Copyright
©The Author(s) 2016.
World J Biol Chem. Feb 26, 2016; 7(1): 44-63
Published online Feb 26, 2016. doi: 10.4331/wjbc.v7.i1.44
Published online Feb 26, 2016. doi: 10.4331/wjbc.v7.i1.44
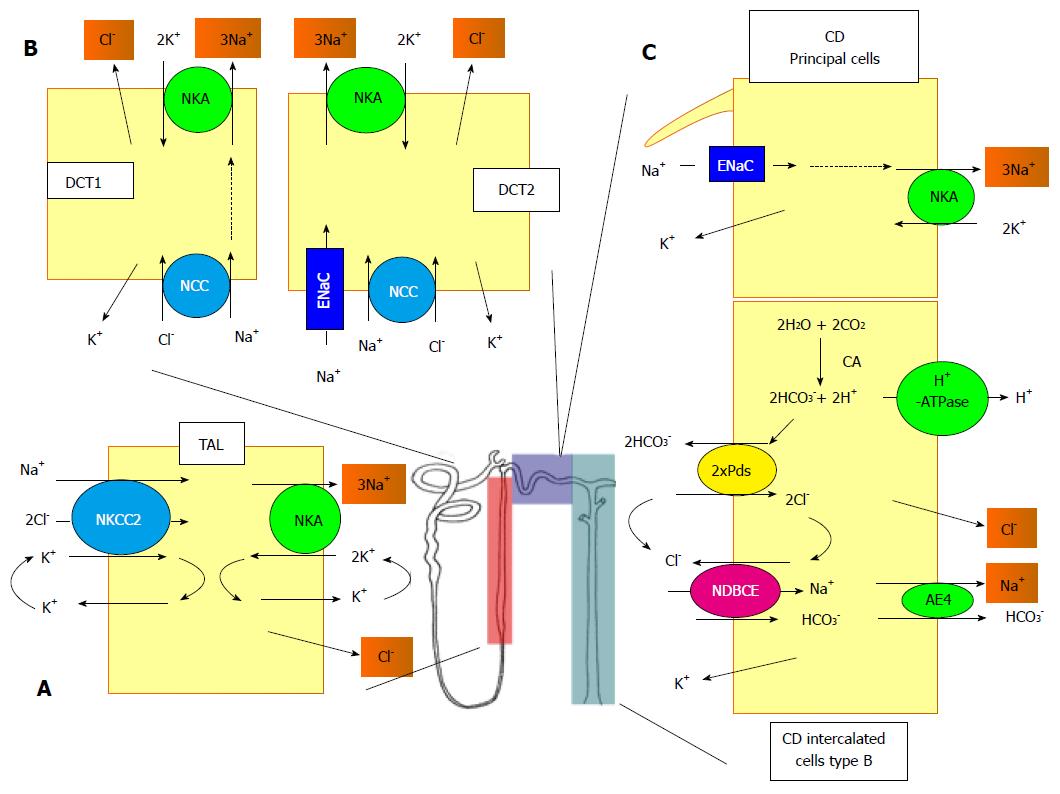
Figure 1 Schematic representation of the different sodium transport systems along the nephron.
A: In TAL, the apical entry of Na+ is mediated by the NKCC2. At the basolateral side, the Na+ exits the cell through the NKA and the Cl- through a channel of the CLC-K family; B: DCT consists of two different sub-structures, the DCT1 and the DCT2. In DCT1, the entry of Na+ and Cl- is mediated by the NCC and in DCT2, a ENaC is also involved. In both structures, the exit of Na+ and Cl- is mediated by the NKA and a CLC-K conductance; C: In the CD, the Na+ enters the principal cells through ENaC and exits through the NKA. The system is more complex in the B-intercalated cells. The apical entry of of Na+ and Cl- is mediated by the functional association between the pendrin, bicarbonate/Cl- exchanger (Pds), and NDBCE. The system is energized by the vacuolar proton pump (H+-ATPase) and the generation of bicarbonate mediated by a CA. The exit of Na+ and Cl- is mediated by an AE4 and a Cl--conductance, respectively. TAL: Thick ascending limb; NKCC2: Na+/K+/2Cl--cotransporter; NKA: Na,K-ATPase; DCT: Distal convoluted tubule; NCC: Na+/Cl--cotransporter; ENaC: Na+ channel; CD: collecting duct; Pds: Bicarbonate/Cl- exchanger; CA: Carbonic anhydrase; NDBCE: Na+-driven bicarbonate/Cl- exchanger; AE4: Anion exchanger.
