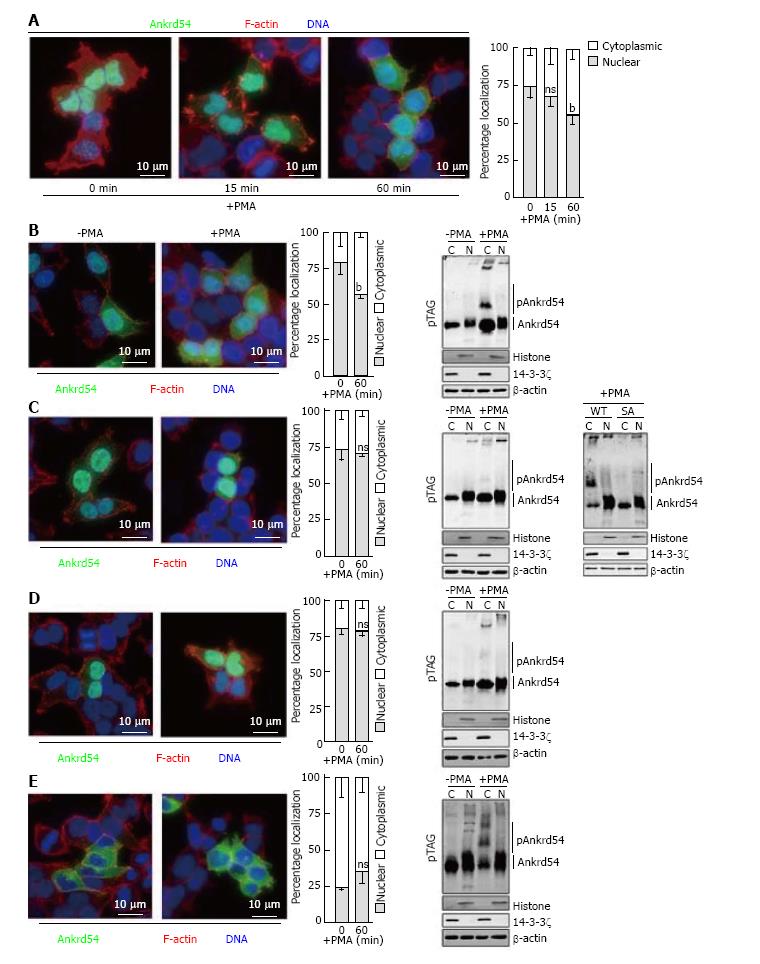
Figure 3 Amino terminal serine cluster (Ser14, Ser17, Ser18, Ser19) of Ankrd54 is required for phorbol 12-myristate 13-acetate stimulated nuclear export.
A: Localization analysis of eGFP-tagged Ankrd54 (green) in HEK293 cells stimulated with PMA (200 nmol/L) for the indicated times. Delineation of the nucleus by Hoechst staining (blue), and F-actin with TRITC-phalloidin (red). Nuclear and cytoplasmic localization of Ankrd54 was enumerated (graph at right), bP < 0.01, ns: Not significant (P > 0.05); B-E: Localization analysis of eGFP-tagged Ankrd54 by immunofluorescence of cells (left panels) and by Phos-Tag™ SDS-PAGE (pTag) analysis (right panel), in HEK293 cells with and without PMA treatment (200 nmol/L, 60 min). Delineation of the nucleus by Hoechst staining (blue), and F-actin with TRITC-phalloidin (red). Nuclear and cytoplasmic localization of Ankrd54 was enumerated (graph in centre), bP < 0.01. Right panel, immunoblot analysis of nuclear (N) and cytoplasmic (C) fractionation of transfected HEK293 cells were immunoblotted using anti-Ankrd54, anti-Histone (H3), anti-14-3-3ζ, and anti-β-actin antibodies. Mobility shifted Ankrd54, due to phosphorylation, is indicated (pAnkrd54). Cell immunofluorescence and immunoblot analysis was undertaken for cells expressing wild-type (B), serine-alanine (S14A, S17A, S18A, S19A) mutated (C), NLS deleted (D) and NES deleted (E) constructs of eGFP-tagged Ankrd54. For panel (C) a direct in-gel comparison of wild-type and SA mutant Ankrd54, before and after PMA stimulation is shown in the far-right panel. PMA: Phorbol 12-myristate 13-acetate.
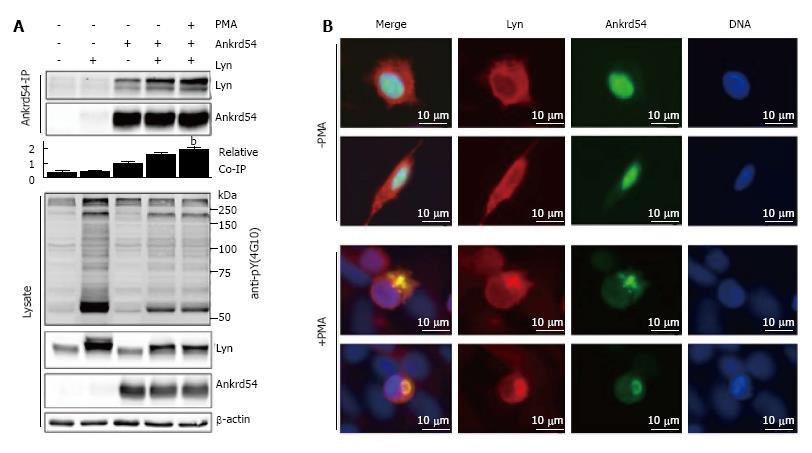
Figure 4 Phorbol 12-myristate 13-acetate stimulation of cells co-expressing Ankrd54 and Lyn promotes their co-immunoprecipitation and co-locations.
A: Immunoblot analysis of Ankrd54 immunoprecipitates from cells transfected with eGFP-Ankrd54 and/or Lyn with or without PMA stimulation (200 mmol/L, 1 h). Immunoprecipitates and cell lysates were immunoblotted using anti-Ankrd54, anti-Lyn (sc-15, sc-7274), anti-pY-4G10, and anti-β-actin antibodies, bP < 0.01; B: Localization analysis of eGFP-tagged Ankrd54 (green) and Lyn (sc-15, alexaFlour546) by immunofluorescence of HEK293 cells with and without PMA treatment (200 nmol/L, 1 h). Delineation of the nucleus by Hoechst staining (blue). Scale bar = 10 μm; PMA: Phorbol 12-myristate 13-acetate.
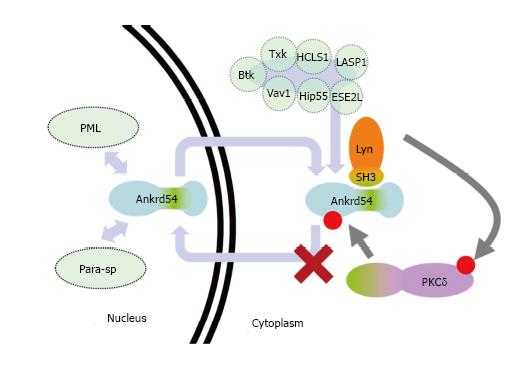
Figure 5 Schematic of proposed model of PKCδregulation of Ankrd54 nuclear-cytoplasmic shuttling.
Model of potential mechanism by which PKCδ regulated the cytoplasmic accumulation of Ankrd54. Ankrd54 shuttles between the nuclear and cytoplasmic compartments, promoted by its NLS and NES motifs. Ankrd54 can interact with Lyn, Btk, Txk, HCLS1, LASP1, Vav1, Hip55 and ESE2L. Nuclear Ankrd54 can localize to PML nuclear bodies and paraspeckles (Para-sp). Lyn, and potentially Btk/Txk, can phosphorylate and activate PKCδ, which can then phosphorylate Ankrd54, promoting cytoplasmic accumulation.
- Citation: Samuels AL, Louw A, Zareie R, Ingley E. Control of nuclear-cytoplasmic shuttling of Ankrd54 by PKCδ. World J Biol Chem 2017; 8(3): 163-174
- URL: https://www.wjgnet.com/1949-8454/full/v8/i3/163.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v8.i3.163