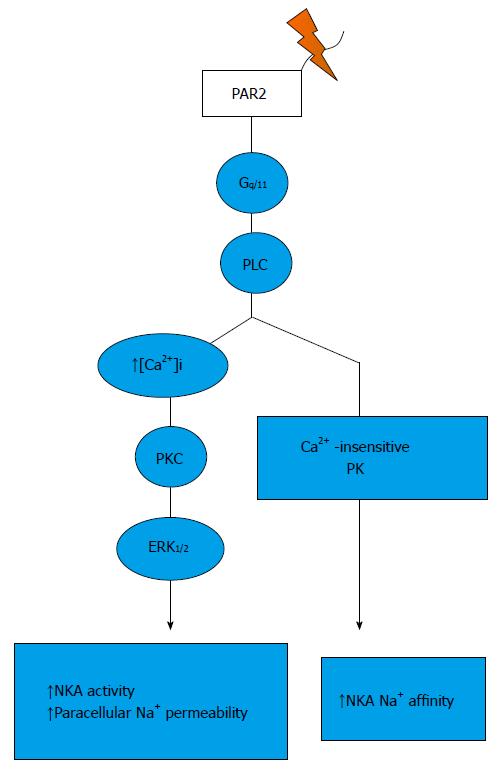
Figure 2 Signalling pathways trigger by protease-activated receptor 2 activation in the thick ascending limb.
PAR: Protease-activated receptor.
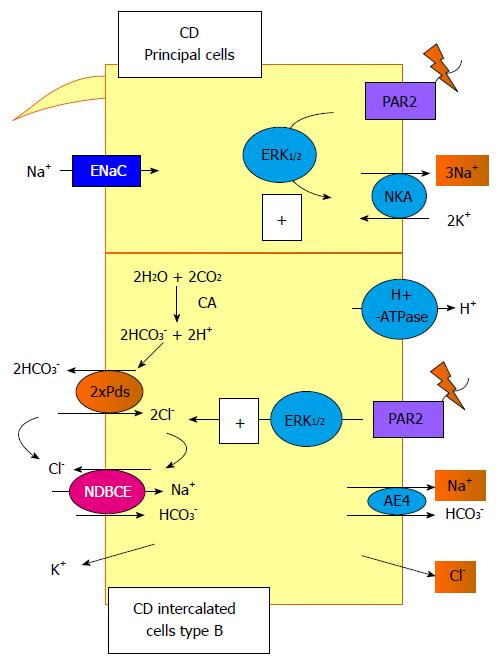
Figure 3 Signalling pathways trigger by protease-activated receptor 2 activation in the collecting duct.
In this segment, protease-activated receptor (PAR) 2 activation increases the NKA activity in the principal cells and the Pds/Na+-driven bicarbonate/Cl- exchanger (NDBCE) system in the B-IC. Both processes involved the activation of the ERK1/2 pathway. CD: Collecting duct; PAR: Protease-activated receptor.
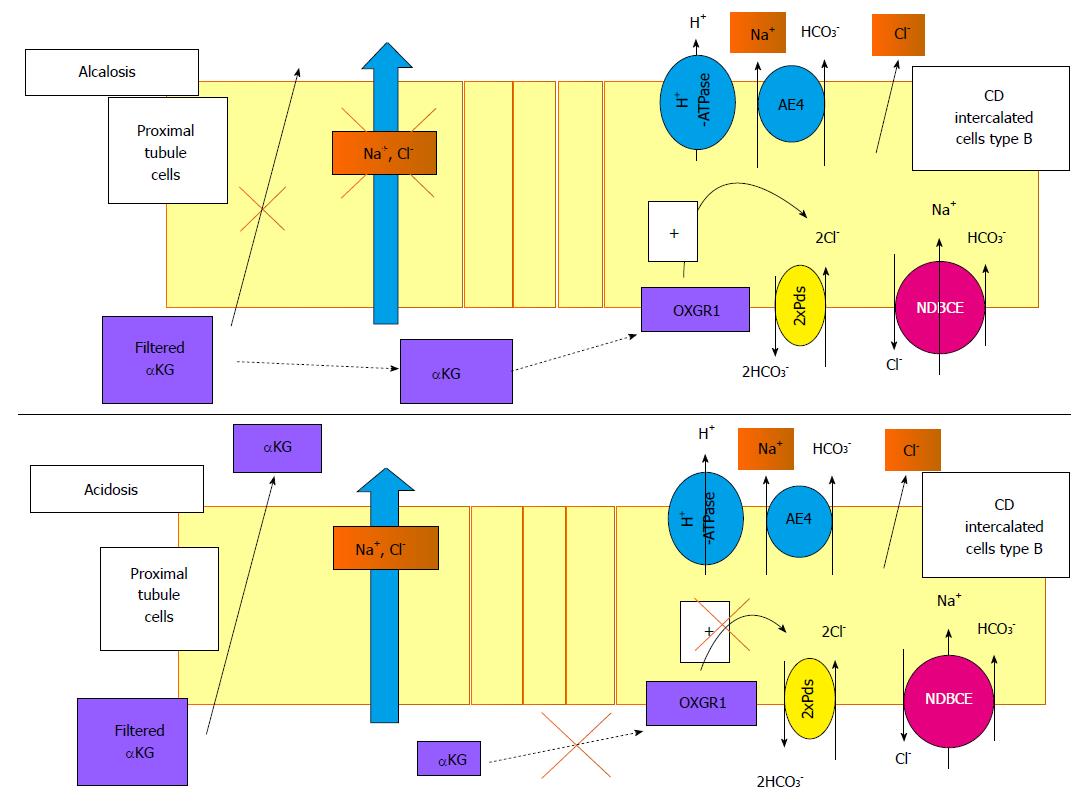
Figure 4 Role of α-ketoglutarate and its receptor, OXGR1, in the regulation of renal Na+ transport.
α-ketoglutarate (αKG) is freely filtered by the glomerulus. During alkalosis, Na+ reabsorption in the proximal segment is reduced and the reabsorption of αKG is blunted, increasing its concentration in the lumen. This high level of αKG delivered to the distal tubule may activate its receptor in the B-intercalated cells. The signalling pathway triggered by this activation is still unknown but activates the Pds/NDBCE system, leading to an increased Na+ reabsorption. In case of acidosis, the filtered pool of αKG is reabsorbed by the proximal tubule, decreasing its luminal concentration and blunting the activation of OXGR1 in the CD. In this case, the reabsorption of Na+ in the distal nephron is not stimulated. CD: Collecting duct; NDBCE: Na+-driven bicarbonate/Cl- exchanger; OXGR1: 2-oxoglutarate receptor 1; αKG: α-ketoglutarate.
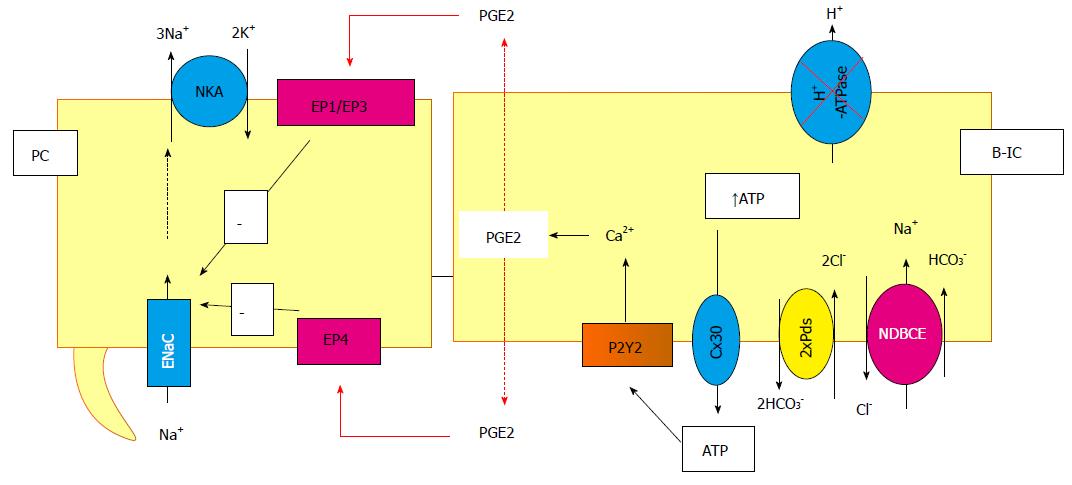
Figure 5 Cross-talk between principal and intercalated cells, example of the inhibition of the vacuolar proton pump.
In this particular situation, the ATP is released by the B-intercalated in the lumen cell through connexion and then binds and activates the P2Y2 receptors. This activation leads to prostaglandin (PGE2) synthesis and release in both side of the cells. It binds to the EP receptors in principal cells, inducing, then, the inhibition of the Na+ reabsorption. NDBCE: Na+-driven bicarbonate/Cl- exchanger; PGE2: Prostaglandin; B-IC: Type B intercalated cells.
- Citation: Morla L, Edwards A, Crambert G. New insights into sodium transport regulation in the distal nephron: Role of G-protein coupled receptors. World J Biol Chem 2016; 7(1): 44-63
- URL: https://www.wjgnet.com/1949-8454/full/v7/i1/44.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v7.i1.44